Abstract
Background:
The aim of this study was identification of the epidemiology of Prototheca zopfii species from the milk samples of dairy cattle in Isfahan, central Iran.
Methods:
Milk samples were obtained from 230 dairy cattle, 130 with and 100 without mastitis, in Isfahan. The samples were cultured in Prototheca Isolation Medium (PIM) and Sabouraud’s dextrose agar. All P. zopfii isolates were identified by morphological and biochemical methods. Then, as a confirmatory test they were examined by genotype-specific PCR.
Results:
Four P. zopfii strains (3.07%) were isolated from the 130 samples of dairy cattle with clinical mastitis and there was no isolation from totally 100 samples of healthy bovines without mastitis. Specific PCR product (about 946 bp) was detected in four isolates.
Conclusion:
It seems that P. zopfii genotype II plays a key role in affecting bovine mastitis that confirmed other previous studies. Our study was the first, which identified the Prototheca species by traditional and molecular methods in Iran and Middle East as well.
Keywords: Bovine mastitis, Prototheca zopfii, Iran
Introduction
The algae of the Prototheca (P.) genus are in a close relation to green algae Chlorella but they lack of chlorophyll (1). There are five species of prototheca including P. moriformis, P. stagnora, P.ulmea, P. wicherhamii, and P. zopfii in the nature in saprophyte form and can be isolated from different environmental sources such as stool, soil, lakes, and mires. Protothecosis is a zoonotic infection as well as some species of Prototheca e.g., P. zopfii, and P. wicherhamii are etiological agents of human protothecosis (2). Krüger distinguished these species for the first time (1894) and in 1952 P. zopfii was identified as a bovine mastitis pathogen which affected and reduced milk production in dairy cattle (3). There are many reports for endemic incidence as well as some case reports of the infection in many regions of the world (4). In last decade, the incidence of the bovine mastitis has been increased (5–9). Bovine mastitis due to Prototecha species has been demonstrated by some other investigations. in these studies, different aspects of the infection have considered such as epidemiological aspects (4,7,10,11), clinical symptoms (5,6,9,12), anti-microbial sensitivities (13,14) and histopathologically(15,16). P. zopfii genotype II as a predominant pathogen for the mastitis (3,17,18). Prototheca zopfii has been differentiated to three biotypes (17). Studies using 18S rRNA gene sequencing, obviously confirmed that the P. zopfii had three different biotypes. Recently biotypes I and II are considered as Genotypes I and II, respectively and biotype III as a P. blaschkeae (18). In the most cases of Prototheca infections that can lead to mastitis in caw, P. zopfii genotype II was identified as a predominant causative pathogen in bovine mastitis (3).
The aim of this study was identification of the epidemiology of the disease through biochemical and PCR methods in isolated P. zopfii species from the milk samples of dairy cattle with and without mastitis in Isfahan, Iran.
Materials and Methods
Milk samples obtained from 230 dairy cattle, 130 with and 100 without mastitis, in Isfahan. The samples first were cultured in Prototheca Isolation Medium (PIM), supplemented with chloramphinicol (100μg/ml) and Subouraud-dextrose-agar for 7 days at 27°C. Then the P. zopfii species were identified by morphological and biochemical methods. Genotypes of the species isolates from mastitis were analyzed by genotype-specific PCR. In order to perform PCR, certain primers for the genotypes were employed (19). In order to consider the three genotypes of P. zopfii and primer designing through the analysis of the 18S rRNA gene sequencing, acquired from GeneBank # AY973040, AY 940456, AY973041 (specific for genotypes I, II, and III, respectively), was used Gene runner, MEGA 4 and oligo analyzer software were employed to achieve this goal (18).
Four isolated P. zopfii samples recultured in Subouraud’s Dextrose agar medium at 27°C for 48 hours for DNA extraction by using Invisorb Spin Plant Kit (Invitek GmbH). Afterwards, 18S rRNA gene was amplified by PCR. Genotype-specific primers are listed in Table 1.
Table 1:
Genotype-specific primers used in this study
| Specific primer | Target | Sequences |
|---|---|---|
| PZGT 1-A/f PZGT 1-A/r |
Genotype 1-specific PCR | 5′-CGCGCAAATT ACCCAATCC-3′ 5′-GCCAAGGCC CCCCGAAG-3′ |
| PZGT 2-A/f PZGT 2-A/r |
Genotype 2-specific PCR | 5′-CGCGCAAATT ACCCAATCC-3′ 5′-GTCGGCGGG GCAAAAGC-3′ |
| PZGT 3-A/f PZGT 3-A/r |
Genotype 3-specific PCR | 5′-CAGGGTTCGA TTCCGGAGAG-3′ 5′-GTTGGCCC GGCATCGCT-3′ |
PCR amplification (25 μl/reaction) was carried out with the master mix (0.3 μl (5 U/μl) TAQ Polymerase, 3 mM MgCl2, 1 μl (65 ng) genomic DNA, 1 μl (50 μM) each primer and 0.5 μl (10 mM) (dNTP), 2.5 μl buffer (10X) .The PCR was started with an initial denaturation step at 95 °C for 4 min followed by 40 thermal cycles of denaturation at 95 °C for 1 min, annealing at 66 °C for 55 s, and DNA synthesis at 72 °C for 55 s, and finally at 72 °C for 8 min.
