Abstract
Objective:
Recent work shows that the time from the initial use of nicotine, cannabis, and alcohol to the onset of dependence on these substances is shorter (“telescoped”) in anxiety-disordered individuals. Previously, we hypothesized that telescoping may result from a shared neurobiology underlying both anxiety disorders and dependence. This hypothesis implies that telescoping occurs because individuals with an anxiety disorder transition to dependence with less overall drug exposure (“dependence susceptibility”). To investigate this further, we examined an estimate of the amount smoked (rather than the time transpired) from smoking initiation milestones to the onset of nicotine dependence in those with and without an anxiety disorder.
Method:
We used the subset of respondents in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Wave 1 who reported having smoked at least 100 cigarettes (N = 18,013). All data were based on face-to-face interviews.
Results:
Individuals with any anxiety disorder transitioned to nicotine dependence after smoking fewer total cigarettes than did individuals with no anxiety disorder. Furthermore, those with more than one anxiety disorder transitioned to nicotine dependence after smoking fewer cigarettes than did those with one anxiety disorder only. Several potentially confounding covariates were controlled for in these analyses.
Conclusions:
Dependence susceptibility is a novel concept with the potential to inform theoretical accounts of and prevention strategies for substance dependence among those with an anxiety disorder. In addition to nicotine, our theory and past data suggest that dependence susceptibility for other addictive substances (e.g., alcohol) also would be found among those with an anxiety disorder.
Multiple epidemiological surveys have found that those with an anxiety disorder are at high risk for developing dependence on alcohol and other drugs relative to the general population (Compton et al., 2007; Grant et al., 2004b; Hasin et al., 2007; Kessler et al., 1997; Regier et al., 1990). There also is evidence that those with an anxiety disorder transition to dependence on alcohol, nicotine, and cannabis in less time (i.e., “telescoped”) following the initiation of the use of these substances than do those with no anxiety disorder (e.g., Kushner et al., 2011; Lopez-Quintero, et al., 2011). A better understanding of dependence telescoping among those with an anxiety disorder may be both practically and theoretically important.
We recently suggested that telescoping (and comorbidity in general) may result from a shared neurobiology underlying both anxiety disorders and substance dependence (Kushner et al., 2011). We extrapolated this idea, in part, from the work of George Koob and others who point to dysregulation in the brain’s stress/anxiety systems (e.g., the extended amygdala and hypothalamic–pituitary–adrenal axis) resulting from chronic substance use as a core process (“allostasis”) in the development of physical dependence (e.g., Koob, 2003; Koob and Le Moal, 2008; Schepis et al., 2011). Considering the neurobiological dysregulations associated alcoholinduced allostasis and the dysregulations in these same brain systems identified as neurobiological hallmarks of anxiety disorders (Holsboer, 1989; LeDoux, 2007; Pervanidou, 2008; Van den Bergh et al., 2008), we hypothesized that the neuro-patho-developmental processes leading to dependence should be more efficient, and therefore accelerated, in those with an anxiety disorder. Kushner et al. (2011) termed this hypothesized phenomenon dependence susceptibility.
The telescoped development of dependence among those with an anxiety disorder (above) is consistent with the dependence susceptibility hypothesis. However, there would be no need to infer a biological vulnerability to dependence if telescoping simply reflects a higher quantity of drug and/or alcohol use over a given period by those with an anxiety disorder. In the present work, we attempted to clarify these alternative explanations by contrasting the amount smoked (rather than the time transpired) from several smoking initiation milestones to the onset of nicotine dependence between those with and without an anxiety disorder. We predicted that individuals with anxiety disorders would smoke fewer packs of cigarettes from both the initiation of use and from the onset of daily smoking to the onset of dependence compared with other smokers.
Method
Participants
Data in this study were drawn from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Wave 1 data set, a nationally representative survey of U.S. citizens with response data from 43,093 individuals (cf., Grant, 2004b). Fifty-seven percent of the sample was female, and ages ranged from 18 to 98 years (M = 46.4, SD = 18.2). In the present study, we examined only the subset of individuals who reported having smoked at least 100 cigarettes in their lifetime (N = 18,013). In this subsample, 20.5% met lifetime criteria for one anxiety disorder (panic disorder, social phobia, specific phobia, or generalized anxiety disorder); ages for the subsample ranged from 18 to 98 years (M = 49.6, SD = 17.4).
Measures
In the NESARC, lifetime psychopathology and substance use disorders were assessed using the National Institute on Alcohol Abuse and Alcoholism’s Alcohol Use Disorder and Associated Disabilities Interview Schedule–IV (AUDADISIV; Grant et al., 2003), a highly structured diagnostic interview designed for administration by trained laypersons and possessing good reliability (Grant et al., 2004a). The AUDADIS-IV contains questions designed to assess the presence of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), psychopathology (including anxiety disorders) and substance use disorders (including nicotine dependence), as well as age at initiation of use, age at onset of disorder, and frequency and quantity of use. For example, participants were asked at what age they first smoked a full cigarette, at what age they began smoking daily, their usual frequency of smoking (e.g., daily), and their usual quantity of cigarettes smoked.
