Abstract
In pathological conditions, the amount of DJ-1 determines whether a cell can survive or engage a cell death program. This is exemplified in epithelial cancers, in which DJ-1 expression is increased, while autosomal recessive early onset Parkinson's disease mutations of DJ-1 generally lead to decreased stability and expression of the protein. We have shown previously that DJ-1 is cleaved by caspase-6 during induction of apoptosis. We demonstrate here that the N-terminal cleaved fragment of DJ-1 (DJ-1 Nt) is specifically expressed in the nucleus and promotes apoptosis in SH-SY5Y neuroblastoma cell lines. In addition, overexpression of DJ-1 Nt in different cell lines leads to a loss of clonogenic potential and sensitizes to staurosporin and 1-methyl-4-phenylpyridinium (MPP+)-mediated caspase activation and apoptosis. Importantly, inhibition of endogenous DJ-1 expression with sh-RNA or DJ-1 deficiency mimics the effect of DJ-1 Nt on cell growth and apoptosis. Moreover, overexpression of DJ-1 Nt increases reactive oxygen species (ROS) production, and sensitizes to MPP+-mediated apoptosis and DJ-1 oxidation. Finally, specific exclusion of DJ-1 Nt from the nucleus abrogates its pro-apoptotic effect. Taken together, our findings identify an original pathway by which generation of a nuclear fragment of DJ-1 through caspase 6-mediated cleavage induces ROS-dependent amplification of apoptosis.
Keywords: DJ-1, caspase 6, apoptosis, ROS production
DJ-1/PARK7 has been identified as an oncogene capable to transform fibroblasts either alone or in cooperation with H-Ras.1 Later, DJ-1 mutations or deletions have been shown to be responsible for rare cases of autosomal recessive early onset Parkinson's disease (EOPD).2 DJ-1 is a highly conserved protein, expressed in nearly all organisms from human to bacteria.3 Recent consensual data suggest that DJ-1 could harbor a protective function.4 This phenotype has been linked to its ability to stimulate Akt phosphorylation and activity through inhibition of PTEN.4 DJ-1 protective effect has also been associated to its capacity to interact with the MAPK kinase cascade5 or to sequestrate nuclear factor E2-related factor 2 (Nrf2). In addition, there are also compelling evidences suggesting that DJ-1 promotes cell survival by protecting cells from oxidative stress.6, 7 Indeed, DJ-1 over-expression confers resistance to oxidative stress, and conversely, DJ-1 silencing by si-RNA or knockdowned cells derived from DJ-1-deficient mice triggers increased sensitivity to stress-inducing agents.8 The level of expression of DJ-1 controls the cell death and survival balance in neurodegenerative diseases or cell survival in cancer and rare deletion or loss of function mutations in DJ-1 gene have been linked to autosomal recessive EOPD.9, 10 Point mutations or deletion of DJ-1 gene yield proteins with decreased stability and thereby, abolish their protective and survival-associated functions. In addition, in several models of cancer cells, DJ-1 expression is increased and positively associated with the severity of the disease.11, 12, 13
Caspases define a family of cysteine proteinases with crucial function in the initiation and execution of apoptosis.14 They cleaved their substrates specifically after an aspartate residue leading either to inhibition or activation of their functions.15 Among caspases, caspase 6 has been shown to have an important role in neurodegenerative diseases, as ablation of caspase 6 in mice can protect them from Huntington's disease.16 We have shown recently that DJ-1 is cleaved by caspase 6 in TSM1 neurons and neuroblastoma cell lines undergoing staurosporin (STS) or 6-hydroxydopamine-mediated apoptosis, and overexpression of the DJ-1 C-terminal fragment was found to exert anti-apoptotic function by inhibition of the p53 pathway.17
In the present study, we investigated the possibility that the caspase-6-generated N-terminal fragment of DJ-1 could have influenced the biological function of this protein in different cell line models. We report that during induction of apoptosis by various stimuli, this fragment accumulates into the nucleus of Hela or MEF-DJ-1−/− cells. Overexpression of DJ-1 Nt in SH-SY5Y neuroblastoma cell line leads to a loss of clonogenic potential. Inhibition of DJ-1 expression by specific Sh-RNA or using MEF deficient for DJ-1 mimics the effect of DJ-1 Nt on both colony formation and induction of apoptosis. Moreover, overexpression of DJ-1 Nt in SH-SY5Y cells increased reactive oxygen species (ROS) production, induced DJ-1 oxidation and sensitizes to MMP+ mediated apoptosis. Finally, specific exclusion of DJ-1 Nt from the nucleus was shown to abrogate its pro-apoptotic effect.
Results
Loss of DJ-1 or DJ-1 knockdown inhibits clonogenic cell growth and sensitizes SH-SY5Y cells to STS-mediated apoptosis
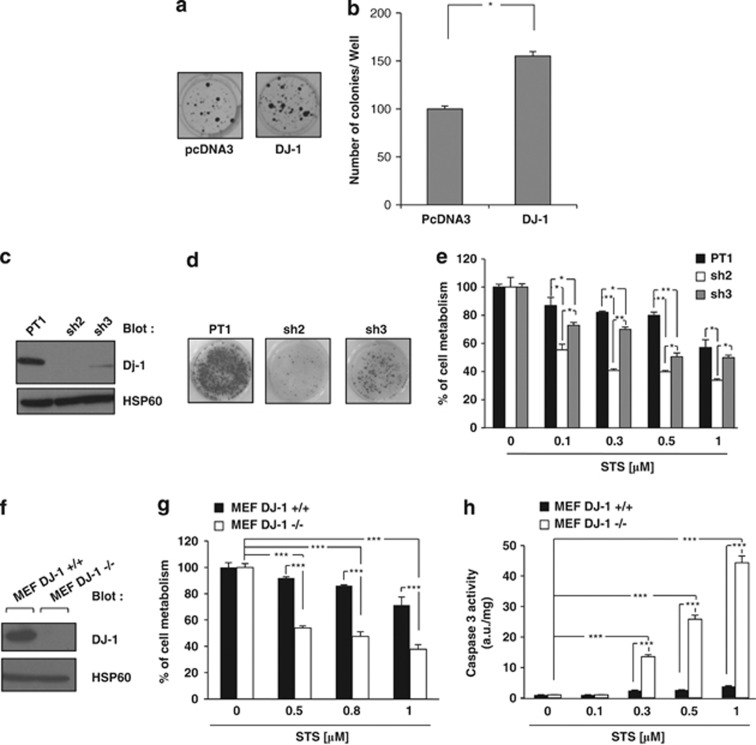
We used the neuroblastoma cell line SH-SY5Y as a model to investigate the function of DJ-1. Over-expression of DJ-1 increased the ability of SH-SY5Y cells by 60% to form colonies, confirming the oncogenic function for this protein (Figures 1a and b). We next generated different SH-SY5Y cell clones in which DJ-1 expression was knockdowned using the most potent DJ-1 Sh-RNA. DJ-1 expression was inhibited by 90% by DJ-1 Sh3-RNA and abrogated by Sh2-RNA (Figure 1c). Both cell clones exhibited reduced clonogenic potential that matched well with the level of extinction of DJ-1 (Figure 1d). DJ-1 knocks down by both Sh-RNA sensitized SH-SY5Y cells to STS-mediated loss of cell viability (Figure 1e). These results were confirmed using DJ-1-deficient mouse embryo fibroblasts (MEF), in which the absence of DJ-1 (Figure 1f) also increased sensitivity to STS-mediated loss of cell viability (Figure 1g).18 Accordingly, an increase in caspase 3 activity upon STS treatment was detected in DJ-1−/− cells compared to DJ-1+/+ MEFs (Figure 1h). Finally, we investigated the effect of 1-methyl-4-phenylpyridinium (MPP+) on cell metabolism in DJ-1+/+ and DJ1−/− MEF. MPP+ interferes with oxidative phosphorylation, causing depletion of ATP and cell death.19 Therefore, this compound has been widely used as an agent causing Parkinson-like disease in primates by killing certain dopamin-producing neurons in the substantia nigra. We found that conversely to STS, DJ-1+/+ MEF were poorly sensitive to 2 mM MPP+, whereas DJ-1−/− MEF exhibited increased sensitivity to both drugs (Supplemental Figures 1A and B).
