Abstract
Background:
Protozoa related to Trypanosome family including Leishmania, synthesize enzymes to escape from drug therapy. One of them is PTR1 that its enzymatic activity is similar to dihydrofolate reductase (DHFR). Dihydrofolate reductase - thymidylate synthase has a major role in DNA synthesis, if it is inhibited, the result would be the death of parasite. Since PTR1 activity is similar to DHFR, causes the decrease of inhibition effect of drug. The aim of this study was inhibition of Iranian L. major PTR1 expression with mRNA antisense in prokaryotic system as an approach to appear of the drugs therapeutic effects more.
Methods:
PTR1 gene was ligated to pACYCDuet-1 and pcDNA3 plasmids as sense and antisense plasmids, respectively. Simultaneously transfer of sense and antisense plasmids was done in E. coli strain M15. SDS-PAGE and western blot analysis were carried out to analyze the expression.
Results:
Sense and antisense plasmids were prepared and confirmed by restriction analysis and PCR then simultaneously transfer of them was done. SDS-PAGE and western blot analysis showed PTR1 gene was inhibited by mRNA antisense in bacterial cells.
Conclusion:
Expression of PTR1 gene in sense plasmid was inhibited successfully by antisense plasmid.
Keywords: PTR1, Leishmania major, Antisense plasmid, Dihydrofolate reductase, Expression
Introduction
Leishmania major protozoa are the causative agent of human zoonotic cutaneous leishmaniasis. Studies have shown that the growth of protozoa related to Trypanosome family depends on (folate-pteridine) (1). Protozoa related to Trypanosome family including Leishmania synthesizes enzymes to escape from drug therapy. One of these enzymes is PTR1 (1–9). The enzymatic activity of PTR1 is similar to dihydrofolate reductase (DHFR) (10, 11). DHFR plays a role in activating the enzyme of thymidylate synthase (TS) that catalyzes the conversion of deoxyuridylate in to deoxythymidylate and another role of DHFR is the reduction of dihydrofolate (H2-folate) to tetrahydrofolate (H4-folate) (9); the recent product is co-factor of thymidylate synthase (9–11). Thus DHFR-TS has a major role in DNA synthesis, if it is inhibited, according to the mentioned reactions of DNA synthesis, the result would be the death of parasite (1). Genesis drugs on metabolism in mediating cell proliferation are important affect; one of the mechanisms of drug is inhibition of DHFR-TS (1). Since PTR1 activity is similar to DHFR causes the decrease of inhibition effect of drug (10, 11). On the other hand, PTR1 causes synthesis and reduction of pteridine that increases the resistance of parasite against oxidative stress-related drug or reactions of the host (1, 2, 12). Because of production of enzymes by Leishmania including PTR1, the drug efficacy has been decreased and no response to treatment is observed, therefore, it is suggested that in addition of prescription of anti-parasite drugs, these enzymes inhibit in such way that the therapeutic effects of drugs appear more (1).
The aim of this study was inhibition of Iranian L. major PTR1 expression with antisense RNA in prokaryotic system.
Materials And Methods
Sense and antisense plasmids construction
In order to transfer of sense and antisense plasmids in one bacterial cell, two different plasmids with two different origins of replication, and two different antibiotic resistance genes were needed. The position of restriction enzyme cut sites used on the plasmids was in two different directions.
BamH I and Hind III were considered on the sense plasmid, as the expression, PTR I protein was produced. In addition, BamH I and KpnI were considered on antisense plasmid, in this case gene order placement is in the reverse mode.
For this purpose, plasmids pACYCDuet-1 and pcDNA3 were used for the sense and antisense plasmids, respectively.
Plasmid pQE–ptr that has been described previously (13), was digested by Hind III and BamH I to release the PTR1 gene. The fragment was cloned into Hind III and BamH I digested pACYCDuet-1. This vector was transformed in E. coli strain M15 then extracted and confirmed by restriction analysis and PCR.
For preparing of antisense plasmid, pQE–ptr (13) was digested by kpn I and BamH I to release the PTR1 gene. The fragment was cloned into pcDNA3, which was lineared by kpn I and BamH1. Recombinant plasmid was transformed in E. coli strain M15.
