Abstract
Background:
Both increased arterial stiffness and hyperuricaemia are associated with elevated cardiovascular risks. Little is known about the relations of serum uric acid (UA) level to regional arterial stiffness and wave reflection. The aim of the study was to investigate the gender-specific association of serum UA and indices of arterial function in a community-based investigation in China.
Methods:
Cross-sectional data from 2374 adults (mean age 58.24 years) who underwent routine laboratory tests, regional pulse wave velocity (PWV) and pulse wave analysis measurements were analyzed in a gender-specific manner. None of the participants had atherosclerotic cardiovascular disease, chronic renal failure, systemic inflammatory disease, gout, or were under treatment which would affect serum UA level.
Results:
Men had higher serum UA level than women. Subjects with hyperuricaemia had significantly higher carotid-ankle PWV in both genders (P< 0.05), and the carotid-femoral PWV (PWVc-f) was higher in women (P< 0.001) while the augmentation index was marginally lower in men (P = 0.049). Multiple regression analysis showed that serum UA was an independent determinant only for PWVc-f in women (β = 0.104, P = 0.027) when adjusted for atherogenic confounders. No other independent relationship was found between UA level and other surrogates of arterial stiffness.
Conclusions:
Serum UA levels are associated with alterations in systemic arterial stiffness that differ in men and women. Women might be more susceptible to large vascular damage associated with hyperuricaemia.
Keywords: Arterial stiffness, Gender, Public health, Pulse wave velocity, Uric acid, Wave reflection
Introduction
Uric acid (UA) is the end product of endogenous and dietary purine nucleotide metabolism in humans. Many previous studies have confirmed an independent association between hyperuricaemia and adverse cardiovascular outcomes, especially in high-risk individuals (1–3). However, the Framingham Heart Study (4) and the Atherosclerosis Risk in Communities Study (5) failed to link UA with atherosclerotic cardiovascular diseases (CVD). Thus, the specific role of serum UA in relation to atherosclerosis remains unclear.
Arterial stiffness occurs early in the atherosclerosis process and carries a poor prognosis for CVD (6). Pulse wave velocity (PWV) and pulse wave analysis are all noninvasive indices for early detection of arterial stiffness. PWV is an accepted gold-standard assessment of stiffness index and is measured at different sites of the arterial tree, such as carotid-femoral (PWVc-f), carotid-radial (PWVc-r) and carotid-ankle PWV (PWVc-a)(7,8). The augmentation index (AIx) measured by pulse wave analysis is a composite parameter reflecting both large and distal arterial properties.
Accumulating studies have suggested of the relationship between the serum UA level and some of these surrogate markers for atherosclerosis. However, the results are far from sufficient and conclusive, while some even controversial (9–11). Most of the previous studies only analyzed in selected populations (11), including just one or two estimates of artery stiffness targeted (12) or in a relatively small sample. Whether higher UA contributes equally to central and peripheral arterial stiffness in the general subjects remains unclear. Because the differences in arterial stiffness among different ethnic groups have been documented (5), it is necessary to investigate the associations between UA concentration and indices of arterial stiffness in Chinese inhabitants. The present investigation was designed to explore their relationship in a community-based population participating in a health-screening programme.
Materials and Methods
Study Population
All participants were selected from a population-based cross-sectional investigation study that included 5116 Chinese inhabitants of Haidian or Daxing District, Beijing, China in 2007. Participants with CVD, chronic kidney disease, systemic inflammatory disease, gout, or under treatment which would affect UA levels were excluded. All measurements were obtained at the same time. The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital, and each subject provided written informed consent prior to participation.
Questionnaire and anthropometric measurements
A questionnaire was filled out for each subject at inclusion using a face-to-face interview method. The survey assessed traditional cardiovascular risk factors, including age, family history of premature cardiovascular events, cigarette smoking, and history of hypertension, CVD and diabetes. Subjects were considered non-smokers if they had never smoked or if they had given up smoking for at least three consecutive years. The investigation was completed by physicians trained by the research team.
Physical examinations, including anthropometry and blood pressure (BP) measurements, were performed after an overnight fast in the morning for each patient in the supine position. Brachial BP was measured with a mercury sphygmomanometer (Yuyue, Armamentarium Limited Company, Jiangsu, China) after 15 minutes of recumbent rest. Phases I and V of the Korotkoff sounds were used as the systolic (SBP) and diastolic blood pressure (DBP), respectively. Two measurements at an interval of 3 minutes were averaged. Anthropometric measures (height, body weight, and waist and hip circumferences) were recorded by a standardized protocol. Body mass index (BMI) was calculated as weight (kg) / height (m2).