Analytic approach
Estimates of number of packs of cigarettes smoked.
We estimated the total number of packs of cigarettes smoked for each respondent from the age at which specific initiation milestones first occurred through the age at which nicotine dependence was first diagnosed or, for those never diagnosed with nicotine dependence, to their age at the time of the NESARC Wave 1 interview. One initiation milestone was the first full cigarette smoked, and a second was the point at which daily smoking began. To obtain the smoking exposure estimates, we (a) extrapolated the typical number of cigarettes smoked per year by multiplying the number of cigarettes respondents reported typically smoking per day (this could be <1 for nondaily smokers) by 365, (b) estimated the number of packs smoked per year by dividing the estimate of the number of cigarettes smoked per year by 20 (the number of cigarettes in a pack), and (c) determined the number of years relevant to the estimate by subtracting the age at which the initiating milestone was reached from the age at which the terminating milestone was reached (either nicotine dependence onset or the Wave 1 interview, whichever came first). We then obtained the final estimates of packs of cigarettes smoked between each of the two initiating milestones and the terminating milestone by multiplying the estimated number of packs smoked per year (b) by the number of years between the initiating and terminating milestones (c). (Individuals who reported age at onset of dependence—or age at Wave 1 for those not nicotine dependent—to be the same as age at the first full cigarette smoked [n = 344] or first daily cigarette smoking [n = 755] had these intervals coded as 1 year, meaning that they converted to dependence within the first year of their initiation of smoking.) Finally, our nicotine exposure estimates were log-transformed to correct for significantly positively skewed distributions.
Data analytic method.
We used SPSS (Version 18) Complex Sampling Module (SPSS Inc., Chicago, IL) to compare individuals with and without a lifetime anxiety disorder on gender distribution and rates of lifetime nicotine dependence (using cross-tabulations) as well as nicotine use milestones and nicotine exposure variables (using general linear modeling). We also used SPSS (Version 18) Complex Sampling Module to conduct Cox regressions comparing the risk of transition to nicotine dependence per units of packs of cigarettes smoked (log-transformed) between those with and without lifetime anxiety disorders and between those with no lifetime anxiety disorder, one lifetime anxiety disorder, and more than one lifetime anxiety disorder. All analyses incorporated adjustments (Taylor series linearization) for the complex sampling design used in the NESARC survey.
Covariates.
All analyses included the following covariates: age, gender, age at initiating milestone (first full cigarette smoked or first daily smoking), and significant nicotine use other than cigarettes (50 or more lifetime uses of pipe tobacco or cigars, 20 or more lifetime uses of snuff or chewing tobacco).
Results
Group characteristics
Individuals with a lifetime anxiety disorder diagnosis were significantly younger than non-lifetime-anxiety-disordered individuals (M = 45.11 years, SE = 0.12 vs. M = 48.06, SE = 0.10, p < .001, Cohen’s d = 4.78). Additionally, anxiety-disordered individuals were younger when they (a) smoked their first full cigarette (M = 15.71 years, SE = 0.05, vs. M = 16.19 years, SE = 0.02, p < .001, d = 2.31), (b) began smoking daily (among those who endorsed ever smoking daily) (M = 18.16, SE = 0.05, vs. M = 18.50, SE = .03, p < .001, d = 1.75), and (c) first met nicotine-dependence criteria (among those with lifetime nicotine dependence) (M = 29.53, SE = 0.19, vs. M = 31.73, SE = 0.14, p < .001, d = 2.37). Anxiety-disordered individuals also smoked more packs of cigarettes per year (M = 315.49, SE = 2.50, vs. M = 283.78, SE = 1.44, p < .001, d = 2.66), smoked more cigarettes per day (M = 17.73, SE = 0.13, vs. M = 16.01, SE = 0.08, p < .001, d = 2.65), were more likely to be female (percentage female = 60.1% vs. 41.6%, odds ratio [OR] = 2.11, 95% CI [2.01, 2.21], p < .001), and were more likely to have met criteria for nicotine dependence in their lifetime (percentage ever dependent = 58.5% vs. 34.1%, OR = 2.73, 95% CI [2.60, 2.86], p < .001). (As noted in the Method section, covariates were used in the Cox regressions below to ensure that these demographic and clinical variables did not account for group differences in the primary analyses.)
Lifetime anxiety disorder predicting risk of transition from first cigarette to nicotine dependence per pack of cigarettes smoked
Lifetime anxiety disorder present versus absent.