Figure 1.
DJ-1 expression level is important for clonogenic capacity and sensibility to apoptosis. (a) SH-SY5Y cells were cotransfected with puromycin-resistant vector and with either an empty vector or a vector carrying wild-type DJ-1. After 48 h, cells were treated with puromycin (2 μg/ml) every 2 days. Ten days later, cells were fixed and colonies detected by adding crystal violet. (b) Colonies were scored by Image J quantification software. (c) SH-SY5Y cells with stable expression of the empty vector (PT1) or DJ-1 shRNA vector (sh2, sh3) were lysed and analyzed by western blot for DJ-1 expression. (d) The various SH-SY5Y were analyzed for their clonogenic potential. (e) SH-SY5Y clones were incubated for 24 h at 37 °C with increasing concentrations of STS. Cell metabolism was measured by the XTT assay as described in Materials and Methods section. (f) MEF DJ-1 −/− or DJ-1 +/+ cells were lysed and analyzed by western blot for DJ-1 expression. (g) MEF DJ-1 −/− or +/+ cells were incubated for 6 h at 37 °C with increasing concentrations of STS. Cell metabolism was measured by the XTT assay. (h) MEF DJ-1 −/− or DJ-1 +/+ cells were incubated as described above, and caspase-3 activity was evaluated as described in Materials and Methods section
DJ-1 is cleaved by caspase 6 in cells undergoing apoptosis
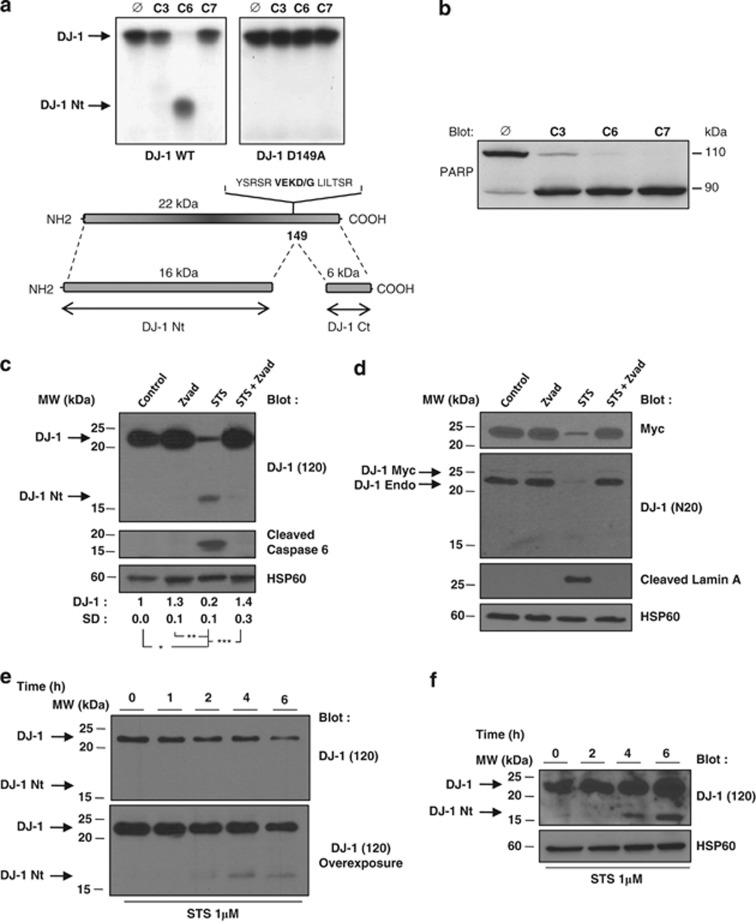
As described earlier DJ-1 was cleaved after Asp149 by recombinant caspase-6,17 but not by any other caspases including caspase 3 and 7, and accordingly mutation of Asp149 by an alanine prevented cleavage (Figure 2a). The activity of recombinant caspases was confirmed by assessing the cleavage of poly-ADP-ribose polymerase (PARP) in identical conditions (Figure 2b). Moreover, cleavage of DJ-1 was readily detected in wild-type DT40 cells but not in their caspase 6−/− counterparts, confirming that DJ-1 is a caspase 6-preferential substrate (Supplemental Figure 2).20 Importantly, the caspase 6 consensus cleavage site in DJ-1 (VEKD) was highly conserved among different species, suggesting its possible importance during phylogeny (Supplemental Figure 3).
Figure 2.