Laboratory measurements
All subjects were advised not to eat, smoke, or drink coffee before examination. A blood sample was collected by venipuncture after an overnight fast. The baseline plasma total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), creatinine and UA were measured by a qualified technician using enzymatic assays (Roche Products Ltd., Switzerland) on a fully automatic biochemical autoanalyzer (COBAS c6000, Roche Products Ltd., Switzerland). The glycemic status of the participants was determined according to guidelines, with the use of an oral glucose tolerance test where appropriate. Hyperuricaemia was defined as a serum uric acid level of at least 420 μmol/L in men and at least 357 μmol/L in women (13).
Arterial stiffness and wave reflections evaluation
PWV was assessed by using the automatic waveform analyzers (Complior, Artech Medical, Pantin, France) as previously described (14, 15). All individuals were examined after resting in the supine position for at least 5 minutes. Different pressure waveforms were obtained simultaneously at four sites: the right carotid, radial, femoral and ankle arteries. Transit distances were assessed between each pulse-recording site. PWVc-f, PWVc-r and PWVc-a were then automatically calculated from measurements of pulse transit time and the distance between the 2 sites / from tonometry waveforms and body surface measurements as previously described. The mean PWV of at least 10 consecutive pressure waveforms was calculated for further analysis.
AIx was measured by using a validated system (SphygmoCor; AtCor Medical, Sydney, Australia) that employs the principle of applanation tonometry and appropriate software for noninvasive recording and analysis of the arterial pulse, as previously described (7, 16). Peripheral pulse waves were recorded from the radial artery and transformed into the aortic pulse wave through pulse wave analysis. From this aortic pressure waveform, the augmentation pressure (AP) and AIx were calculated. The AP is defined as the height of the late systolic peak above the inflection point on the waveform and may be positive or negative depending on the relative heights of the two peaks. AIx is defined as AP divided by central pulse pressure and is expressed as a percentage. AIx was averaged from 10 to 12 successive waves and was corrected for a steady heart rate (HR) of 75 beats/min (17).
The same observer, unaware of the subjects’ clinical and biochemical data, performed all the measurements. The interclass correlation coefficients between the first and second measurements were 0.95 for the AIx-75, 0.93 for PWVc-f, 0.91 for PWVc-r and 0.89 for PWVc-a. The coefficients of variation for the AIx-75 and PWV were less than 5%.
Statistical analyses
The data are presented as mean values ± standard derivation or percentages, unless otherwise stated. Student t test was used to compare groups for continuous variables and the chi-square test to compare categorical variables. In addition, differences in non-parametric variables were compared using the Manne Whitney U-test. Pearson correlation analyses between arterial stiffness and serum UA, or other variables of interest were calculated to examine potential relationships. Then, multivariate stepwise regression analyses were performed to look for independent associations between arterial stiffness and the variables that were found to have a significant association with arterial stiffness in a univariate analysis. A P value of less than 0.05 (two-tailed) was considered statistically significant. Statistical analyses were performed with SPSS 11.0 software (Statistical Package for the Social Science, Inc., Chicago, IL, USA).
Results
Baseline characteristics
Table 1 shows the demographic, clinical, and hemodynamic characteristics of the study participants by gender. Of 2374 participants included in the present analyses, there were 1138 men and 1236 women. Mean ages were 58.24 ± 12.38 years for all participants (range 35 to 96 years). The mean serum UA level was found to be significantly higher in men (326.76 ± 72.96 μmol/L) than in women (263.68 ± 64.4 μmol/L, P < 0.001). The mean PWVc-f, PWVc-r and PWVc-a values were significantly higher, while AIx-75 was much lower in men than in women (P all < 0.05). Men, compared with women, had higher brachial BP, higher proportions of current smokers, higher UA and TG, but lower serum TC, LDL-C and 2 h postprandial blood glucose (2h BG) level.
