Abstract
Background:
The frequency of invasive opportunistic mycoses has increased significantly over the past decades especially in immunocompromised patients. Invasive aspergillosis (IA) has become a major cause of morbidity and mortality among these patients. As bronchoalveolar lavage (BAL) fluid samples are generally useful specimens in the diagnosis of invasive pulmonary aspergillosis (IPA), this study was designed to evaluate the incidence of fungal elements in at-risk patients by direct microscopy and culture of BAL samples.
Methods:
In a 16-month period, 400 BAL samples were obtained from several groups of different patients with pulmonary and respiratory disorders and examined by using both direct microscopy and culture.
Results:
Of the 400 samples, 16 (4%) were positive direct examination with branching septate hyphae and 46 (11.5%) were positive culture: 25 (54%) Aspergillus flavus, 6 (13%) A. fumigatus, 5 (10.9%) A. niger, 1 (2.2%) A. terreus, 3 (6.5%) Penicillium spp. and 6 (13%) mixed A. flavus/A. niger. A. flavus was the most common cause of Aspergillus infection or colonization. Bone marrow transplant (BMT) recipients were the most susceptible group to fungal infection and/or colonization.
Conclusion:
Among Aspergillus species, A. flavus was the most common isolate in both infections and colonization in Iran. More studies are needed to clarify the epidemiological aspect of aspergillosis in Iran.
Keywords: Aspergillus, Bronchoalveolar lavage, Fungus, Iran
Introduction
Pulmonary fungal infections are expanding because of vast using of antibiotics and corticosteroids particularly in hematologic malignancy, bone marrow, and solid organ transplantation, autoimmune diseases, intensive care unit (ICU), solid-organ cancer (tumor) and pulmonary disorders (1–4). The most common fungi that cause opportunistic infections in transplant recipients and other immunocompromised patients are Candida and Aspergillus species. The incidence of these infections varies among different patient groups, although the mortality of invasive Candida infections appears to be less than the mortality due to Aspergillus infections (5). Invasive mycoses represent a major cause of morbidity and mortality in patients with malignancy or undergoing hematopoietic stem cell transplantation (HSCT) (6). Invasive aspergillosis (IA) occurs in 1 to 15% of organ transplant recipients, and its mortality rate ranges from 65 to 92% (7). The disease in lung transplant recipients is more frequent (3–23.3%) than pancreas, liver, heart, kidney, and small bowel transplant recipients (8). Incidence rates among patients at risk vary according to local epidemiology and presumably many other factors such as strict isolation of patients, use of high-efficiency particulate air (HEPA) filters, and the use of strict diagnostic criteria. Among Aspergillus isolates from large diagnostic reference centers, A. fumigatus is by far the most common species (50–60%), followed by A. flavus, A. niger and A. terreus (10–15% each), whereas A. nidulans, A. ustus and other rare Aspergillus species represent <2% of isolates (9). Nevertheless, non-fumigatus Aspergillus species such as A. flavus and A. terreus are becoming more common (10, 11). Diagnosis of invasive pulmonary aspergillosis (IPA) is difficult due to the lack of rapid, sensitive and accurate test methods. Therefore, efforts to improve diagnosis are ongoing and need to be further intensified. Other methods, such as tissue biopsy are often not optimal due to the fragile condition of the patient with respect to sustaining an invasive procedure.
Although the diagnostic value of bronchoalveolar lavage (BAL) for aspergillosis is controversial, BAL is a relatively safe and useful tool in high-risk patients suspected to have IPA and has been used in many clinical practices. In many cases, the evaluation of multiple findings from microscopy of BAL samples is helpful in the effort to improve the diagnosis of the disease (9).
The present preliminary study evaluated the incidence of fungal elements detected or isolated from different patients who are at-risk for fungal infections, by direct microscopy and culture of BAL samples taken from patients referred to some of hospitals in Tehran.