DJ-1 protein is cleaved under in vivo and in vitro caspase-activated condition. (a and b) DJ-1 Wt, DJ-1 D149A and PARP were transcribed and translated in vitro and incubated for 16 h at 37 °C with recombinant caspases (25 ng). Proteins were then electrophoresed on 12% PAGE and autoradiographed. (c) Jurkat T-leukemic cells were treated with 1 μM STS in the presence or absence of Z-VAD-fmk (50 μM) for 6 h and analyzed by immunoblotting with anti-DJ-1 (120) and the cleaved DJ-1-specific antibody. (d) Jurkat T cells were transfected with DJ-1-Myc. After 48 h, cells were treated with 1 μM STS with or without Z-VAD-fmk (50 μM) for 6 h and analyzed by immunoblotting with anti-Myc, anti-DJ1 (N20) and anti-cleaved lamin A/C antibody. (e) Jurkat T cells were treated for the times indicated with 1 μM STS. Cells were lysed and proteins were analyzed by immunoblotting with anti-DJ-1 (120). (f) SH-SY5Y cells were treated for the times indicated with 1 μM STS. Cells were lysed and proteins were analyzed by immunoblotting with anti-DJ-1 antibody (120). HSP60 was used as a loading control
In the present study, we investigated the function of the large N-terminal fragment of DJ-1 on proliferation and apoptosis of different cell line models. Experiments using several commercially available antibodies revealed the disappearance of intact DJ-1 in these cell line models but failed to detect the N or C-terminal cleaved fragments of DJ-1. Although this was expected concerning DJ-1 Ct, due to the poor stability of this fragment,17 the lack of recognition of DJ-1 Nt by various anti-DJ-1 antibodies was puzzling. Therefore, we generated a rabbit-antiserum specifically directed against the recombinant N-terminal form of DJ-1. This DJ-1-specific antibody (DJ-1 (120)) consistently detected a 16-kDa protein (hereafter referred to as DJ-1 Nt) in the T-leukemic cell line Jurkat, used here for its high sensitivity to STS-mediated killing, likely corresponding to cleavage by caspases after Asp149 (Figure 2c). Accordingly, cleavage of DJ-1 was prevented by zVAD-fmk-implicating caspases in this process. Interestingly, zVAD-fmk was also found to increase DJ-1 level in the absence of any apoptotic signal, suggesting a constitutive turnover of DJ-1 through basal caspase activation. Cleavage of DJ-1 correlated with accumulation of active cleaved caspase 6 and the appearance of a cleaved lamin A fragment, which is a specific nuclear caspase 6 substrate (Figure 2d). Cleavage of endogenous DJ-1 was detected also using a commercially available antibody in Myc-tagged-DJ-1 transfected Jurkat T cells (Figure 2d). Although both endogenous and exogenous DJ-1 were cleaved in the presence of STS, the commercially available DJ-1 (N20) antibody failed to recognize the cleaved form of DJ-1, in contrast to the DJ-1 (120) antibody (Figure 2c). In addition, time-course experiments showed that DJ-1 was cleaved as soon as 2 h after STS addition in Jurkat T cells (Figure 2e). Finally, DJ-1 cleavage was also detected in the SH-SY5Y cell line treated with STS (Figure 2f).
Distinct cellular localization of the native and cleaved forms of DJ-1
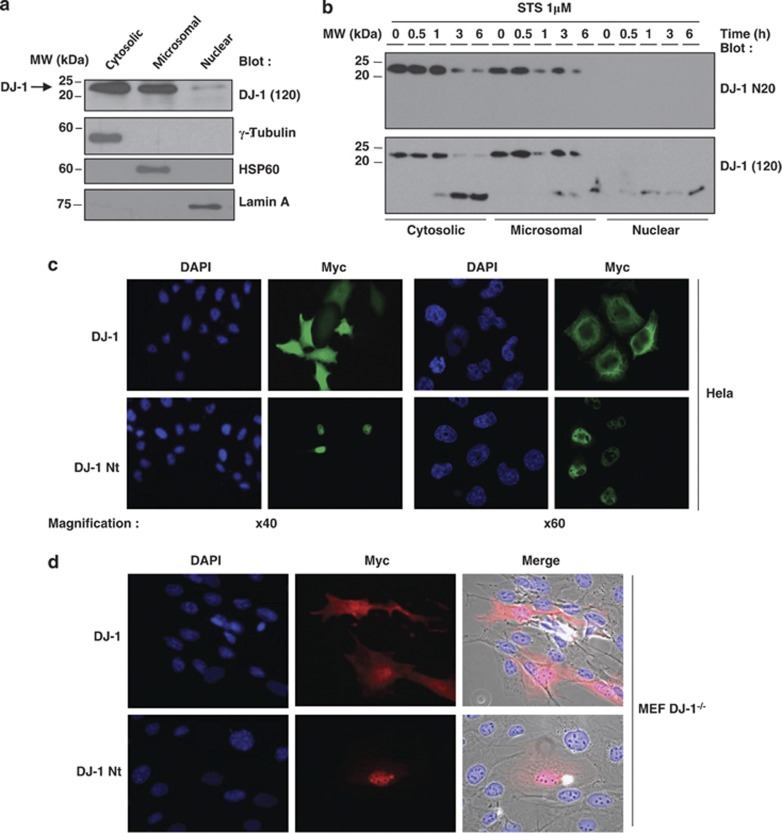
As DJ-1 cleavage may alter its function, we performed subcellular fractionnements to analyze the localization of the native and cleaved forms of DJ-1. Western blot experiments indicated that native DJ-1 was present both into the cytosolic and the microsomal fraction of SH-SY5Y neuroblastoma cells (Figure 3a). The purity of each fraction was checked with specific protein markers including γ-tubulin, HSP60 and lamin A (Figure 3a). Interestingly, both calreticulin and HSP60 staining partially colocalized with Myc-DJ-1 in transfected Hela cells, suggesting that DJ-1 is associated with both the endoplasmic reticulum and the mitochondria (Supplemental Figure 4A). We also performed a kinetic of DJ-1 cleavage in the different subcellular fractions of Jurkat T cells following STS treatment. The data presented in Figure 3b confirmed that the DJ-1 N20 antibody did not recognize the cleaved form of DJ-1, irrespectively of the considered fraction. Importantly, both full-length DJ-1 and cleaved DJ-1 were found in the cytoplasmic and to a lesser extent for cleaved DJ-1 microsomal fraction of Jurkat cells; whereas as previously shown, the cleaved DJ-1, but not the full-length DJ-1 fragment, accumulates in the nuclear fraction. Confocal microscopy analysis of Hela cells transfected with wild-type Myc-DJ-1 or Myc-DJ-1 Nt constructs confirmed the nuclear localization of tagged-Myc-DJ-1 Nt and the cytoplamic distribution of tagged-Myc-DJ-1 (Figure 3c). A similar pattern was observed using DJ-1−/− MEF transfected with DJ-1 or DJ-1 Nt (Figure 3d). Taken together, these findings support the notion that DJ-1 cleavage by caspase 6 results in nuclear accumulation of the N-terminal fragment of DJ-1. Finally, we transfected GFP-DJ-1 and GFP-DJ-1 Nt constructs in Hela cells and analyzed live cells under a fluorescence microscope before fixing them. Data in Supplemental Figure 4B showed that the location of GFP-tagged constructs was identical in live cells as compared with the fixed ones (Figure 3c).
Figure 3.