Table 1:
Selected clinical, demographic and hemodynamic characteristics of the survey population according to gender
| Total (n = 2374) | Men (n = 1138) | Women (n = 1236) | P value | |
|---|---|---|---|---|
| Age, yr | 58.24 ± 12.38 | 56.74 ± 13.37 | 59.61 ± 11.22 | < 0.001 |
| Current smoker, n/ % | 620 (26.1%) | 522 (45.9%) | 98 (7.9%) | < 0.001 |
| Hypertension, n/ % | 798 (33.6%) | 447 (39.3%) | 351 (28.4%) | < 0.001 |
| SBP, mmHg | 128.88 ± 17.87 | 130.59 ± 17.13 | 127.32 ± 18.39 | < 0.001 |
| DBP, mmHg | 76.8 ± 10.41 | 78.71 ± 10.34 | 75.04 ± 10.18 | < 0.001 |
| BMI, kg/m2 | 25.45 ± 3.4 | 25.5 ± 3.11 | 25.41 ± 3.65 | 0.539 |
| HR, bpm | 76.03 ± 9.79 | 75.83 ± 9.98 | 76.16 ± 9.68 | 0.622 |
| TC, mmol/L | 5.04 ± 0.93 | 4.93 ± 0.91 | 5.14 ± 0.94 | < 0.001 |
| TG, mmol/L | 1.81 ± 1.31 | 1.89 ± 1.52 | 1.73 ± 1.08 | 0.003 |
| LDL-C, mmol/L | 2.91 ± 0.74 | 2.79 ± 0.7 | 3.02 ± 0.75 | < 0.001 |
| UA, μmol/L | 293.93 ± 75.52 | 326.76 ± 72.96 | 263.68 ± 64.4 | < 0.001 |
| FBG, mmol/L | 5.39 ± 1.6 | 5.36 ± 1.4 | 5.42 ± 1.76 | 0.357 |
| 2 h BG, mmol/L | 7.52 ± 3.94 | 7.16 ± 3.78 | 7.86 ± 4.06 | < 0.001 |
| PWVc-f, m/s | 11.21 ± 2.89 | 11.34 ± 2.94 | 11.1 ± 2.84 | 0.044 |
| PWVc-r, m/s | 9.46 ± 1.46 | 10 ± 1.43 | 8.96 ± 1.31 | < 0.001 |
| PWVc-a, m/s | 9.24 ± 2.18 | 9.35 ± 1.83 | 9.14 ± 2.45 | 0.019 |
| AIx-75, % | 25.24 ± 10.39 | 21.06 ± 10.25 | 29.09 ± 8.93 | < 0.001 |
Continuous variables are expressed as mean ± SD; dichotomous variables, as n (%)
Abbreviations: AIx-75, augmentation index at heart rate 75/min; 2 h BG, 2 h post blood glucose in OGTT; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HR, heart rate; LDL-C, low density lipoprotein cholesterol; PWVc-a, carotid-ankle pulse wave velocity; PWVc-f, carotid-femoral pulse wave velocity; PWVc-r, carotid-radical pulse wave velocity; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid
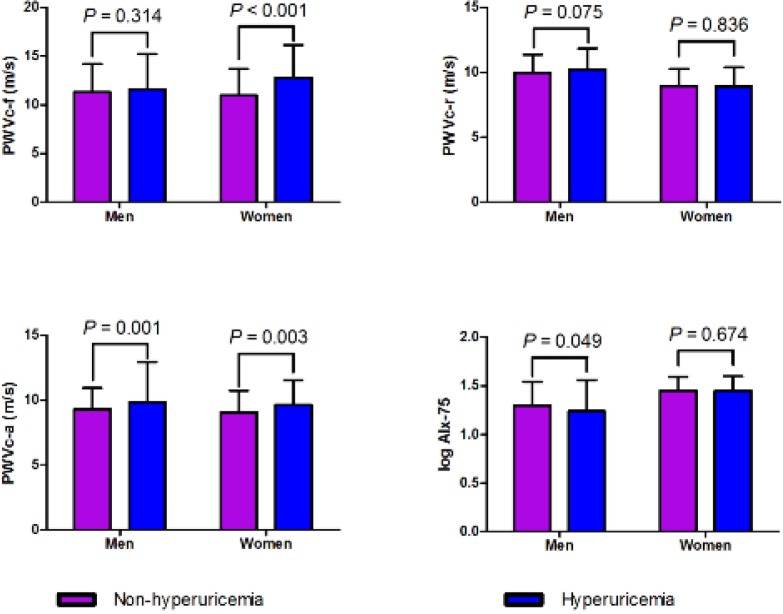
Sub-group analyses showed that hyperuricemic subjects, compared with those with normal serum UA, had significantly higher PWVc-f in women (P < 0.001), significantly higher PWVc-a in both genders (P < 0.05) and marginally lower log AIx-75 in men (P = 0.049). PWVc-r didn’t differ in subjects with hyperuricaemia or normal UA level in both genders (Fig. 1).