Materials and Methods
This report is a cross-sectional study that was performed during a 16-month period (from June 2009 to October 2010). According to standard techniques (12), 400 BAL fluid specimens were collected from different patients who referred to bronchoscopy section of Shariati and Imam-Khomeini Hospitals, Tehran University of Medical Sciences. The BAL fluid samples were obtained by a specialist physician, collected in sterile tubes without any conservation media, and transferred to the laboratory within 2 hours. An aliquot of 4–7 ml of each sample was centrifuged at 3000 rpm for 20 min, the supernatant was transferred to a separate tube, and the sediment was used for further experiments. A seventy-five microliters aliquot of the sediment was inoculated on each 9-cm petri dishes containing Sabouraud glucose (4%) agar and Brain Heart Infusion (BHI) agar, equally and separately. The plates were incubated for 3–7 days at 30°C. A 150 μl aliquot of the sediment was prepared with 20% potassium hydroxide (KOH) by using 24×50 mm coverslip and was microscopically examined with magnification ×400 and ×100. Progressive dichotomous branching hyphae that were seen in sediment of BAL samples and rarely in tissue biopsy was defined as positive results in direct microscopy. For definition of IPA, we used the diagnostic criteria described by European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) (13, 14).
Results
According to the clinical backgrounds, the patients were divided into seven groups. Table 1 summarizes the predisposing groups and demographic data of the patients as well as their final results of diagnosis of IPA. Frequencies of isolated Aspergillus species were separately shown in Table 2.
Table 1:
Demographic and clinical features of the patients in this study
| Groups of patients | No of Cases, | Age mean,% | Age range | No. male/female | Proven IPA,% | Probable IPA,% | Possible IPA,% |
|---|---|---|---|---|---|---|---|
| Bone marrow transplant recipients | 18 | 33.4 | 14–57 | 11/7 | 5.5 | 22.2 | 33.3 |
| Patients with haematological malignancies | 24 | 38.3 | 15–72 | 17/7 | - | 25 | 46 |
| Solid organ transplant recipients* | 52 | 40.8 | 32–59 | 31/21 | 3.8 | 11.6 | 67.3 |
| Solid-organ cancer patients | 28 | 62.6 | 29–94 | 20/8 | - | 11 | 46 |
| Patients with autoimmune diseases and corticosteroids consumer | 43 | 42.8 | 15–79 | 18/25 | - | 7 | 35 |
| Patients in intensive care unit (ICU) | 29 | 47.3 | 18–81 | 17/12 | - | 6.9 | 34.5 |
| Patients with pulmonary and respiratory disorders ** | 206 | 54.5 | 7–86 | 120/86 | - | - | - |
IPA, invasive pulmonary aspergillosis
This group had also two patients with probable IFD (3.8%) (caused by Penicillium spp.)
Patients without the criteria of host factor: 2% positive direct examination + positive culture, 7.3% positive culture but with negative direct examination
Table 2:
Frequency of Aspergillus species isolated from 400 BAL samples
| Aspergillus species | A. flavus | A. niger | A. fumigatus | A. terreus | Mixed A. flavus/A. niger |
|---|---|---|---|---|---|
| No. of cases | 25 | 5 | 6 | 1 | 6 |
| Percent (%) of cases | 58 | 11.7 | 14 | 2.3 | 14 |
Bone marrow transplant (BMT) recipients:
Of 18 patients, 61.1% was men (n=11) and 38.9% women (n=7), one patient with proven IPA (5.5%) (Caused by A. flavus), four patients with probable IPA (22.2%) (Two by A. fumigatus and two by A. flavus), six patients with possible IPA (33.3%) and seven patients with not IPA (39%).
Patients with hematological malignancies:
Of 24 patients, 70% was men (n=17) and 30% women (n=7), six patients with probable IPA (25%) (caused by A. flavus), eleven patients with possible IPA (46%) and seven patients with not IPA (29%).
Solid organ transplant recipients:
Of 52 patients, 60% was men (n=31) and 40% women (n=21), two patients with proven IPA (3.8%) (Caused by A. flavus), six patients with probable IPA (11.6%) (Three by A. flavus, two by A. niger and one by mixed A. flavus/A. niger), two patients with probable IFD (3.8%) (Caused by Penicillium spp.), thirty-five patients with possible IPA (67.3%) and seven patients with not IPA (13.5%).