DJ-1 and DJ-1 Nt exhibit differential subcellular localization. (a) SH-SY5Y cells were collected and protein samples from different subcellular fractions were prepared, separated by gel electrophoresis. Expression of DJ-1 was visualized by western blotting. As expected, γ-tubulin, HSP60 and Lamin A were, respectively, found only in the cytoplasmic, microsomal and nuclear fractions. (b) Jurkat T cells were treated for the times indicated with 1 μM STS. Then, protein extracts from the indicated subcellular fractions were prepared and separated by SDS-PAGE. Expression of DJ-1 was visualized by western blotting using either the N20 anti-DJ-1 (upper panel) or the (120) anti-DJ-1 antibody, respectively (lower panel). (c) Hela cells were transfected with DJ-1-Myc or DJ-1 Nt-Myc. Fourty eight hours later cells were fixed and permeabilized. Slides containing cells were mounted and analyzed under a fluorescence microscope (left panel) or confocal microscope (right panel). (d) MEF DJ-1−/− were transfected with DJ-1-Myc or DJ-1 Nt-Myc and analyzed as described above
Expression of DJ-1 in SH-SY5Y cells increased ROS production
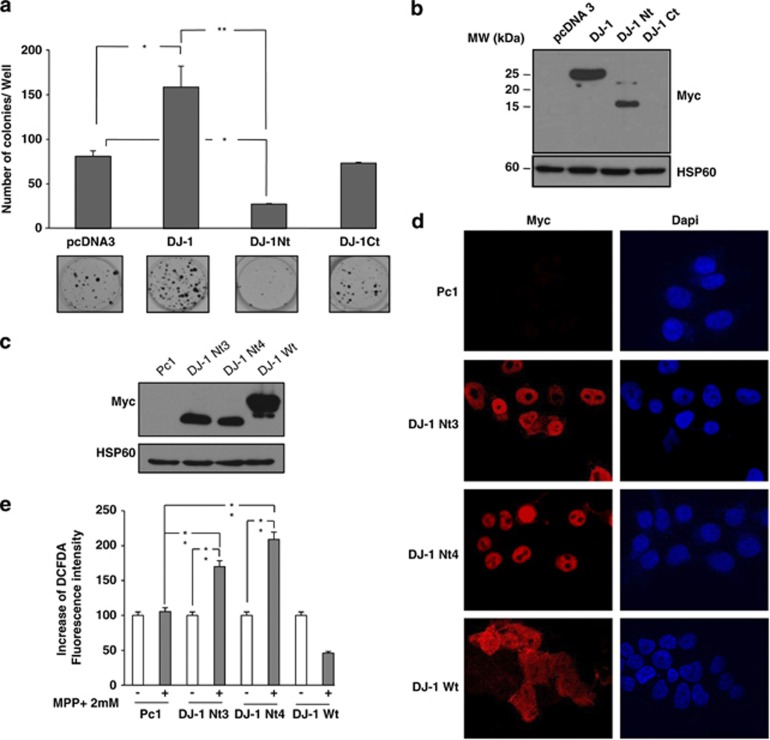
To investigate the function of the DJ-1 Nt fragment, we next transfected the different forms of DJ-1 in SH-SY5Y cells. As expected, expression of Myc-tagged DJ-1 Wt but not Myc-tagged DJ-1 Ct increased the clonogenic potential of SH-SY5Y cells (Figure 4a). In contrast, transfection of Myc-tagged DJ-1 Nt decreased by 75% the number of SH-SY5Y cell colonies. Western blot analysis of cell extract confirmed expression of both DJ-1 Wt and DJ-1 Nt but not DJ-1 Ct, likely owing to its poor stability (Figure 4b). To investigate further the function of DJ-1 Nt, we established SH-SY5Y cells stably expressing DJ-1 Nt. Compared to SH-SY5Y cell lines expressing DJ-1 Wt, the establishment of the DJ-1 Nt clones was considerably longer. This can be due to the lower growth capacity of these cells (Figure 4a). The pro-apoptotic effect of DJ-1 Nt while detectable in basal conditions was, however, less pronounced than in stress conditions and can explain why we were able to obtain DJ-1 Nt clones. As compared to SH-SY5Y cells transfected with an empty vector PC1 or DJ-1 Wt, DJ1 Nt3 and 4 clones exhibited high amount of Myc-Tagged DJ-1 Nt, even though expression of DJ-1 Wt was higher than that of DJ-1 Nt3 and 4 (Figure 4c). Confocal microscopy experiments also confirmed a clear nuclear localization of DJ-1 Nt and cytoplasmic localization for DJ-1 Wt (Figure 4d). Finally, expression of DJ-1 Nt3 or 4 in SH-SY5Y cells was accompanied by increase in MPP+-mediated ROS production as assessed by dichloro-dihydrofluorescein diacetate (DCFDA) staining followed by flow cytometry (Figure 4e). By contrast, ROS production was reduced in SH-SY5Y cells expressing DJ-1 Wt treated with MPP+, suggesting a protective function of DJ-1 Wt in this context (Figure 4e).
Figure 4.