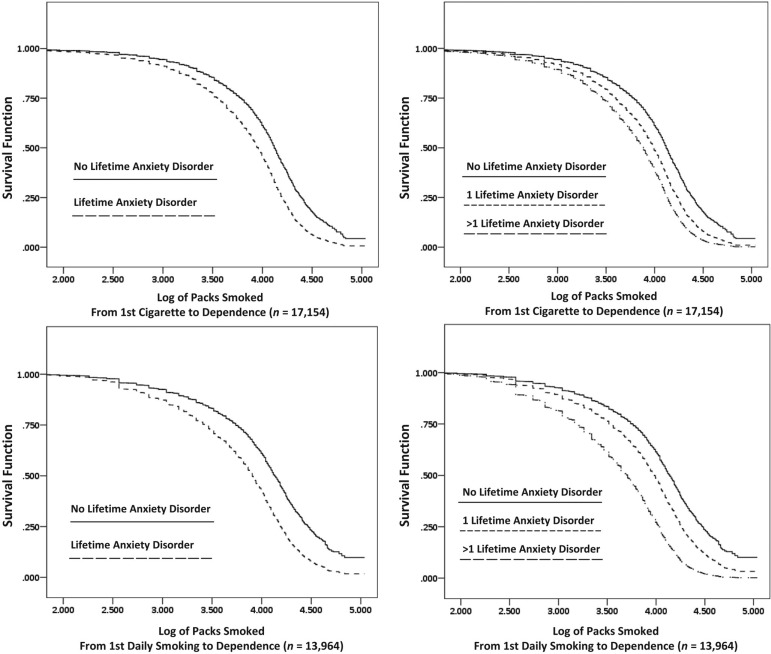
As shown in the upper left panel of Figure 1, we found that individuals with a lifetime anxiety disorder diagnosis transitioned from the first full cigarette smoked to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did individuals with no lifetime anxiety disorder (hazard ratio [HR] = 1.60, 95% CI [1.54, 1.67]), t(65) = 23.52, p < .001.
Figure 1.
Conversion to nicotine dependence by amount of nicotine exposure
Lifetime anxiety disorder count.
As shown in the upper right panel of Figure 1, we found that individuals with more than one lifetime anxiety disorder transitioned from the first full cigarette smoked to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did those with only one lifetime anxiety disorder diagnosis (HR = 1.33, 95% CI [1.27, 1.40]), t(65) = 10.99, p < .001. Additionally, we found that each anxiety group (one lifetime anxiety diagnosis and more than one lifetime diagnosis) transitioned from the first full cigarette smoked to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did individuals with no lifetime anxiety disorder—one lifetime anxiety diagnosis: (HR = 1.47, 95% CI [1.40, 1.54]), t(65) = 16.18, p < .001; more than one lifetime anxiety diagnosis: (HR = 1.95, 95% CI [1.86, 2.04]), t(65) = 29.27, p < .001.
Lifetime anxiety disorder predicting risk of transition from the first daily smoking to nicotine dependence per pack of cigarettes smoked
Lifetime anxiety disorder present versus absent.
As shown in the lower left panel of Figure 1, we found that individuals with a lifetime anxiety disorder diagnosis who typically smoked daily transitioned from the point of their first daily smoking to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did individuals with no lifetime anxiety disorder (HR = 1.73, 95% CI [1.66, 1.80]), t(65) = 27.31, p < .001.
Lifetime anxiety disorder count.
As shown in the lower right panel of Figure 1, we found that individuals with more than one lifetime anxiety disorder who typically smoked daily transitioned from the first daily smoking to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did those with only one lifetime anxiety disorder diagnosis (HR = 1.80, 95% CI [1.71, 1.90]), t(65) = 22.04, p < .001. Additionally, we found that each anxiety group (one lifetime anxiety diagnosis and more than one lifetime diagnosis) transitioned from first daily smoking to the onset of nicotine dependence after smoking significantly fewer packs of cigarettes than did individuals with no lifetime anxiety disorder—one lifetime anxiety diagnosis: (HR = 1.49, 95% CI [1.42, 1.56]), t(65) = 16.54, p < .001; more than one lifetime anxiety diagnosis: (HR = 2.68, 95% CI [2.56, 2.80]), t(65) = 44.16, p < .001.
Discussion
According to several neurobiological models (e.g., Koob and Le Moal, 2008; Schepis et al., 2011), dysregulation of the brain’s stress/anxiety systems caused by repeated episodes of substance use and withdrawal underlies the development of substance dependence. Because the same brain systems are dysregulated in those with anxiety disorders even before any drug or alcohol use (Holsboer, 1989; LeDoux, 2007; Pervanidou, 2008; Van den Bergh et al., 2008), we theorized that the development of substance dependence should be more efficient and, therefore, accelerated in this group (dependence susceptibility). Consistent with this, Kushner et al. (2011) demonstrated telescoped development of alcohol dependence among those with anxiety disorders, and Lopez-Quintero et al. (2011) demonstrated telescoped transition to alcohol, nicotine, and cannabis dependence among those with an anxiety disorder. However, this past work does not clarify whether telescoping among those with an anxiety disorder results from a lower threshold of substance exposure needed to develop dependence or whether anxiety-disordered individuals tend to use a greater quantity of substance per unit of time and therefore achieve dependence more quickly. Clarifying this issue is the central purpose of the study described in this report.