Solid-organ cancer (tumor) patients:
Of 28 patients, 71.4% was men (n=20) and 28.6% women (n=8), three patients with probable IPA (11%) (Two by A. flavus and one by A. niger), thirteen patients with possible IPA (46%) and twelve patients with not IPA (43%).
Patients with autoimmune diseases and corticosteroids consumer:
Of 43 patients, 40.5% was men (n=18) and 59.5% women (n=25), three patients with probable IPA (7%) (Two by A. flavus and one by A. niger), fifteen patients with possible IPA (35%) and twenty-five patients with not IPA (58%).
Patients in intensive care unit (ICU):
Of 29 patients, 59% was men (n=17) and 41% women (n=12), two patients with probable IPA (6.9%) (Caused by A. flavus), ten patients with possible IPA (34.5%) and seventeen patients with not IPA (58.6%).
Patients with pulmonary and respiratory disorders:
Of 206 patients, 58.3% was men (n=120) and 41.7% women (n=86), none had the host predisposing factors but had one of clinical criteria (cough, dyspnea, chest pain, hemoptysis, bronchial asthma, cavity within area of consolidation in CT imaging) with mould fungi in direct microscopy and recovery by culture of BAL specimen (5 positive direct microscopy and 19 positive cultures). As the patients were not immunocompromised, this group is outside the scope of definitions drawn up (no-IFD episodes).
Among the 206 episodes, 4 patients had both positive direct examination (branching septate hyphae) and positive culture (2%) synchronously (two A. flavus, one A. fumigatus and one mixed A. flavus/A. niger) and 15 patients had negative direct examination but with positive culture (7.3%) (six A. flavus, one A. niger, two A. fumigatus, one A. terreus, one Penicillium spp. and four mixed A. flavus/A. niger).
Discussion
We have reported a screening survey to evaluate the frequency of mold infections of the lungs or respiratory tract specially IPA in different patients using BAL as the clinical specimen, by direct microscopy examination and culture. Despite recent advances in the molecular diagnosis of Aspergillus and aspergillosis, direct microscopy remains an essential tool for both detection of the organisms in the sample, and identification of the growing culture to the species level. Molecular methods are invaluable when an organism cannot be isolated in culture, when subtle differences within a complex are not readily visible under light microscopy or when unstable phenotypic characteristics have been lost as may happen in chronic infections. However, in most cases, molecular methods are difficult to perform and their results are difficult to interpret (15).
The present study is a relatively large survey for diagnosis IFD by BAL samples in Iran. Accounting for approximately 90% of Aspergillus infections worldwide, the most common species causing aspergillosis is A. fumigatus. Depending on regional distinctions, A. flavus, A. nidulans and A. terreus are reported as well, and there is evidence that these non-fumigatus pathogens are increasing (16, 15). For example, a study revealed that A. terreus was exclusively found in hospital samples from Austria (17) or A. niger was the most isolated non-fumigatus species in Spain (17). There are differences in the clinical presentations produced by these different species. However, unlike most reports in the world indicating A. fumigatus is dominant agent, in our study A. flavus was the most common causative agent among Aspergillus isolates (Table 2). The high frequency of A. flavus isolation from our patients might be due to higher prevalence of the fungus in surrounding environment like air, water, or soil. Similarly, there is an agreement on higher prevalence of A. flavus isolation from patients (18) and air (19) in Iran. In the current research, the most prevalent Aspergillus species causing human diseases were A. flavus, A. niger, A. fumigatus and A. terreus, respectively. Among immunocompromised patients, BMT recipients are the most susceptible group to IPA. This group is at risk because of relapse of the underlying condition (e.g. malignancy), as well as complications that arise from the transplant itself (20). Our data showed similar results and this group had superlative IPA with incidence 27.7% and the next group was patients with haematological malignancies with incidence 25%.