DJ-1 Nt reduces the clonogenic potential of SH-SY5Y and increases intracellular ROS. (a) SH-SY5Y cells were cotransfected with puromycin-resistant vector and different constructs of DJ-1. After 48 h, cells were treated with puromycin (2 μg/ml) every 2 days. After 10 days cells were fixed and colonies detected by adding crystal violet. The numbers of colonies were next scored by Image J quantification software. (b) SH-SY5Y cells with transient expression of the empty vector or different constructs of DJ-1 were lysed and analyzed by western blot for Myc expression. (c) Cell lines stably expressing DJ-1 Wt, DJ-1 Nt or empty vector were lysed and analyzed by western blot for Myc expression. (d) Immunostaining of Myc in SH-SY5Y clones. Nuclei were visualized with DAPI. (e) SH-SY5Y clones were treated or untreated with 2 mM MPP+. Twenty four hours later, cells were incubated with DCFDA (5 μM) for 30 min at 37 °C and cells were analyzed with cytometer. HSP60 was used as a loading control
DJ-1 Nt induces mitochondrial cell death in SH-SY5Y
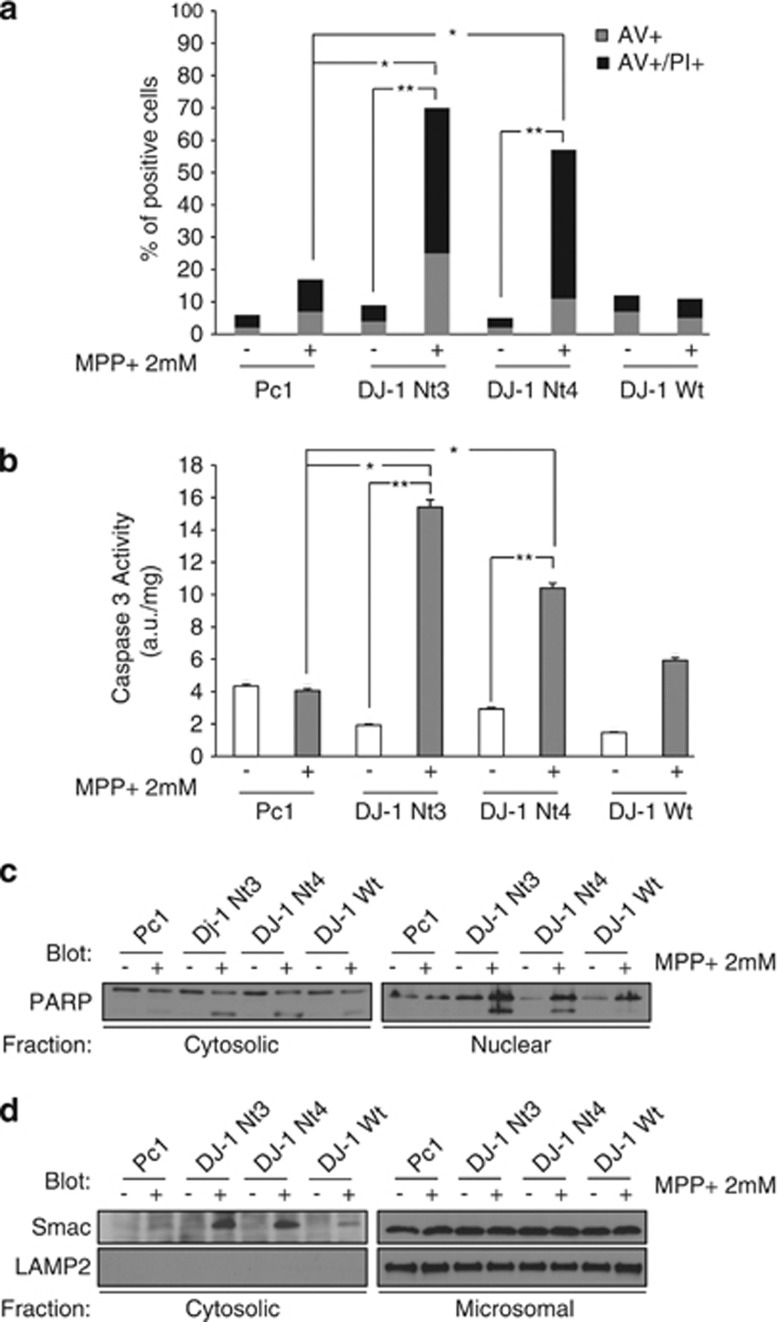
We next wanted to determine how DJ-1 Nt killed SH-SY5Y cells. MPP+ at a 2 mM concentration slightly induced apoptosis in parental SH-SY5Y cells as judged by Annexin V/PI staining, while expression of DJ-1 Nt3 or 4 sensitized drastically to MPP+ killing (Figure 5a). Increased cell death of DJ-1 Nt3- and 4-expressing cells was also confirmed by phase contrast microscopy (Supplemental Figure 5). MPP+ increased caspase 3 activity and poly-ADP polymerase cleavage in DJ-1 Nt-expressing cells (Figures 5b and c). Finally, Smac release from mitochondria, a hallmark of mitochondrial apoptosis, was drastically increased in DJ-1 Nt3- or 4-expressing SH-SY5Y cells (Figure 5d). In response to MPP+, cells overexpressing DJ-1 Wt exhibited unchanged levels of ROS production, Annexin V/PI staining, caspase 3 activation, PARP cleavage and SMAC release from mitochondria (Figures 4e, 5a–d).
Figure 5.
Cells with stable expression of DJ-1 Nt show drastic upregulation of caspase-mediated apoptotic cell death. (a) SH-SY5Y cells stably expressing the different forms of DJ-1 were incubated with 2 mM MPP+. Twenty four hours later, cells were stained using the Annexin V FITC Apoptosis Detection Kit and analyzed with cytometer. (b) After 24 h of exposure to MPP+, stable clones were harvested, washed and lysed in caspase buffer before determination of caspase-3 activity. (c) Stable SH-SY5Y clones were treated with MPP+ and subcellullar fractions prepared. Protein samples were separated by electrophoresis and expression of PARP was visualized by western blotting. (d) Stable SH-SY5Y clones were incubated with 2 mM MPP+. Twenty four hours later, cells were harvested, washed and subcellular fractions were prepared. Protein samples were separated by electrophoresis and expression of Smac visualized by western blotting. LAMP2 serves as a microsomal marker
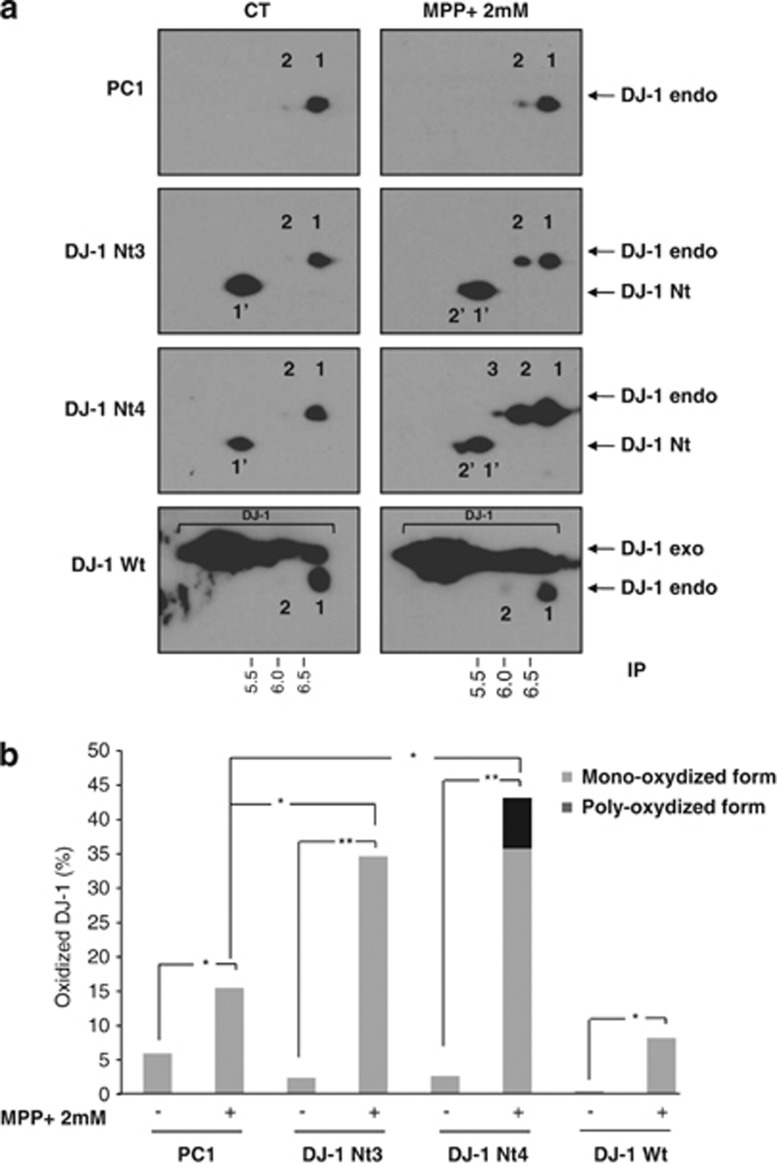