Results
The P. zopfii species cultured in Prototheca Isolation Medium (PIM), supplemented with chloramphinicol (100μg/ml) (Fig. 1) and Subouraud- dextrose- agar (Fig. 2).
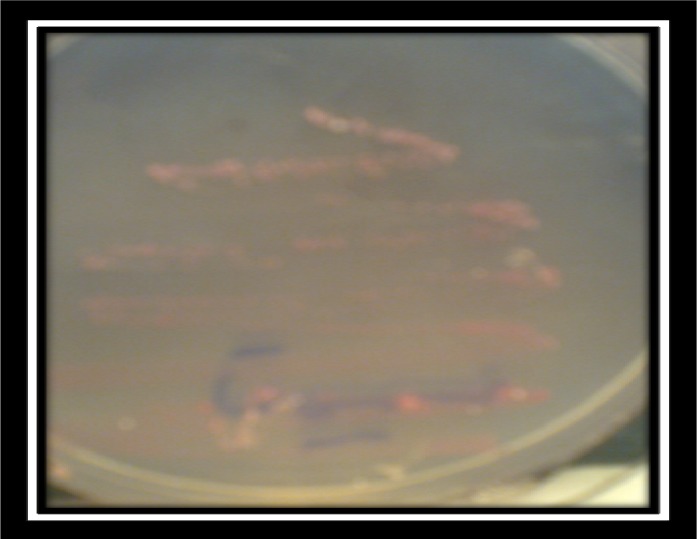
Fig. 1:
Characteristic colony morphology of P.zopfii genotype 2 on differential Prototheca Isolation Medium at 27°C after 7 days (Source: Authors)
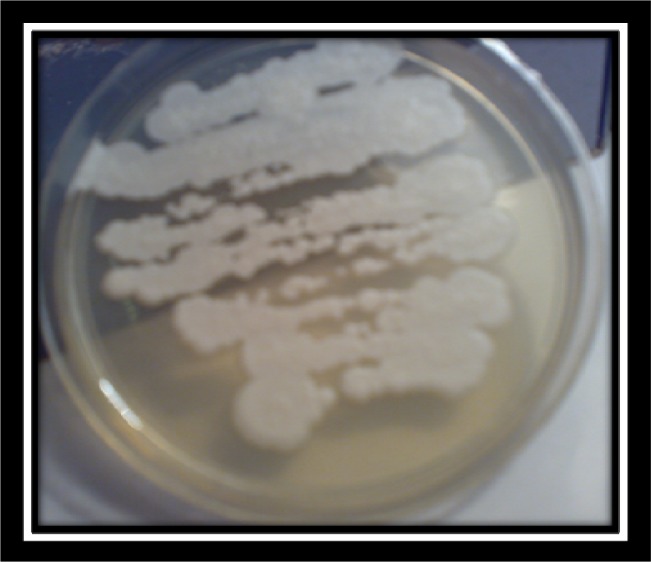
Fig. 2:
Colony morphology of P.zopfii genotype 2 on Subouraud Medium (Source: Authors)
Then the P. zopfii species were identified by morphological (Fig. 3) and biochemical methods (Table 2).