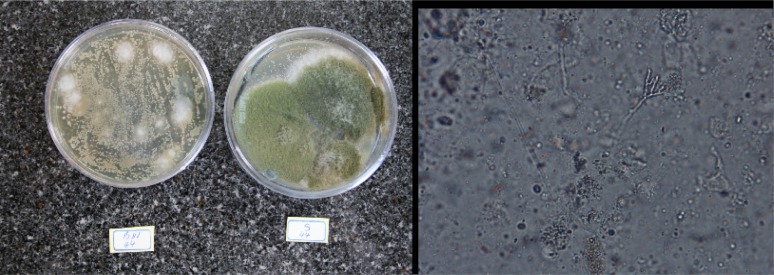
Early and definitive diagnosis of pulmonary fungal infections in severely immunocompromised patients is difficult, and a high index of suspicion is necessary in patients with risk factors for invasive disease (21). Histopathological diagnosis by examining lung tissue obtained by thoracoscopy or open-lung biopsy remains the “gold standard” in the diagnosis of IPA (22). However, lung biopsies are not performed very often because of concern about complications in patients who are often thrombocytopenic and in respiratory distress. Unfortunately, in this study we did not received enough number of biopsy tissues for examination. However, in immunocompromised patients with the characteristic clinical presentation, demonstration of Aspergillus in culture or direct microscopy, even if obtained from sputum or BAL fluid (a non-sterile site), has a high predictive value for IPA and for almost all practical purposes it is valuable for diagnosis and treatment (23). We considered the presence of septate, acute, branching hyphae invading the lung tissue samples, along with a positive culture for Aspergillus from the same site, as an IPA (Fig. 1).
Fig. 1:
Right: KOH preparation of BAL sample, slender septate hyphae with angular dichotomous branching ×400; Left: A. flavus colony in Sabouraud glucose (right) and Brain heart infusion agar (left)
In this study, among cases with positive direct examination, two had negative culture that may be because of antifungal therapy used for prophylaxis or empirical therapy. The biggest group of patients in this study was patients with pulmonary and respiratory disorders (51.7%). As only 2% of samples of these patients had both direct examination and culture positive simultaneously, it is possible that their symptoms are due to fungal allergies (24). Despite effective mechanisms for elimination of inhaled conidia from the respiratory tracts of healthy individuals, Aspergillus conidia are capable of colonizing injured lung tissue and epithelia. Although such colonization often has no clinical consequences, Aspergillus conidia can cause a variety of clinical manifestations depending on the immune status of the host (25). Therefore, isolation of Aspergillus spp. from respiratory samples of a patient with suspected aspergillosis does not necessarily indicate that the identified species is the etiologic agents of the infection. Although the diagnostic significance of the isolation of Aspergillus spp. from respiratory cultures has been extensively examined in immunocompromised hosts who develop IPA (26), little is known in respect to the presence of Aspergillus spp. in the respiratory tracts of immunocompetent or mildly immunocompromised patients with other forms of aspergillosis (27). As a limitation of this study, histopathological observations were not available for most patients, and we could not differentiate between IPA and colonization or other forms of chronic pulmonary aspergillosis. Consequently, the results of our study show that isolation of saprophytic fungi from respiratory samples does not confirm that they represent the etiologic pathogen, because airway colonization by saprophytic fungi is a common feature in patients with chronic lung disease.