Recombinant plasmids were extracted and confirmed by restriction analysis and PCR.
Simultaneously transformation of sense and antisense plasmid in one bacterial cell
The E.coli strain M15 was transformed with pACYCDuet–ptr and selected on Luria Bertani agar containing 30 μg/ml chloramphenicol. These cells were transformed again with pcDNA–Rptr and selected on Luria Bertani agar containing 50 μg/ml ampicillin and 30 μg/ml chloramphenicol.
Transformation of pACYCDuet–ptr and pcDNA–Rptr in the E. coli strain M15 was done separately as control and selected on Luria Bertani agar containing selective antibiotics.
E. coli strain M15 was suseptible to used selective antibiotics.
Gene expression
Gene expression was done as previously described (13, 14) by a little modification. Briefly, the transformant E. coli strain M15 with both of sense and antisense plasmids was inoculated into 3 ml culture tube containing X medium (1.2% bacato trypton, 2.4% yeast extract, 0.04% glycerol, 1% M9 salts) (M9 salt containing 6.4% Na2HPO4 – 7H2oO, 1.5% KH2PO4, 0.025% NaCl, 0.05% NH4Cl) and allowed to grow at 37°C in a shaker incubator at 200 rpm overnight. The day after, it was inoculated in to 50 ml flask and allowed at 37ºC in a shaker incubator at 200 rpm. The culture in the logaritmic phase (at OD 600 = 0.6) was inducted with 0.8mM ispropyl-B-D-thiogalactopyranosid (IPTG) for 5 hours. After induction, cells were withdrawn and analyzed by 10% SDS–PAGE (15). Induced and uninduced the transformant bacterial cells with pcDNA-Rptr and pACYC Duet–ptr were analyzed in parallel.
Western blot analysis
For immunoblotting, proteins resolved by SDS-PAGE were electrophoretically transferred onto nitrocellulose membrane (13). Then UV cross linker was used for protein fixation. The membrane was blocked with 3% BSA (Bovine Serum Albumin) at room temperature and washed twice with TBS (Tris-Buffered Saline) after 1 h. After that incubated for 1 h at 37 °C with the rabbit anti-PTR1 antiserum at a 1:1000 dilution as the primary antibody. The membrane was washed three times with TBS-T (TBS-TWEEN 20) and incubated for 1 h at 37 °C with the goat anti-rabbit IgG HRP conjugate solution at a 1:5000 dilution as secondary antibody (13, 16). Antibody binding was visualized using calorimetry with diaminobenzidine (DAB) and H2O2.
Results
Preparation of sense and antisense plasmids:
Plasmid pQE-PTR1 that was the source of the L. major PTR1 gene (13) was digested with restriction enzymes and released the PTR1 gene. The PTR1 gene was ligated to pACYCDuet-1 and pcDNA3 then confirmed by restriction analysis and PCR (Fig. 1, Fig. 2, Fig. 3, Fig. 4) and named pACYC Duet–ptr and pcDNA–Rptr, respectively.