Fig. 1:
Arterial stiffness changes according to hyperuricemia status and gender
Relationship between serum UA, PWV and AIx-75
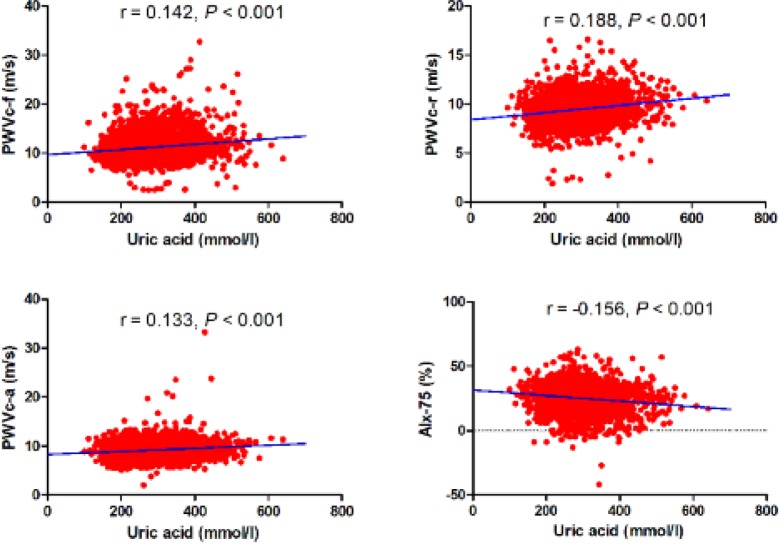
The associations of serum UA level with regional PWV and AIx-75 are presented in Table 2, Fig. 2 and Table 3. Figure 2 shows the relationship between serum UA, PWVc-f, PWVc-r, PWVc-a and AIx-75 in the entire population. These scatter charts clearly show that serum UA concentration positively correlates with PWVc-f, PWVc-r and PWVc-a, but negatively correlates with AIx-75 (P all <0.001). Table 2 lists univariate correlation coefficients between arterial stiffness and various clinical parameters by gender. The results showed that PWVc-f and PWVc-a significantly correlated with UA concentration in both genders, while had no relationship with PWVc-r and AIx-75 in either gender. Parameters that represent cardiac risk factors, such as age, HR, SBP, BMI, serum UA, lipid profiles, blood glucose and eGFR, had different effects on arterial stiffness with gender difference. When these parameters were used as explanatory variables in the multivariate stepwise regression analysis for PWV and AIx-75, results showed that UA was a significant independent variable for PWVc-f in women though the contributions of age, SBP, FBG and BMI were large. On the contrary, UA level had no independent contribution to PWVc-f in men and to other regional arterial stiffness in both gender. All the explanatory variables included are listed in Table 3.
Table 2:
Pearson’s correlation coefficients between arterial stiffness, serum UA and other variables in male and female subjects
| Variable | PWVc-f (m/s) | PWVc-r (m/s) | PWVc-a (m/s) | AIx-75 (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| UA | 0.072* | 0.209** | 0.051 | 0.042 | 0.071* | 0.16** | 0.005 | 0.01 |
| Age | 0.517** | 0.564** | −0.117** | 0.089* | −0.032 | 0.383** | −0.054 | 0.137** |
| BMI | −0.061* | 0.074* | −0.009 | −0.012 | −0.054 | 0.115** | −0.025 | −0.007 |
| SBP | 0.33** | 0.417** | 0.075* | 0.184** | 0.041 | 0.394** | 0.021 | 0.146** |
| DBP | −0.005 | 0.05 | 0.19** | 0.196** | 0.133** | 0.218** | 0.07* | 0.136** |
| HR | 0.106* | 0.134* | 0.137* | 0.003 | 0.114* | 0.034 | 0.077 | −0.049 |
| TC | −0.032 | 0.075** | 0.048 | 0.051 | 0.048 | 0.069* | −0.011 | 0.064* |
| TG | −0.025 | 0.156** | 0.081* | 0.018 | 0.016 | 0.126** | −0.018 | 0.008 |
| LDL-C | 0.062* | 0.145 | 0.012 | 0.060* | 0.041 | 0.087* | −0.021 | 0.072** |
| FBG | 0.084* | 0.115** | 0.051 | 0.029 | −0.004 | 0.119** | 0.057 | 0.011 |
| 2 h BG | 0.168** | 0.279** | 0.046 | 0.060* | 0.022 | 0.235** | −0.031 | 0.026 |
Fig. 2.