Although we found that individuals with anxiety disorders smoke approximately 70 more packs of cigarettes per year, we showed that individuals with anxiety disorders transition to dependence after smoking fewer total packs of cigarettes than do individuals without anxiety disorders (Figure 1). These findings suggest that increased exposure to cigarettes does not account for nicotine dependence telescoping among those with an anxiety disorder. In addition, more anxiety diagnoses led to greater dependence susceptibility. This latter finding lends strength to the conclusion that the extent to which an individual has the underlying trait(s) associated with risk for an anxiety disorder directly relates to his or her degree of dependence susceptibility. This conclusion also is consistent with recent findings by Kushner et al. (2012) showing that risk for alcohol dependence increases linearly with internalizing disorder load.
This study does not clarify whether a fully manifest anxiety disorder versus an endophenotypic neurobiological proneness to an anxiety disorder alone promotes dependence susceptibility. In this regard, it is relevant to note that post hoc tests (not shown) confirm dependence susceptibility even among the minority of our sample (approximately 18%) for whom an anxiety disorder manifested after the onset of nicotine dependence. Similarly, Kushner et al. (2011) reported that telescoping to alcohol dependence was evident among those for whom the onset of the anxiety disorder was after alcohol dependence. That the neurobiological stress systems relevant to dependence susceptibility are already dysregulated or are especially prone to becoming dysregulated before the onset of an anxiety disorder is consistent with these data and our theory.
This study has a number of potentially important limitations. The data were based on retrospective recall, which could be increasingly inaccurate for respondents recalling cigarette use patterns from many years ago. Also, the data set provided respondents’ age when daily smoking first began but not the age when the typical pattern of smoking first began. This suggests that the estimate of the smoking amount beginning with the first full cigarette smoked is likely to be somewhat less accurate than the estimate of the smoking amount beginning with the onset of daily smoking. This is because respondents probably smoked less than their typical amount (but possibly more) for an unknown period before achieving the typical amount of smoking reported at Wave 1. With this said, we have no reason to believe that the accuracy of the estimates systematically differed between the groups, and we have some affirmative reasons to conclude that they did not. For example, the time from the first cigarette smoked to the onset of a typical pattern of daily smoking was very similar between the two groups (about 2.6 years vs. 2.5 for those with vs. those without a lifetime anxiety disorder). Finally, although our theory is based on a neurobiological model of dependence that is pertinent to multiple addictive substances (e.g., Koob and Le Moal, 2008), we focused only on nicotine dependence because the NESARC did not include sufficient information on the lifetime use of substances such as alcohol and marijuana to allow for the tests conducted in this report related to nicotine. Future epidemiological and clinical data collection efforts should include such data.
From an applied perspective, the dependence susceptibility view suggests that those with one, and especially more than one, lifetime anxiety disorder should be made aware that the same amount of substance use is more likely to result in dependence for them compared with other individuals. From a neuroscientific perspective, future studies using pharmacological, neural imaging, and neurocognitive assessment will be needed to study the neurobiological processes implied by the dependence susceptibility model supported by epidemiological data in this report.
Footnotes
This research was supported by National Institute on Alcoholism and Alcohol Abuse Grants RO1 AA0105069 and KO2 AA0017886 (to Matt G. Kushner).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (4th ed.) Washington, DC: Author; 1994. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and Alcohol Dependence. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004a;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004b;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Psychiatric implications of altered limbic-hypothalamic-pituitary-adrenocortical activity. European Archives of Psychiatry and Clinical Neuroscience. 1989;238:302–322. doi: 10.1007/BF00449812. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Maurer E, Menary K, Thuras P. Vulnerability to the rapid (“telescoped”) development of alcohol dependence in individuals with anxiety disorder. Journal of Studies on Alcohol and Drugs. 2011;72:1019–1027. doi: 10.15288/jsad.2011.72.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Wall MM, Krueger RF, Sher KJ, Maurer E, Thuras P, Lee S. Alcohol dependence is related to overall internalizing psychopathology load rather than to particular internalizing disorders: evidence from a national sample. Alcoholism: Clinical and Experimental Research. 2012;36:325–331. doi: 10.1111/j.1530-0277.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. Journal of Neuroendocrinology. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, Adinoff B. The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcoholism: Clinical and Experimental Research. 2011;35:595–605. doi: 10.1111/j.1530-0277.2010.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]