Fig. 1:
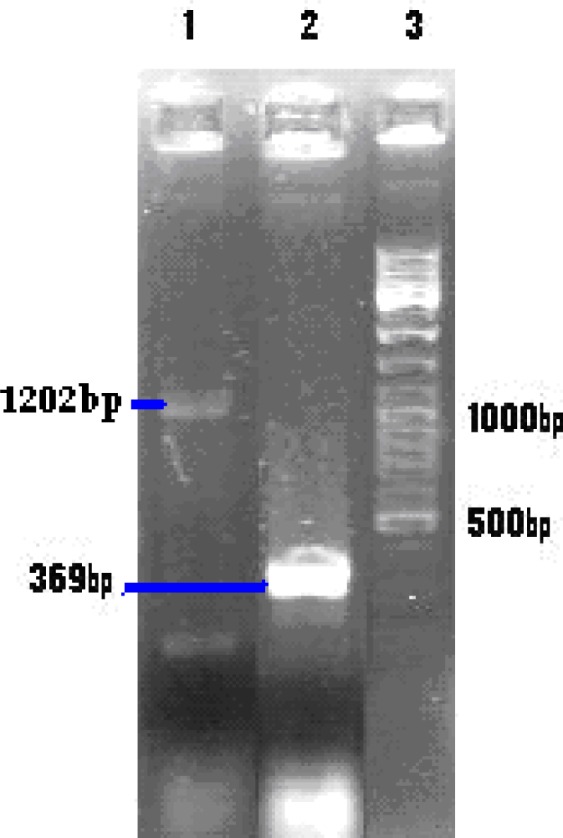
Electrophoresis of PCR product on 1% agarose gel
Lane 1: The 1202 bp as PCR product of pACYCDuet-ptr
Lane 2: The 369 bp as PCR product of pACYCDuet-1
Lane 3: 100 bp DNA ladder marker
Fig. 2:
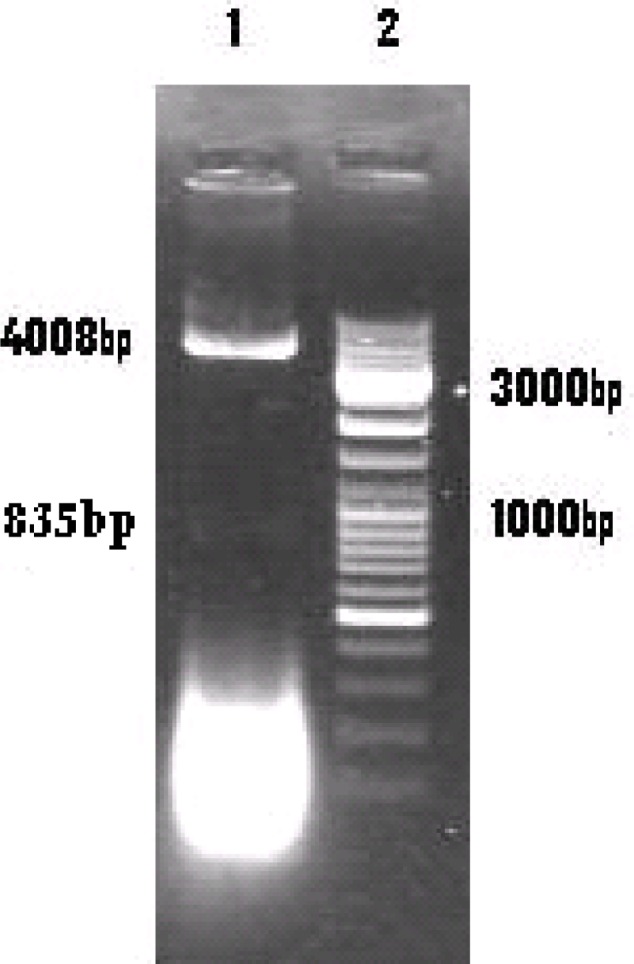
1.5% Agarose gel electrophoresis
Lane 1: Digested pACYCDuet-ptr by BamH I and Hind III
Lane 2: 100 bp DNA ladder marker
Fig. 3:
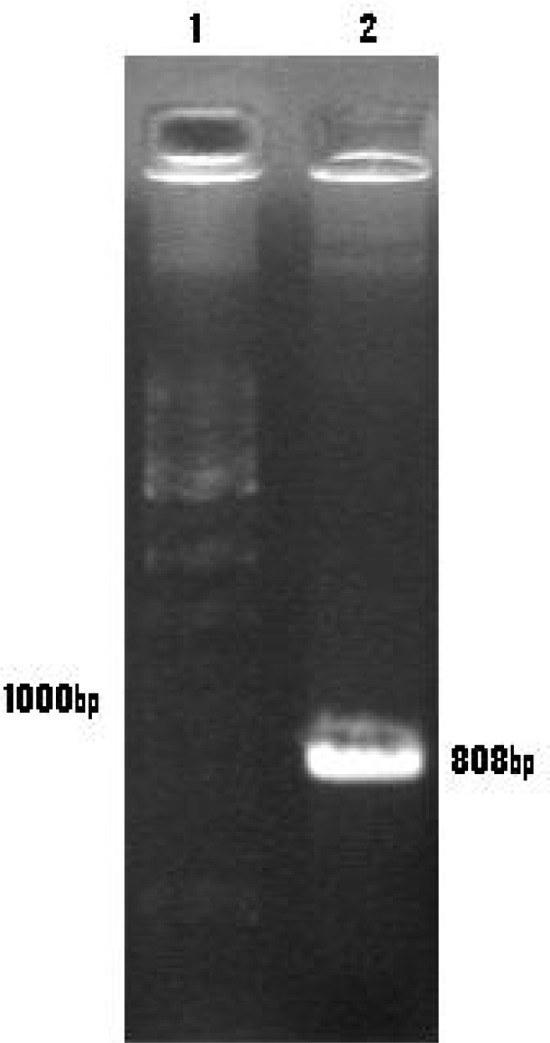
Electrophoresis of PCR product on 1% agarose gel
Lane 1: 100 bp DNA ladder marker
Lane 2: The 808 bp as PCR product of pcDNARptr