Scatter plot of arterial stiffness parameter against serum uric acid level r expresses the Pearson correlation coefficient.
Table 3:
Multivariate stepwise regression analysis showing independent contributions to arterial stiffness in men and women
| Independent variable | B | SE | β | t | P |
|---|---|---|---|---|---|
| PWVc-f men (R2 = 0.333, SE = 1.873, F = 71, P < 0.001) | |||||
| Age, yr | 0.092 | 0.008 | 0.413 | 11.142 | < 0.001 |
| SBP, mmHg | 0.024 | 0.005 | 0.205 | 5.326 | < 0.001 |
| HR, bpm | 0.03 | 0.008 | 0.127 | 3.617 | < 0.001 |
| 2h BG, mmol/L | 0.064 | 0.02 | 0.118 | 3.24 | 0.001 |
| PWVc-f women (R2 = 0.286, SE = 2.143, F = 28.615, P < 0.001) | |||||
| Age, yr | 0.094 | 0.011 | 0.415 | 8.852 | < 0.001 |
| SBP, mmHg | 0.029 | 0.006 | 0.224 | 4.669 | < 0.001 |
| FBG, mmol/l | 0.228 | 0.071 | 0.147 | 3.195 | 0.002 |
| BMI, kg/m2 | −0.11 | 0.038 | −0.14 | −2.917 | 0.004 |
| UA, μmol/l | 0.004 | 0.002 | 0.104 | 2.22 | 0.027 |
| PWVc-r men (R2 = 0.051, SE = 1.34, F = 7.195, P < 0.001) | |||||
| BMI, kg/m2 | −0.062 | 0.022 | −0.146 | −2.757 | 0.006 |
| HR, bpm | 0.019 | 0.007 | 0.14 | 2.656 | 0.008 |
| Current smoker | 0.34 | 0.152 | 0.118 | 2.244 | 0.026 |
| PWVc-r women (R2 = 0.024, SE = 1.266, F = 7.959, P < 0.001) | |||||
| TC, mmol/L | 0.172 | 0.06 | 0.12 | 2.848 | 0.005 |
| SBP, mmHg | 0.007 | 0.003 | 0.102 | 2.428 | 0.016 |
| PWVc-a men (R2 = 0.02, SE = 1.496, F = 4.44, P = 0.012) | |||||
| HR, bpm | 0.018 | 0.008 | 0.12 | 2.222 | 0.027 |
| Current smoker | 0.34 | 0.17 | 0.108 | 2.001 | 0.046 |
| PWVc-a women (R2 =0.062, SE = 2.897, F = 19.371, P < 0.001) | |||||
| Age, yr | 0.052 | 0.013 | 0.179 | 4.093 | < 0.001 |
| SBP, mmHg | 0.02 | 0.007 | 0.131 | 2.989 | 0.003 |
| Aix-75 men (R 2 = 0.003, SE = 10.212, F = 4.503, P = 0.034) | |||||
| Current smoker | −1.348 | 0.635 | −0.066 | −2.122 | 0.034 |
| Aix-75 women (R2 = 0.038, SE = 8.6, F = 22.782, P < 0.001) | |||||
| SBP, mmHg | 0.063 | 0.015 | 0.132 | 4.185 | < 0.001 |
| Age, yr | 0.082 | 0.024 | 0.108 | 3.433 | 0.001 |
B, Unstandardized Coefficients; SE, standard error for B; β, Standardized Coefficients.
For other abbreviations, see Table 1 footnote.