It has been previously reported that oxidative stress-inducing agents such as MPP+ might induce oxidation of DJ-1 that can be detected by 2D-gel electrophoresis.21 Accordingly, we observed that in untreated cells, non-oxidized DJ-1 migrated with an isoelectric point (IP) of 6.4 (spot1), whereas in cells stimulated with MPP+, its oxidized and superoxidized counterparts exhibited IPs of 6 (spot 2) and 5.5 (spot 3), respectively (Figure 6a). DJ-1 Nt for its own migrated with an IP of 5 (spot 1'). Interestingly, increased oxidation of both endogenous and DJ-1 Nt was detected in DJ-1 Nt3 and 4 clones treated with MPP+ (spot 2'). Importantly, when the same western blot was overexposed, the cleaved form of DJ-1 was clearly detected but only in cells over-expressing DJ-1 Nt3 or 4 (Supplemental Figure 6). We performed quantification analysis of the different spots using Image J software. Results in Figure 6b represent the percentage of DJ-1 oxidized forms versus total DJ-1 in the four different clones. In control and DJ-1 Wt-expressing cells treated with MPP+, the level of oxidized DJ-1 reached 10–15%, whereas 35–45% of DJ-1 was oxidized in clones stably expressing DJ-1 Nt3 and 4 in identical conditions.
Figure 6.
DJ-1 Nt increases oxidation state of DJ-1. (a) SH-SY5Y cells stably expressing the different forms of DJ-1 were stimulated with 2 mM MPP+ for 24 h. Protein extracts were prepared and separated by 2D gel electrophoresis. From top to bottom, panels correspond to SH-SY5Y stably expressing a pcDNA3 empty vector, to two SH-SY5Y clones expressing DJ1 Nt (clone 3 and 4) and to SH-SY5Y clone expressing DJ1 Wt. (b) Histogram plot of relative DJ-1 oxidized state quantified with image J software
In addition, we transfected either an empty (pcDNA3), DJ-1, DJ-1 Nt vector or the combination of a DJ-1 and DJ-1 Nt constructs in DJ-1−/− MEF cells (Supplemental Figure 7). We found that DJ-1 overexpression is not able to counteract DJ-1 Nt-mediated apoptosis in MPP+-treated cells.
DJ-1 Nt nuclear localization is necessary for its pro-apoptotic function
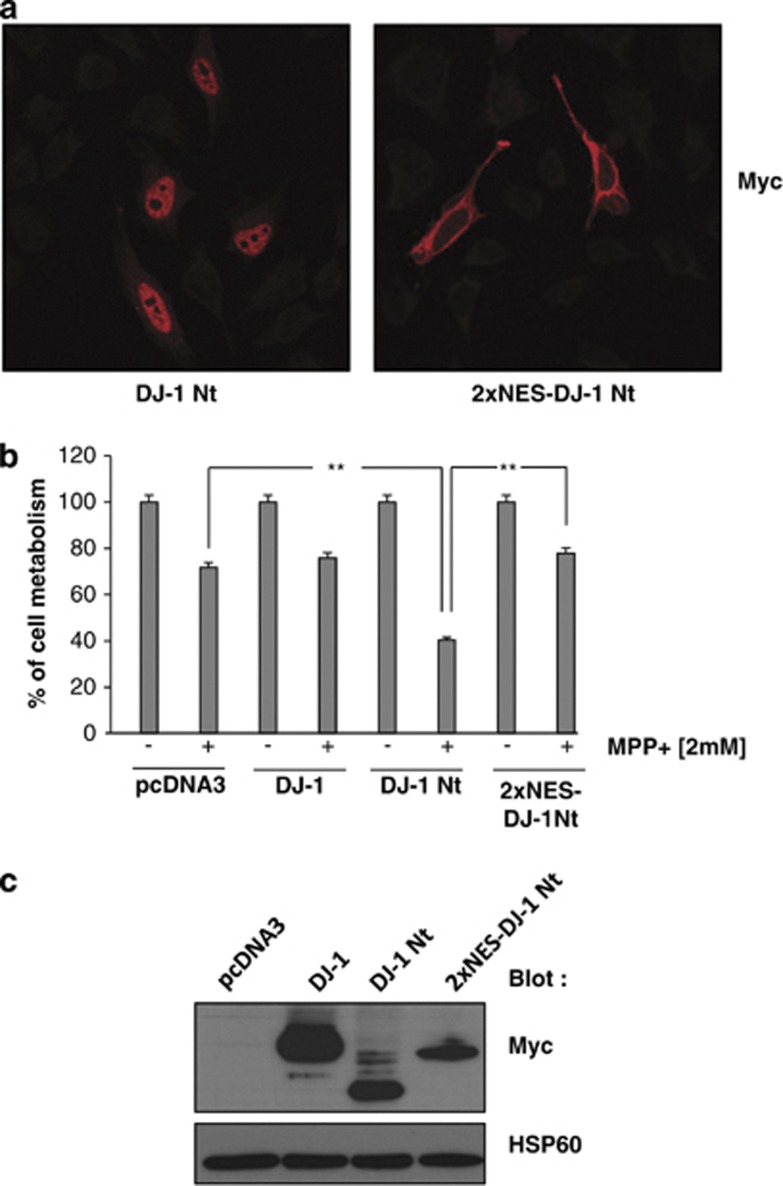
To investigate the role of the nuclear localization of DJ-1 Nt, we transfected SH-SY5Y cells with either an empty vector (pcDNA3), DJ-1 Wt, DJ-1 Nt or a vector encoding a construct of DJ1 Nt arboring two nuclear export sequence signal (2xNES-DJ-1 Nt) that is therefore totally excluded from the nucleus (Figure 7a). Thus, if the nuclear localization of DJ-1 Nt is crucial for its pro-apoptotic effect, exclusion from the nucleus should inhibit DJ-1 Nt effect's on cell metabolism in cells treated for 24 h with MPP+. As expected, SH-SYH5Y cells transfected with DJ-1 Nt were more sensitive to MPP+ treatment than cells transfected with either control or DJ-1 Wt vector (Figure 7b). Importantly, SH-SY5Y cells transfected with the 2xNES-DJ-1 Nt construct showed a drastic loss of sensitivity to MPP+ treatment compared with cells transfected with DJ-1 Nt. Finally, we established that the expression of DJ1 Nt and 2xNES-DJ-1 Nt was equivalent in SH-SY5Y cells (Figure 7c). In conclusion, the nuclear location of DJ-1 Nt seems to be crucial for its pro-apoptotic function.
