Abstract
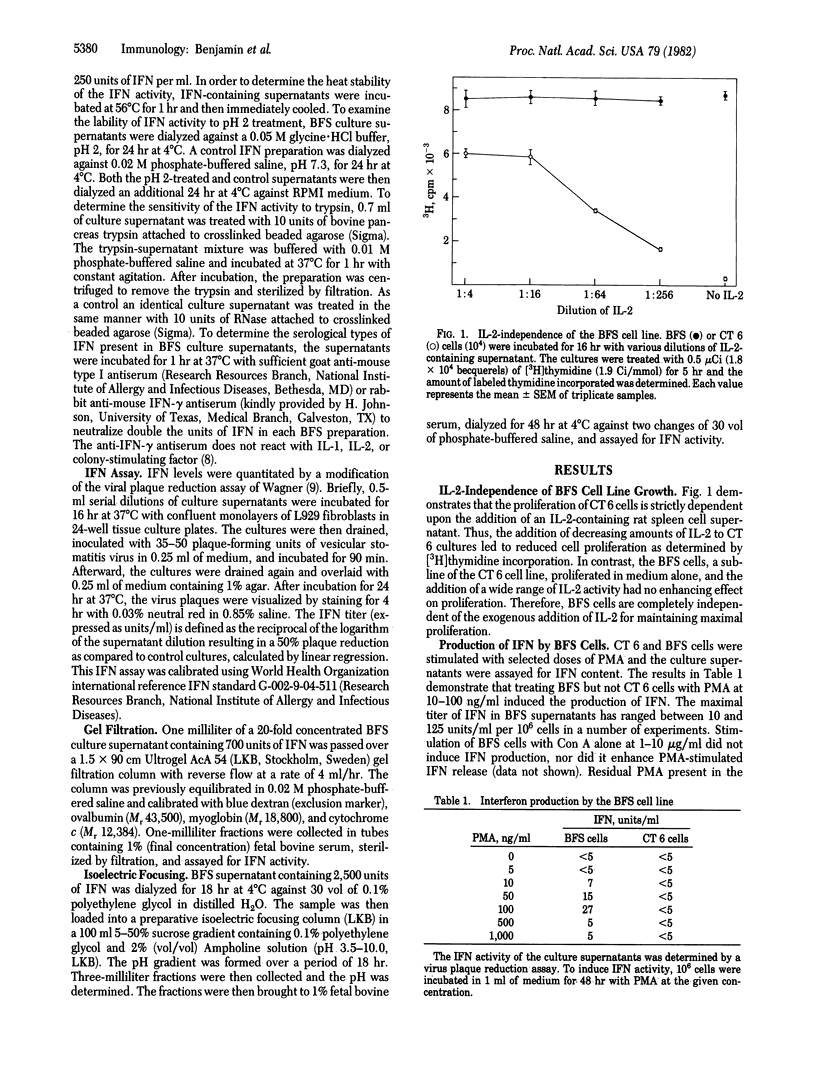
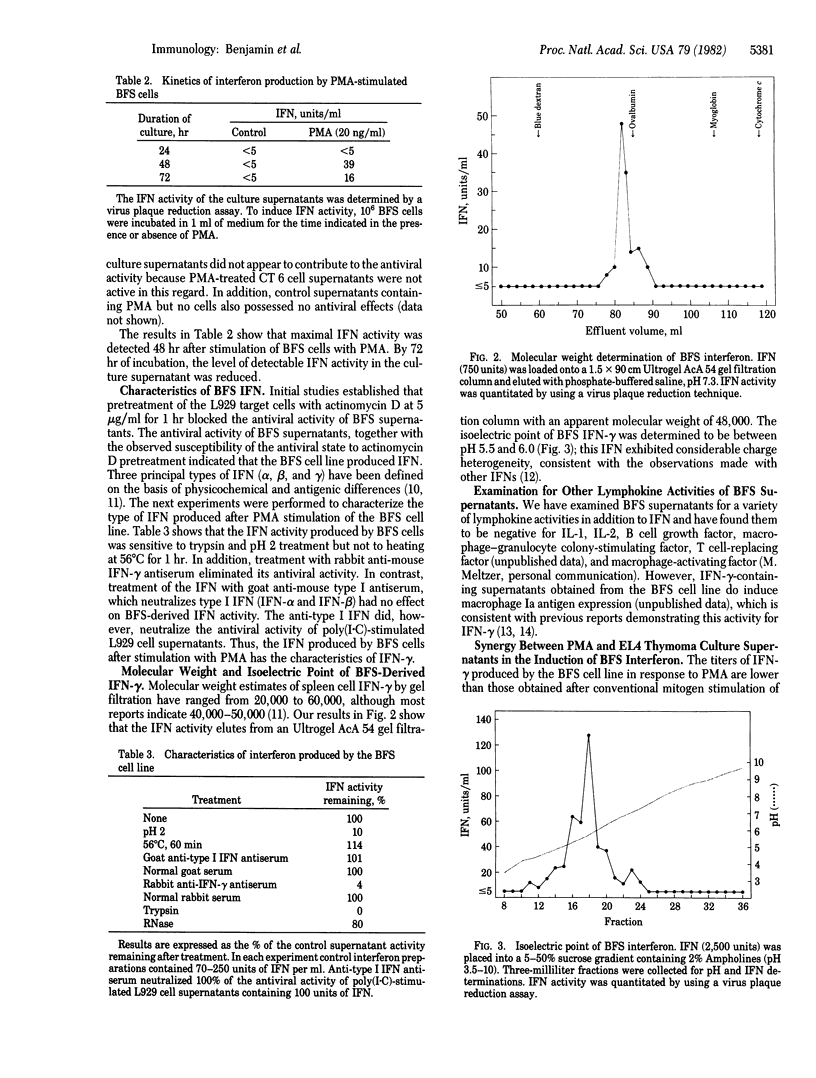
An interleukin 2-independent murine T cell line (BFS) was isolated that produced immune interferon after stimulation with phorbol 12-myristate 13-acetate. The BFS cell line did not produce detectable levels of interleukin 1, interleukin 2, B cell growth factor, macrophage-granulocyte colony-stimulating factor, macrophage-activating factor, or T cell replacing factor. Maximal interferon was induced 48 hr after stimulation with phorbol myristate acetate at 10-100 ng/ml. Production of interferon by phorbol myristate acetate-stimulated BFS cell cultures was synergistically increased by the addition of EL4 thymoma cell culture supernatants. BFS-derived interferon activity was sensitive to pH 2 treatment and was neutralized with antiserum to immune interferon but was resistant to heating at 56 degrees C and to treatment with antiserum to type I interferon. In addition, the interferon activity was sensitive to trypsin but resistant to RNase. BFS-derived interferon had an apparent molecular weight of 48,000 and a pI of 5.5-6.0. Each of these properties is consistent with the conclusion that the BFS cell line produces immune interferon after stimulation with phorbol myristate acetate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Fuller-Farrar J., Hilfiker M. L., Farrar W. L., Farrar J. J. Phorbol myristic acetate enhances the production of interleukin 2. Cell Immunol. 1981 Feb;58(1):156–164. doi: 10.1016/0008-8749(81)90157-x. [DOI] [PubMed] [Google Scholar]
- Hilfiker M. L., Moore R. N., Farrar J. J. Biologic properties of chromatographically separated murine thymoma-derived Interleukin 2 and colony-stimulating factor. J Immunol. 1981 Nov;127(5):1983–1987. [PubMed] [Google Scholar]
- Interferon nomenclature. Nature. 1980 Jul 10;286(5769):110–110. doi: 10.1038/286110a0. [DOI] [PubMed] [Google Scholar]
- Kawade Y., Yamamoto Y. Induction and production of L cell interferon. Methods Enzymol. 1981;78(Pt A):139–143. doi: 10.1016/0076-6879(81)78107-2. [DOI] [PubMed] [Google Scholar]
- Landolfo S., Kirchner H., Simon M. M. Production of immune interferon is regulated by more than T cell subset: Lyt-1,2,3 and Qat-5 phenotypes of murine T lymphocytes involved in IFN-gamma production in primary and secondary mixed lymphocyte reaction. Eur J Immunol. 1982 Apr;12(4):295–299. doi: 10.1002/eji.1830120408. [DOI] [PubMed] [Google Scholar]