Fig. 4:
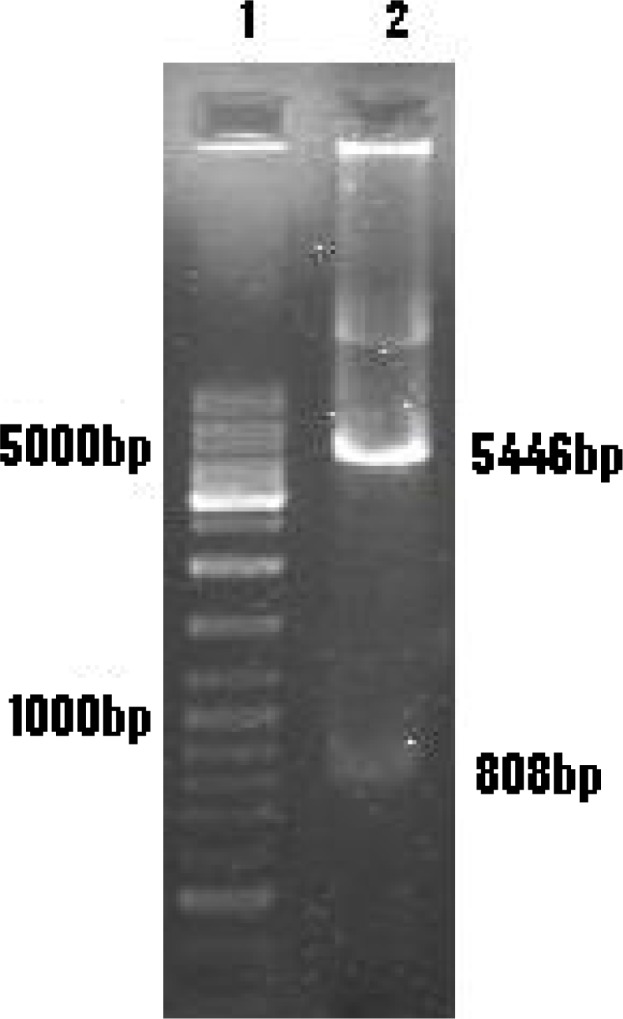
1.5% Agarose gel electrophoresis
Lane 1: 100 bp DNA ladder marker
Lane 2: Digested pcDNA-Rptr by BamH I and KpnI
The results of the simultaneously transfer of sense and antisense plasmids:
Plate from simultaneously transfer of pACYCDuet-ptr and pcDNA-Rptr plasmids contained colonies. Since the plate contained selective antibiotics (ampicillin, chloramphenicol) in every plasmid, the presence of colonies in such plate indicated the simultaneous presence of both plasmids in one bacterial cell.