As conclusion, while we could not show a clear correlation between direct examination and culture particularly in patients with pulmonary and respiratory disorders, our results showed that A. flavus might be the most common causative agent among Aspergillus species, as a human pathogen or respiratory colonizer in Tehran. Combination of molecular diagnostic methods and/or antigen detection by serological methods with conventional methods can improve the diagnosis of IFD.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
We thank all personnel at Respiratory Ward of Shariati Hospital and Medical Mycology laboratory in School of Public Health, who helped us to prepare BAL samples. This research was financially supported by Tehran University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- 1.Perea S, Patterson TF. Invasive Aspergillus infections in hematologic malignancy patients. Semin Respir Infect. 2002;(17):99–105. doi: 10.1053/srin.2002.33443. [DOI] [PubMed] [Google Scholar]
- 2.Goldani LZ, Maia AL. Aspergillus Thyroiditis. In: Pasqualotto AC, editor. Aspergillosis: From Diagnosis to Prevention. New York: Springer; 2010. pp. 905–906. [Google Scholar]
- 3.Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am. 2003;(17):113–134. doi: 10.1016/s0891-5520(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS, Kontoyiannis DP. The Significance of Isolation of Saprophytic Molds from the Lower Respiratory Tract in Patients with Cancer. Cancer. 2004;(100):165–72. doi: 10.1002/cncr.11876. [DOI] [PubMed] [Google Scholar]
- 5.McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis. 2001;(33):641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 6.Van Burik J, Weisdorf D. Infections in recipients of hematopoietic stem cell transplantation. In: Mandell GL, Bennet JE, Doolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: C Livingstone; 2005. pp. 3486–3501. [Google Scholar]
- 7.Gavalda J, Len O, San Juan R, et al. Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin Infect Dis. 2005;(41):52–59. doi: 10.1086/430602. [DOI] [PubMed] [Google Scholar]
- 8.Zaas AK, Alexander BD. Invasive pulmonary aspergillosis. In: Latge JP, Steinbach WJ, editors. Aspergillus fumigatus and aspergillosis. Washington, DC: ASM Press; 2009. pp. 293–300. [Google Scholar]
- 9.Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis: disease spectrum, treatment practices, and outcomes I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79(4):250–60. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lass-Florl C, Griff K, Mayr A, et al. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol. 2005;(131):201–7. doi: 10.1111/j.1365-2141.2005.05763.x. [DOI] [PubMed] [Google Scholar]
- 11.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;(34):909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 12.German Society of Pneumology Recommendations for diagnostic bronchoalveolar lavage. Pneumologie. 1994;48(Suppl1):311–23. [PubMed] [Google Scholar]
- 13.Pauw BD, Walsh TJ, Donnelly JP, et al. Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;(46):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;(46):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson EM, Borman AM. The Importance of Conventional Methods: Microscopy and Culture. In: Pasqualotto AC, editor. Aspergillosis: From Diagnosis to Prevention. Spinger Science+Business Media B V; 2010. p. 56. [Google Scholar]
- 16.Marr KA, Patterson T, Denning D. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect Dis Clin North Am. 2002;(16):875–894. doi: 10.1016/s0891-5520(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen KL, Mellado E, Lass-Florl C, Rodriguez-Tudela JL, Johansen HK, et al. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother. 2010;(54):4545–4549. doi: 10.1128/AAC.00692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badiee P, Alborzi A. Detection of Aspergillus species in bone marrow transplant patients. J Infect Dev Ctries. 2010;4(8):511–516. doi: 10.3855/jidc.807. [DOI] [PubMed] [Google Scholar]
- 19.Hedayati MT, Mayahi S, Denning DW. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor. Aspergillus Environ Monit Assess. 2010;(1–4):168. 481–7. doi: 10.1007/s10661-009-1128-x. [DOI] [PubMed] [Google Scholar]
- 20.Marr KA. Fungal Infections in Blood and Marrow Transplant Recipients. In: Wingard JR, Anaissie EJ, editors. Fungal Infections in the Immunocompromised patient. New York: Taylor & Francis Group; 2005. pp. 75–86. [Google Scholar]
- 21.Zmeili OS, Soubani AO. Pulmonary aspergillosis: a clinical update. Q J Med. 2007;(100):317–334. doi: 10.1093/qjmed/hcm035. [DOI] [PubMed] [Google Scholar]
- 22.Ruhnke M, Bohme A, Buchheidt D, et al. Diagnosis of invasive fungal infections in hematology and oncology–guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;(82):141–148. doi: 10.1007/s00277-003-0768-0. [DOI] [PubMed] [Google Scholar]
- 23.Oren I, Goldstein N. Invasive pulmonary aspergillosis. Current Opinion in Pulmonary Medicine. 2002;(8):195–200. doi: 10.1097/00063198-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Nobbe S, Denk B, Poll U, Rid V, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008;(145):58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;(46):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 26.Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;(33):1824–1833. doi: 10.1086/323900. [DOI] [PubMed] [Google Scholar]
- 27.Soubani AO, Khanchandani G, Ahmed HP. Clinical significance of lower respiratory tract Aspergillus culture in elderly hospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;(23):491–494. doi: 10.1007/s10096-004-1137-1. [DOI] [PubMed] [Google Scholar]