- Marcucci F., Waller M., Kirchner H., Krammer P. Production of immune interferon by murine T-cell clones from long-term cultures. Nature. 1981 May 7;291(5810):79–81. doi: 10.1038/291079a0. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Pyhälä L., Törmä E., Cantell K. No evidence for a carbohydrate moiety affecting the clearance of circulating human leukocyte interferon in rabbits. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):305–310. doi: 10.1111/j.1699-0463.1974.tb02331.x. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Conzelmann A., Acuto O., North M., Haas W., Pohlit H., von Boehmer H., Hengartner H., Mach J. P., Engers H. Established murine cytolytic T-cell lines as tools for a somatic cell genetic analysis of T-cell functions. Immunol Rev. 1980;51:125–156. doi: 10.1111/j.1600-065x.1980.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Nathan I., Groopman J. E., Quan S. G., Bersch N., Golde D. W. Immune (gamma) interferon produced by a human T-lymphoblast cell line. Nature. 1981 Aug 27;292(5826):842–844. doi: 10.1038/292842a0. [DOI] [PubMed] [Google Scholar]
- Pang R. H., Yip Y. K., Vilcek J. Immune interferon induction by monoclonal antibody specific for human T cells. Cell Immunol. 1981 Nov 1;64(2):304–311. doi: 10.1016/0008-8749(81)90482-2. [DOI] [PubMed] [Google Scholar]
- Simon P. L., Farrar J. J., Dind P. D. Biochemical relationship between murine immune interferon and a killer cell helper factor. J Immunol. 1979 Jan;122(1):127–132. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. In vitro production and cellular origin of murine type II interferon. Immunology. 1979 Apr;36(4):883–890. [PMC free article] [PubMed] [Google Scholar]
- Torres B. A., Farrar W. L., Johnson H. M. Interleukin 2 regulates immune interferon (IFN gamma) production by normal and suppressor cell cultures. J Immunol. 1982 May;128(5):2217–2219. [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. I. Suppression of cellular infection with eastern equine encephalomyelitis virus. Virology. 1961 Mar;13:323–337. doi: 10.1016/0042-6822(61)90152-0. [DOI] [PubMed] [Google Scholar]