Inhibition of gene expression in the sense plasmid by antisense plasmid: SDS-PAGE and Western blot analysis:
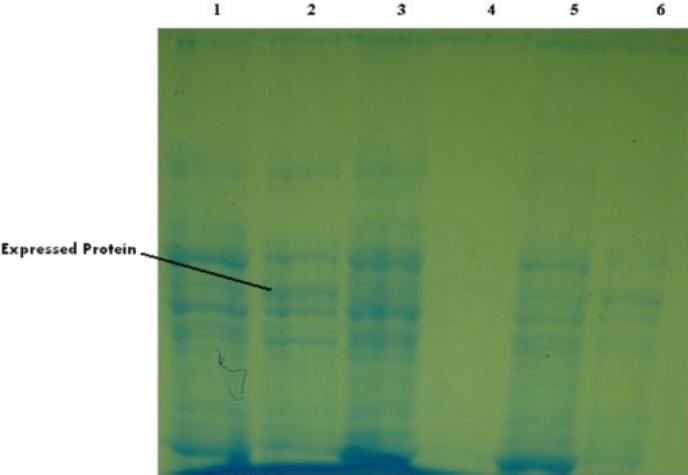
SDS-PAGE on lysate collected transformed bacteria with both of pACYCDuet-ptr and pcDNA-Rptr plasmids before and 5 hours after induction was performed. Induced bacteria containing pACYCDuet-ptr and induced bacteria containing pcDNA-Rptr also were analyzed in parallel. If the cloned PTR1 gene expressed in sense plasmid (pACYCDuet-ptr), the protein with molecular weight of about 30 kDa was expected. While the lysate bacteria containing both sense and antisense plasmids also lysate bacteria containing antisense plasmid, after induction any trace of protein was observed in this range that this results indicate the inhibition of gene expression in sense plasmid by antisense plasmid (Fig. 5). Final confirmation was performed with western blotting (Fig. 6).
Fig. 5:
10% SDS-PAGE of bacterial lysate
Lane 1: Lysate of M15 cell containing pcDNA-Rptr collected 5hrs after induction
Lane 2: Lysate of M15 cell containing pACYCDuet-ptr collected 5hrs after induction
Lane 3: Lysate of M15 cell containing pACYCDuet-ptr and pcDNA-Rptr collected 5hrs after induction
Lane 4: Lysate of M15 cell containing pcDNA-Rptr collected before induction
Lane 5: Lysate of M15 cell containing pACYCDuet-ptr collected before induction
Lane 6: Lysate of M15 cell containing pACYCDuet-ptr and pcDNA-Rptr collected before induction
Fig. 6:
Western blot analysis
Lane 1: Lysate of M15 cell containing pcDNA-Rptr collected 5hrs after induction
Lane 2: Lysate of M15 cell containing pACYCDuet-ptr collected 5hrs after induction
Lane 3: Lysate of M15 cell containing pACYCDuet-ptr and pcDNA-Rptr collected 5hrs after induction
Lane 4: Lysate of M15 cell containing pcDNA-Rptr collected before induction
Lane 5: Lysate of M15 cell containing pACYCDuet-ptr collected before induction
Lane 6: Lysate of M15 cell containing pACYCDuet-ptr and pcDNA-Rptr collected before induction
Discussion
Leishmaniasis is one of the parasitic diseases in most parts of the world. Out of the 30 provinces of Iran in the 15 provinces, leishmaniasis is endemic (17, 18). In addition to reports in the world about Leishmania which is resistant to drugs (19, 20) in Iran 10 to 15 percent of people infected with cutaneous leishmaniasis have been treated with Glucantime; they have no response to treatment that one of the reasons is drug-resistant parasites (21). Studies concerning methods of treatment of cutaneous leishmaniasis was conducted but none has been quite favorable (22, 23). According to the mentioned subjects, there is no completely effective drug therapy for treatment of cutaneous leishmaniasis and some failures are seen in the treatment that the lack of proper medical response is due to drug resistance, which needs more research. As was mentioned DHFR in parasitic DNA synthesis has an important role and inhibit this enzyme is causing the death. Methotrexate, one of anti-metabolite drugs, is an antagonist for folic acid, which competitively will inhibit DHFR, and its result is the death of parasite (24–25). PTR1 enzyme of Leishmania with similar enzymatic activity to DHFR reduced parasite susceptibility to anti-metabolite drugs such as Methotrexate (26) and was shown that deletion of PTR1 gene in Leishmania, which are treated with Methotrexate causes the death of parasite (27).