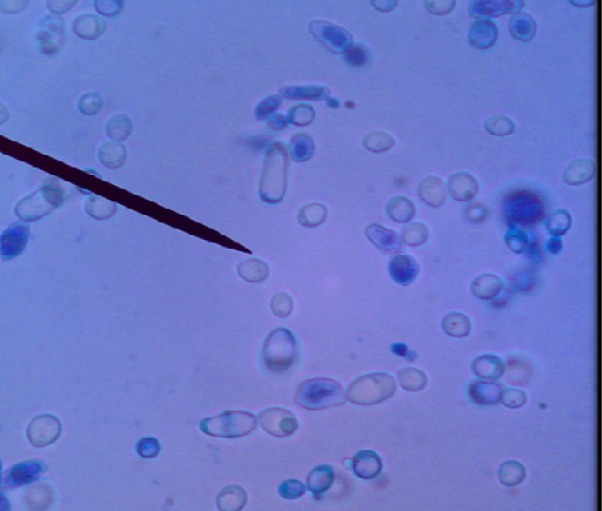
Fig. 3:
Methylene blue staining of P. zopfii genotype II isolated from milk samples obtained from diary cattle with mastitis
Table 2:
Characteristics that discriminate Prototheca zopfii
| Characteristics | Absorption |
|---|---|
| Glucose | + |
| Galactose | - |
| Sucrose | - |
| Trehalose | - |
| Propanol | + |
| Glycerol | + |
(+), absorbtion, (−), no absorbtion
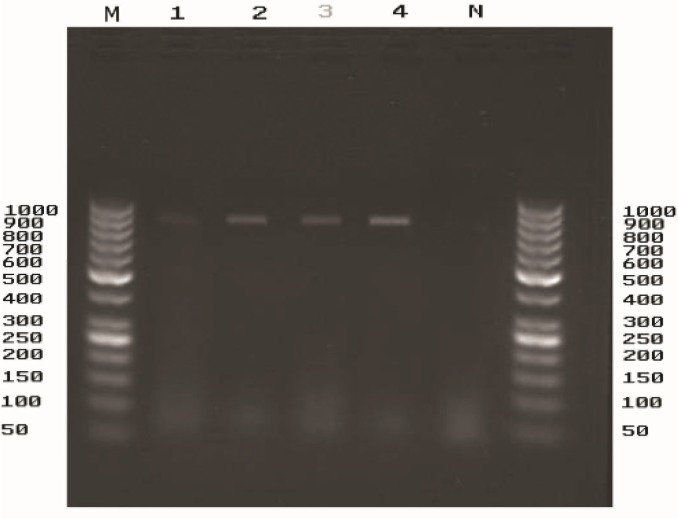
Four P. zopfii strains (3.07%) were isolated from the 130 samples of dairy cattle with clinical mastitis. There was no isolation from totally 100 samples of healthy bovines without mastitis. PCR was performed by newly designed primers for genotype II of P. zopfii and specific PCR product (about 946 bp) was detected in four isolates. Four P. zopfii mastitis isolates were thus identified as P. zopfii genotype II (Fig. 4). The results of genotype-specific PCR also showed that the three genotypes obviously are different in 18S rRNA gene composition. Furthermore, 18S r RNA gene sequencing of genotype III was clearly different from genotypes I and II.
Fig. 4:
The result of the PCR for P. zopfii genotype II. Agarose gel electrophoresis (2%) of amplified PCR products, performed by specific primers for genotype II. Lane M: DNA ladder, lane 1-4: the milk samples of the bovines with mastitis, lane N: negative control
Discussion
Based on the results of this study as well as other previous studies, it seems that P. zopfii genotype II plays a key role in causing bovine mastitis (2–5). This study was performed for the first time in Iran on the bovines with and without mastitis. In order to find P. zopfii in the milk samples, specific medium was used for detection and isolation of Prototheca species (20).
In 1999, Melville et al. performed a study, which considered the sensitivity of P. zopfii to pasteurization. They showed different sensitivity of 40 strains of P. zopfii to different times and temperatures of pasteurization (21).
This study was also important from the point of view of veterinarian studies and humans’ health since bovine mastitis can affect milk production by the cattle and reduce it. On the other hand, the P. zopfii is resistant to pasteurization and, therefore can easily transmit to its consumers and potentially cause health problems. Consequently, more studies should be carried out in this field and in other geographical parts of Iran, using more milk samples from bovines with mastitis in order to coordinate and confirm the results of recent study. The results showed P. zopfii, genotype II, is responsible for causing bovine mastitis in Iran as same as other geographical parts of the world (3, 17, 19).
Protothecosis is a zoonotic disease, which can be transmitted to the human by consuming milk and cause intestinal infections and enteritis because of its resistance to pasteurization (21). As a result, it is important and crucial to consider and identify these microorganisms in milk, because they can be potentially harmful to the human health. This study was the first study, which identified the Prototheca species as causative agents of bovine mastitis, by traditional and molecular methods in Iran and Middle East as well.
We hope that this study paves the way for further studies and investigations to be carried out in this field.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This study was part of MSc thesis supported by Tehran University of Medical Sciences (grand no: 704/P). The authors declare that there is no conflict of interests.