Discussion
In the present study, we explored the associations of UA levels and indices of arterial function in general Chinese population with gender specific analysis. Our findings here demonstrate that while serum UA concentration correlates positively with PWV measured at three different sites and negatively with AIx-75 in the entire population, only PWVc-f is independently associated with elevated UA after correcting for possible confounding factors in women, and no independent association is found between serum UA and PWVc-r, PWVc-a or AIx-75 both in both genders.
Higher serum UA might not have uniform effects along the arterial tree stiffness. Regarding serum UA on large arterial stiffening, our findings are in line and extend observations from selected diseases (11) or general population, (12, 18) which suggested a direct effect of serum UA on elastic arteries stiffness. However, only limited and inconclusive information exists about peripheral arterial changes. Tsai found that UA is an independent predictor of PWVc-r in hypertensive patients (11). On the contrary, in the present study, we found no association between the UA level and PWVc-r or PWVc-a in general residents at low risk for CVD. Differences in the baseline characteristics of a study population may explain the disparate result.
To date, scant data exist regarding the relationship of serum UA and wave reflections. Augmentation index (AIx) has been advocated as an indirect surrogate measure of arterial stiffness, and arterial stiffness is only one contributor to the observed AIx. Though arterial stiffness and wave reflection indices often change in parallel, this is not always the case. Our findings of a negative relationship between UA levels and wave reflections in the presence of a positive association with arterial stiffness only in females are intriguing.
Previous studies showed that though men have much higher serum UA concentration than women, their cardiovascular risks were relatively lower. Hoieggen (19) reviewed the epidemiological data and found an independent relationship between serum UA and cardiovascular risk, at least in women. The mechanisms that underline UA gender-specific effects were not clear but might involve gene discrepancies (20). However, gender-specific relationship between UA level and arterial stiffness, which is also considered as a cardiovascular risk factor, was inconclusively analyzed. In the study of Ishizaka (18) Pearson’s correlation coefficient between the UA level and brachial-ankle PWV is significant in females, but not in males. On the contrary, Lim (21) and Tomiyama (22) found that elevated UA level is not associated with heart-femoral PWV or brachial-ankle PWV in apparently healthy korean adult females or males. Vlachopoulos proved that (10) serum UA levels are independently associated with aortic stiffening (PWVc-f) in both genders while with wave reflections (AI) only in females in never-treated hypertensives. The inconsistency was probably due to different methods in measurement of arterial stiffness and also due to bias in the recruitment of patients.
To the best of our knowledge, this is the first study to explore gender-specific relationships between UA, regional arterial stiffness, and wave reflections in a sample of general Chinese residents. Our findings add to the general impression that UA does not have a uniform impact on both sexes, though we cannot yet explain the underlying mechanism. Our null finding in men could be attributable to the significantly different characteristics between men and women population of our study. In this cross-sectional study, though men had higher proportion of current smokers and hypertension, their mean age was much younger, serum TC, LDL-C and TG were much lower than those in women. These might serve as major underlying factors causing the divergence of the gender-specific associations in our study.
In both univariate correlation and multivariate stepwise regression analysis, we found that lower BMI is weakly but significantly associated with higher PWVc-f in women. This finding is contradictory to other studies (23) and not easy to explain in the present study. When BMI was replaced by waist to hip ratio, the results showed that waist to hip ratio was also an independent predictor for PWVc-f, though only in women (standardized coefficient β 0.084, P = 0.027, data not shown). Our results are in line with Tsai’s (11) findings of negatively correlated BMI and PWVc-f in hypertensive patients. This might indicate that central, but not general adiposity is an important determinant of aortic stiffness. However, our subjects were selected from middle to aged community based samples, which are not representative of the whole population, so the relationship between BMI and PWV should be further clarified.
It has been reported that hyperuricaemia is associated with oxidative stress, (24) endothelial dysfunction (25) and inflammation (26), all of the issues might contribute to arterial stiffening. However, the precise role that UA plays in arterial stiffening is not yet clear. Whether the relationship between serum UA and increased arterial stiffness is circumstantial or causal cannot be determined by this cross-sectional study.
In addition to its cross-sectional nature, our study had several other limitations. First, we ruled out the subjects who used UA-lowering agents or medications which might affect UA levels in our study. However, the relative homogeneity of the study subjects enhancing the internal validity of our findings by reducing confounding factors potentially affecting serum UA and PWV. Second, our study population consisted solely of Chinese subjects, thus the associations observed may not be applicable to other ethnic groups. However, the correlation between UA and PWV demonstrated in our study implied that PWV could be a surrogate marker for evaluation of UA-lowering agents in cardiovascular disease in the future.