To inhibit gene expression, different several ways have been reported such as, oligodeoxy nucleotides (ODNs) (28), mRNA antisense (29) and small interfering RNA (siRNA) molecules (30). The purpose of this study is to identify inhibiting power of sense plasmid by complete antisense plasmid in vitro. After preparing the sense and antisense plasmids, since origin of replication of both plasmids was different and were in compatibility group, transformation of both in a bacterial cell was done successfully and then expression was induced. The results of SDS-PAGE and western blot analysis showed inhibition of sense plasmid by antisense plasmid. In addition, it was shown that the inhibition occurs in the cytosol, as was reported by Dumas et al (31).
In conclusion, expression of PTR1 gene in sense plasmid was inhibited successfully by antisense plasmid. Because the mRNA antisense can be used in gene therapy, the results of this study showed that this method could be successful.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This study was supported by the EMGEN (Eastern Mediterranean Health Genomics & Biotechnology Network) through project number 20091 and carried out in Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, and the authors would like to thank the personal and the director of the center. The authors declare that there is no conflict of interests.
References
- 1.Nare B, Luba J, Hardy LW, Beverley SM. New approaches to Leishmania chemotherapy: pteridine reductase 1 (PTRI) as a target and modulator of antifolate sensitivity. Parasitol. 1997;114(Suppl):S101–S110. [PubMed] [Google Scholar]
- 2.Bello AR, Nare B, Freedman D, Hardy L, Beverley SM. PTRI: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. PNAS USA. 1994;91(24):11442–11446. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellenberger TE, Beverley SM. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989;264(25):15094–15103. [PubMed] [Google Scholar]
- 4.Papadopulou B, Roy G, Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 1992;11(910):3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrebola R, Olmo A, Reche P, Garvey EP, Santi DV, Ruiz-oerez LM, Gonzalez-Pacanowska D. Isolation and characterization of a mutant dihydrofolate reductase–thymidylate synthase from methotrexate– resistant Leishmania cells. J Biol Chem. 1994;269(14):10590–10596. [PubMed] [Google Scholar]
- 6.Beck JT, Ullman B. Biopterin conversion to reduced folates by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1991;49(1):21–28. doi: 10.1016/0166-6851(91)90126-q. [DOI] [PubMed] [Google Scholar]
- 7.Govrley DG, Luba J, Hardy LW, Beverley SM, Hunter WN. Crystallization of recombinant Leishmania major pterdine reductase 1 (PTRI) Acta Cryst D Biol Crystallogr. 1999;55(Pt 9):1608–1610. doi: 10.1107/s0907444999008999. [DOI] [PubMed] [Google Scholar]
- 8.Luba J, Nare B, Liang PH, Anderson KS, Beverley SM, Hardy LW. Leishmania major pteridine reductase 1 belongs to the short chain dehydrogenase family: Stereochemical and kinetic evidence. Biochemistry. 1998;37(12):4093–4104. doi: 10.1021/bi972693a. [DOI] [PubMed] [Google Scholar]
- 9.Nare B, Hardy L, Beverley SM. The roles of pteridine reductese 1 (PTRI) and dihydrofolate reductase – thymidylate synthase (DHFR-TS) in pteridine metabolism in the protozoan parasite Leishmania major. J Biol Chem. 1997;272(21):13883–13891. doi: 10.1074/jbc.272.21.13883. [DOI] [PubMed] [Google Scholar]
- 10.Grogl M, Thomason TN, Franke ED. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47(1):117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham ML, Beverley SM. Pteridine salvage throughout the leishmania infectious cycle: implications for antifolate chemotherapy. Mol Biochem Parasitol. 2001;113(2):199–213. doi: 10.1016/s0166-6851(01)00213-4. [DOI] [PubMed] [Google Scholar]
- 12.Nichol CA, Smith GK, Dutch DS. Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Annu Rev Biochem. 1985;54(1):729–764. doi: 10.1146/annurev.bi.54.070185.003501. [DOI] [PubMed] [Google Scholar]