Figure 7.
DJ-1 Nt nuclear localization is essential for its pro-apoptotic function. (a) SH-SY5Y cells were transfected with Myc-tagged DJ-1 Nt or Myc-tagged 2xNES-DJ-1 Nt. Fourty eight hours later, cells were fixed and permeabilized. Slides containing cells were mounted and analyzed under a fluorescence microscope. (b) SH-SY5Y cells were transfected with control vector or the different forms of DJ-1. Fourty eight hours later, cells were incubated for 24 h with 2 mM MPP+. Cell metabolism was measured by the XTT assay as described in Materials and Methods section. (c) SH-SY5Y cells were as described in Figure 7a. Briefly, cells were lysed and proteins were analyzed by immunoblotting with anti-Myc antibody. HSP90 was used as loading control
Discussion
The protective role of DJ-1 against stress-induced apoptosis is well documented.6, 7 Several mechanisms have been reported that explain the anti-apoptotic function of DJ-1, including activation of the PI3K/AKT pathway consecutive to PTEN inhibition,4 blockage of p53-mediated cell death,17 activation of the MAP kinase cascade22 and regulation of antioxidant genes through Nrf2 stabilization.23 Interestingly, DJ-1 also protects cells against hypoxia-induced cell death and is required for their adaptation to severe hypoxic stress.24 We recently reported that DJ-1 is cleaved by caspase 6 after Asp149 in cell undergoing apoptosis, and that loss of function of DJ-1 triggered by the Parkinson's disease-associated mutation D149A is due to proteolytic resistance to caspase-6.17 We developed one antibody (DJ-1 (120)) that recognized the N-terminal cleavage fragment of DJ-1 in both the human T-lymphoblastic cell line Jurkat and neuroblastoma cell line SH-SY5Y undergoing apoptosis in the presence of STS. Using this antibody, we demonstrate by subfractionation and confocal microscopy experiments that conversely, to DJ-1, the expression of which was restrained to the cytosolic and microsomal compartments, DJ-1-Nt was localized to the nucleus of Hela and MEF-DJ1−/− cells. Such a localization of caspase-cleaved protein fragments to the nucleus is not uncommon, as we reported recently that the caspase-cleaved form of the Lyn tyrosine kinase is delocalized into the nucleus of cells undergoing apoptosis.25 Moreover, several other caspase-derived fragments of substrates including FAK,26 FHOD1,27 Rho-GDI2,28 YY1,29 D4GDJ,30 Huntingtin31 and Beclin 132 are also targeted into the nucleus in identical circumstances. Of note in the present study, we clearly demonstrated that nuclear localization of DJ-1 Nt is essential for its pro-apoptotic function.
We described here that the N-terminal DJ-1 fragment triggers ROS production in different human cell lines. Genetic studies in drosophila have established that DJ-1 is critical for mitochondrial function.33 This observation is in agreement with the finding that DJ-1 Nt expression increases MPP+-induced mitochondrial membrane permeabilization, caspase 3 activation and apoptosis. Therefore, it is tempting to speculate that native DJ-1 protects mitochondrial integrity, whereas cleavage of DJ-1 by caspase 6 leads to mitochondrial membrane permeabilization and therefore favors induction of apoptosis.
To investigate the ability of DJ1 Nt to modulate cell death in the presence or absence of a full-length DJ-1 protein, we transfected either an empty vector (pcDNA3), DJ-1 Wt (full-length DJ-1), DJ-1 Nt alone or the combination of DJ-1 Wt and DJ1 Nt in DJ1−/− MEF cells. A low level of caspase-3 activation was detected in cells transfected with pcDNA3 or DJ-1 Wt plasmid. Conversely, cells transfected with DJ-1 Nt alone or cotransfected with DJ-1 Nt and DJ-1 Wt exhibited a higher level of caspase-3 activation (Supplemental Figure 7). This is a strong indication that the N-terminal fragment of DJ-1 favors caspase-3 activation independently of the presence of the parental full-length DJ-1 protein. Consistent with this is the observation that the presence of DJ-1 Wt does not affect the ability of DJ-1 Nt to increase caspase-3 activity.
In contrast to native DJ-1, overexpression of DJ-1 Nt in different cell lines leads to a loss of clonogenic potential and sensitization to MPP+-mediated apoptosis. Moreover, inhibition of DJ-1 expression with Sh-RNA or DJ-1 deficiency mimics DJ-1 Nt effect on cell growth and apoptosis. Strikingly, we show here that DJ-1 Nt synergizes with MPP+ to mediate mitochondrial membrane permeabilization, ROS production and induction of apoptosis. In the same line, it has been reported recently that cleavage of DJ-1 by matrix metalloprotease 3 mediates oxidative stress-induced dopaminergic cell death.34 This finding is also in agreement with genetic studies in drosophilla that established that DJ-1 is critical for mitochondrial function.31 Cysteine 106 is functionally essential for DJ-1 function and is also subject to oxidation. An interesting observation of the present study is that stable expression of DJ-1 Nt in SH-SY5Y cell line alters the oxidation level of both native and cleaved DJ-1. It has been reported that oxidation can lead to loss of DJ-1 function.35 Thus, an interesting possibility would be that enhanced oxidation status of endogenous DJ-1 by MPP+ in DJ-1 Nt-expressing cells that we do observe in this study altered the functionality of this protein towards mitochondria. Therefore, it is tempting to speculate that production of DJ-1 Nt through caspase cleavage may alter mitochondrial membrane integrity and ROS production.
In conclusion, we describe here an original post-transductional mechanism by which degradation of native DJ-1 and generation by caspase 6 of a new N-terminal fragment of DJ-1 with a nuclear localization co-operate to trigger oxidation of DJ-1, loss of mitochondrial integrity, ROS production and finally apoptosis.