Taken together, our study suggests that serum UA preferentially increases central elastic (carotid) over peripheral muscular (femoral and brachial) arteries stiffness and positive association between serum UA levels and large artery stiffness exists only in females. Though serum uric acid levels are much higher in men than those in women, null findings in men from multiple analyses remain to be elucidated. Further research, involving prospective and intervention studies, would be required to identify gender specific susceptible to vascular damage associated with hyperuricaemia or an elevated serum UA.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors
Acknowledgments
This study was supported by Capital Medical Development Fund (No. 2009–2038) of Beijing and the National Natural Science Fund of Youth (No. 81100107). The authors would like to thank the numerous physicians and nurses who have participated in this program.
References
- 1.Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: The monica/kora cohort study. Arterioscler Thromb Vasc Biol. 2008;28(6):1186–92. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 2.Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Arch Intern Med. 2004;164(14):1546–51. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 3.Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: A chinese cohort study. Arthritis Rheum. 2009;61(2):225–32. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 4.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: The framingham heart study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Iribarren C, Folsom AR, Eckfeldt JH, Mcgovern PG, Nieto FJ. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: The aric study. Atherosclerosis risk in communities. Ann Epidemiol. 1996;6(4):331–40. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 8.Tillin T, Chambers J, Malik I, Coady E, Byrd S, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, Hughes A. Measurement of pulse wave velocity: Site matters. J Hypertens. 2007;25(2):383–9. doi: 10.1097/HJH.0b013e3280115bea. [DOI] [PubMed] [Google Scholar]
- 9.Tsioufis C, Kyvelou S, Dimitriadis K, Syrseloudis D, Sideris S, Skiadas I, Katsi V, Stefanadi E, Lalos S, Mihas C, Poulakis M, Stefanadis C. The diverse associations of uric acid with low-grade inflammation, adiponectin and arterial stiffness in never-treated hypertensives. J Hum Hypertens. 2010;25(9):554–9. doi: 10.1038/jhh.2010.98. [DOI] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Xaplanteris P, Vyssoulis G, Bratsas A, Baou K, Tzamou V, Aznaouridis K, Dima I, Lazaros G, Stefanadis C. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2010;24(1):33–9. doi: 10.1038/ajh.2010.111. [DOI] [PubMed] [Google Scholar]
- 11.Tsai WC, Huang YY, Lin CC, Li WT, Lee CH, Chen JY, Chen JH. Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels. 2009;24(5):371–5. doi: 10.1007/s00380-008-1127-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CF, Yu KH, Luo SF, Ko YS, Wen MS, Lin YS, Hung KC, Chen CC, Lin CM, Hwang JS, Tseng WY, Chen HW, Shen YM, See LC. Role of uric acid in the link between arterial stiffness and cardiac hypertrophy: A cross-sectional study. Rheumatology (Oxford) 2010;49(6):1189–96. doi: 10.1093/rheumatology/keq095. [DOI] [PubMed] [Google Scholar]
- 13.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann ED, Hopkins KD, Gosling RG. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension. 1996;27(5):1188–91. [PubMed] [Google Scholar]
- 15.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26(3):485–90. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 16.Pauca AL, O'rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–7. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson IB, Maccallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in japanese individuals. Atherosclerosis. 2007;192(1):131–7. doi: 10.1016/j.atherosclerosis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Hoieggen A, Os I, Kjeldsen SE. [uric acid--more deleterious than we thought?] Tidsskr Nor Laegeforen. 2005;125(10):1330–2. [PubMed] [Google Scholar]
- 20.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JH, Kim YK, Kim YS, Na SH, Rhee MY, Lee MM. Relationship between serum uric acid levels, metabolic syndrome, and arterial stiffness in korean. Korean Circ J. 2010;40(7):314–20. doi: 10.4070/kcj.2010.40.7.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166(2):303–9. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 23.Nordstrand N, Gjevestad E, Dinh KN, Hofso D, Roislien J, Saltvedt E, Os I, Hjelmesaeth J. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord. 2011;11:7. doi: 10.1186/1471-2261-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174(1 Pt 1):288–91. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 25.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–24. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 26.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–93. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]