- 13.Kheirandish F, Bandehpour M, Haghighi A, Mahboudi F, Mohebali M, Mosaffa N, Kazemi B. Molecular Cloning and Expression of Iranian Leishmania major Pteridine Reductase 1. Iranian J Parasitol. 2008;3(2):1–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Appelbaum ER, Shatzman AR. In: Protein expression, a practical approach. Higgins SJ, editor. BD Hames; 1999. pp. 169–200. [Google Scholar]
- 15.Sambrook J, Russell DW. Molecular cloning A laboratory manual. Cold spring harbor laboratory press; 2001. pp. 4–14. [Google Scholar]
- 16.Kumar P, Kothari H, Singh N. Overexpressoin in Escherichia coli and purification of pteridine reductase (PTR1) from a clinical isolate of Leishmania donovani. Protein Expr Purif. 2004;38(2):228–36. doi: 10.1016/j.pep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10(4–5):591–599. [PubMed] [Google Scholar]
- 18.Mohebali M, Edrissian GH, Nadim A, Hajjaran H, Akhoundi B, Hooshmand S, Sanati AA, Moshfe AA, Charehdar S, Fakhar M. Application of direct agglutination test (DAT) for the diagnosis and seroepidemiological studies of visceral leishmaniasis in the Islamic Republic of Iran. Iranian J Parasitol. 2006;1(1):15–25. [Google Scholar]
- 19.Ouellette M. Biochemical and molecular mechanisms of drug resistance in parasites. Trop Med Int Health. 2001;6(11):874–876. doi: 10.1046/j.1365-3156.2001.00777.x. [DOI] [PubMed] [Google Scholar]
- 20.Sunder S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6(11):849–855. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 21.Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, Mohebali M. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101(5):1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- 22.Asilian A, Jalayer T, Whitworth JAG, Ghasemi RL, Nilforooshzadeh M, Olliaro P. A randomized, placebo, controlled trial of a two-week regimen of aminacidine (paromomycin) ointment for treatment of cutaneous leishmaniasis in Iran. Am J Trop Med Hyg. 1995;53(6):648–651. doi: 10.4269/ajtmh.1995.53.648. [DOI] [PubMed] [Google Scholar]
- 23.Asilian A, Jalayer T, Nilforooshzadeh M, Ghasemi RL, Peto R, Wayling S, Olliaro P, Modabber F. Treatment of cutaneous leishmaniasis with aminosidine (paromomaycin) ointement: double – blind, randomized trial in the lslamic Republic of Iran. Bulletin WHO. 2003;81(5):353–359. [PMC free article] [PubMed] [Google Scholar]
- 24.Coderre AJ, Beverley MS, Schinmke R, Santi DV. Overproduction of a bifunctional thy-midylate synthetase – dihydrofolate reductase and DNA amplification in methotrexate-resistance Leishmania tropica. Proc Natl Acad Sci USA. 1983;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello RA, Nara B, Freedman D, Hardy L, Beverley SM. PTRI: A reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA. 1994;91(24):11442–11446. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nare B, Hardy L, Beverley SM. The roles of pteridine reductese 1 and dihydrofolate reductase–thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J Biol Chem. 1997;272(21):13883–13891. doi: 10.1074/jbc.272.21.13883. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham ML, Beverley SM. Pteridine salvage throughout the Leishmania infectious cycle: implications for antifolate chemotherapy. Mol Biochem Parasitol. 2001;113(2):199–213. doi: 10.1016/s0166-6851(01)00213-4. [DOI] [PubMed] [Google Scholar]
- 28.Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 2002;372(6504):33–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- 29.Mundodi V, Kucknoor AS, Gedamu L. Role of Leishmania (Leishmania) chagasi amastigote cysteine protease in intracellular parasite survival: studies by gene disruption and antisense mRNA inhibition. BMC Mol Biol. 2005;6(1):3. doi: 10.1186/1471-2199-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Chen X, Qui F. An antisense plasmid targeting surviving expression induces apoptosis and sensitizes hepatocarcinoma cells to chemotherapy. J Huazhong Univ Sci Technology Med Sci. 2003;23(4):387–391. doi: 10.1007/BF02829425. [DOI] [PubMed] [Google Scholar]
- 31.Dumas C, Chow C, Muller M, Papadopoulou B. A novel class of developmentally regulated noncoding RNAs in Leishmania. Eukaryot Cell. 2006;5(12):2033–2046. doi: 10.1128/EC.00147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]