References
- 1.Jagielski T, Lagneau P. Protothecosis. A pseudofungal infection. Med Mycol. 2007;17:261–270. [Google Scholar]
- 2.Buzzini P, Turchetti B, Facelli R, Baadino R, Cavarero F, Mattaalia L, et al. First large-scale isolation of Prototheca zopfii from milk produced by dairy herds in Italy. Mycopathologia. 2004;158:427–30. doi: 10.1007/s11046-004-1819-3. [DOI] [PubMed] [Google Scholar]
- 3.Osumi T, Kishimoto U, Kano R, et al. Prototheca zopfii genotypes isolated from cow barns and bovine mastitis in Japan. Vet Microbiol. 2008;131:419–423. doi: 10.1016/j.vetmic.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Hodges RT, Holland JTS, Neilson FJA, Wallace NM. Prototheca zopfii mastitis in a herd of dairy cows. New Zeal Vet J. 1985;33:108–111. doi: 10.1080/00480169.1985.35187. [DOI] [PubMed] [Google Scholar]
- 5.Costa EO, Carciofi AC, Melville PA, Prada MA, Ribeiro AR, Watanabe ET. Bovine mastitis due to algae of the genus Prototheca sp. Mycopathologia. 1996;133:85–88. doi: 10.1007/BF00439118. [DOI] [PubMed] [Google Scholar]
- 6.Costa EO, Ribeiro AR, Watanabe ET, Pardo RB, Silva JAB, Sanches RB. An increase incidence of mastitis caused by Prototheca species and Nocardia species on a farm in Sao Paulo, Brazil. Vet Res Commun. 1996;20:237–241. doi: 10.1007/BF00366921. [DOI] [PubMed] [Google Scholar]
- 7.Costa EO, Carciofi AC, Melville PA, Prada MA, Schalch U. Prototheca spp. outbreak of bovine mastitis. J Vet Med. 1996;43:321–324. doi: 10.1111/j.1439-0450.1996.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Aalbaek B, Stenderup J, Jensen HE, Valbak J, Nylin B, Huda A. Mycotic and algal mastitis in Denmark. Acta Pathol MicrobiolImmunol Scand. 1994;102:451–456. doi: 10.1111/j.1699-0463.1994.tb04898.x. [DOI] [PubMed] [Google Scholar]
- 9.Almeraya AP. Aislamiento de Prototheca en un brote de mastitis bovina. Vet Mex. 1994;25:65–67. [Google Scholar]
- 10.Anderson KL, Walker RL. Sources of Prototheca spp. in a dairyherd environment. J Am Vet Med Assoc. 1998;193:553–556. [PubMed] [Google Scholar]
- 11.Costa EO, Carciofi AC, Melville PA, Prada MS, Ribeiro AR, Watanabe ET. World Buiatrics Congress, 18. Bologna, Italy: Proceedings; 1994. Epidemiological studies on bovine protothecosis; pp. 853–855. [Google Scholar]
- 12.Kirk JH. Diagnosis and treatment of difficult mastitis cases. Agri-Practice. 1991;12:15–20. [Google Scholar]
- 13.Segal E, Padhye AA, Ajello L. Susceptibility of Prototheca species to antifungal agents. Antimicrob Agents Chemother. 1976;10:75–79. doi: 10.1128/aac.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camargo ZP. Algas do ĝ enero Prototheca: caracterısticas microbiologicas e imunologicas [Thesis] Sao Paulo: EscolaPaulista de Medicina; 1978. [Google Scholar]
- 15.Mcdonald JS, Richard JL, Cheville NF. Natural and experimental bovine intramammary infection with. Prototheca zopfii Am J Vet Res. 1984;45:592–595. [PubMed] [Google Scholar]
- 16.Cheville NF, Mcdonald J, Richard J. Ultrastructure of Prototheca zopfii in bovine granulomatous mastitis. Vet Pathol. 1984;21:341–348. doi: 10.1177/030098588402100312. [DOI] [PubMed] [Google Scholar]
- 17.Moller A, Truyen U, Roesler U. Prototheca zopfii genotype2-the causative agent of bovine protothecal mastitis? Vet Microbiol. 2007;120:370–374. doi: 10.1016/j.vetmic.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Roesler U, Moller A, Hensel A, Baumann D, Truyen U. Diversity within the current algal species Prototheca zopfii a proposal for two Prototheca zopfii genotypes and description of a novel species, Prototheca blaschkeae spp. nov. Int J Syst Evol Microbiol. 2006;56:1419–1425. doi: 10.1099/ijs.0.63892-0. [DOI] [PubMed] [Google Scholar]
- 19.Aouay A, Coppee F, Cloet S, Cuvelier P, Belayew A, Lagneau P, et al. Molecular characterization of Prototheca strains isolated from bovine mastitis. Med Mycol. 2008;157:1–4. [Google Scholar]
- 20.Pore RS. Selective medium for the isolation of. Prototheca Appl Microbiol. 1973;26:648–9. doi: 10.1128/am.26.4.648-649.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melville P, Watanabe E, Benites N, Ribeiro A, Silva J, Garino F, et al. Evaluation of the susceptibility of Prototheca zopfii to milk pasteurization. Mycopathologia. 1999;146:79–82. doi: 10.1023/a:1007005729711. [DOI] [PubMed] [Google Scholar]