Materials and Methods
Cell cultures
The human Jurkat leukemic cell line was grown in RPMI 1630 medium (Lonza, Walkersville, MD, USA). Human HeLa cervical carcinoma cell line, human SH-SY5Y neuroblastoma cell line and murine embryonic fibroblast cell line were grown in DMEM (Lonza). DT40 chicken B cell line (a kind gift from Professor Bill Earnshaw) was grown in DMEM supplemented with 1% chicken serum and 10% fetal calf serum (FCS). All other cell cultures were grown at 37 °C under 5% CO2 in the presence of 10% FCS, 50 units/ml penicillin and 50 μg/ml streptomycin to minimize contamination. From the SH-SY5Y cell line, we established different stables clones expressing DJ-1 shRNA or the DJ-1Nter fragment. In each case stable empty vectors (pcDNA3) were also obtained.
Transient transfection experiments
Cells were transfected with DNA vectors using JETPEI reagent in six wells plates containing 40 × 104 cells/well. Cells were lysed and proteins were analyzed 48 h later.
Reagents and antibodies
Sodium fluoride and orthovanadate, phenylmethylsulfonyl fluoride, aprotinin and leupeptin were purchased from Sigma (Saint-Louis, MO, USA). Anti-Caspase 6, anti-Lamin A/C and HRP conjugated anti-rabbit antibodies were from Cell Signaling Technology (Beverly, MA, USA). Anti-Hsp60, anti-Myc, anti-DJ-1 (N20) and anti-calreticulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated anti-mouse and anti-goat antibodies were from Dakopatts (Glostrup, Denmark). DJ-1Nt-specific antibody was obtained from NeoMPS (Strasbourg, France). Rabbits were immunized after the fourth injection of recombinant protein DJ-1 Nt-GST and antibodies were collected from three different bleedings.
Measurement of cell metabolism (XTT)
SH-SY5Y clones or mouse embryo fibroblasts (MEF) cells (10 × 103 cells/100μl) were incubated in a 96-well plate with indicated concentration of STS for 6 h. About 50 μl of XTT reagent (sodium 3'-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4–methoxy–6–nitro) benzene sulfonic acid hydrate) (MPP) was added to each well as described previously.36 The absorbance of the formazan product, reflecting cell viability, was measured at 490 nm. Each assay was performed in quadruplicate.
Immunofluorescence
HeLa or MEF cells were transiently transfected with DJ-1 or DJ-1 Nt constructs in glass chamber slides. Fourty eight hours later, cells were fixed with 3% paraformaldehyde in PBS for 10 min, washed with PBS three times and permeabilized with 0.1% Triton X-100 in PBS for 2 min. After another washing with PBS, cells were incubated with anti-Myc antibody diluted in PBS, 1% BSA at RT for 1 h. Then cells were washed and incubated with secondary anti-Mouse antibody. Finally, DAPI was used to label the nuclei and slides containing cells were mounted and analyzed under a fluorescence microscope (LEICA TCR 5500, Leica, Somls, Germany) or confocal microscope (ZEISS LSM510 META, Zeiss, Oberkochen, Germany).
Western Blot
Western blotting has been described in details elsewhere.37 Briefly, after stimulation, cells were lysed at 4 °C in lysis buffer. Lysates were centrifuged at 10 000 × g for 10 min at 4 °C and supernatants were supplemented with concentrated SDS sample buffer. A total of 30 μg of protein were separated on 12% polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA, USA). After blocking non-specific binding sites, the membranes were incubated with specific antibodies, washed three times and finally incubated with HRP-conjugated antibody for 1 h at room temperature. Immunoblots were revealed using the enhanced chemiluminescence detection kit (Amersham Biosciences, Uppsala, Sweden).
Isoelectric focusing
SH-SY5Y clones were first treated with 2 mM MPP+. After 24 h, cells extracts were prepared in a mixture containing 8.75 M urea, 2.5 M thiourea, 5% (w/v) CHAPS, 50 mM dithiothreitol. Proteins in extracts were separated by isoelectric-focusing phoresis gel (pH 3–10) and 13% polyacrylamide gel electrophoresis (PAGE) containing SDS, transferred onto PVDF membranes and blotted with anti-DJ-1 antibody.
Cellular subfractionnements
Cells were washed with wash buffer (proteoExtract Subcellular Proteome Extractipon KIT, Calbiochem, La Jolla, CA, USA). Then, cells were pelleted by centrifugation (10 min at 300 × g). The wash buffer was aspirated and discarded and Extraction Buffer I was added to extract cytosolic fraction. Cells were incubated for 10 min at 4 °C under gentle agitation, centrifugated for 10 min at 800 × g. The supernatant contains the cytoplasmic fraction. Extraction Buffer II was added to the pellet and cells were incubated for 30 min at 4 °C under gentle agitation. Then, cells were centrifugated for 10 min at 5500 × g and the supernatant containing the microsomal fraction was collected. This crude microsomal fraction contains the plasma membrane, mitochondria, endoplasmic reticulum, gogli and lysosomes. Extraction Buffer III was added to the pellet and cells were incubated for 10 min at 4 °C. Finally, cells were centrifugated for 10 min at 7000 × g and the nuclear fraction was collected. A total of 30 μg of cytosolic, microsomal and nuclear fractions were separated on 15% polyacrylamide gel and transferred onto PVDF membrane. After blocking non-specific-binding sites in saturation buffer, the membranes were incubated with specific DJ-1 antibodies.
Colony formations assay
SH-SY5Y cells were cotransfected with puromycin-resistant vector and different constructs of DJ-1. Fourty eight hours later, cells were treated with puromycin (2 μg/ml) every 2 days. After 10 days cells were fixed with paraformaldehyde 3% and then colonies were detected by adding 1 ml of aqueous crystal violet solution and were scored by Image J quantification software (US National Institutes of Health, Bethesda, MD, USA).
In vitro transcription/translation of WT and mutated DJ-1 and cleavage by recombinant caspases
Wild type and mutated (uncleavable D149A DJ-1) were transcribed and translated using the Promega TNT-coupled reticulocyte lysate system (Promega, Madison, WI, USA) as previously described in the presence of radiolabeled methionine (ICN) as described.38 Briefly, 25 ng recombinant caspases (Sigma) were incubated for 16 h at 37 °C with 2 μl of reticulocyte lysates in 50 μl of 25 mM HEPES (4-(2–hydroxyethyl) piperazine-1-ethanesulfonic acid) buffer pH 7.5 containing 0.1% CHAPS ((3-[(3–cholamidopropyl) dimetylammonio]-1-propanesulfonate)) and 5 mM dithiothreitol. In a subset of experiments, the caspase inhibitor Ac-DEVD-CHO (10 μM) was preincubated with caspases before the addition of the reticulocyte lysates. Proteins were then electrophoresed on 12% PAGE and autoradiographed using Amersham Biosciences hyperfilms.
Caspase 3 activity measurement
Caspase 3 assays have been described in details elsewhere.39 Briefly, after stimulation, cells were lysed at 4 °C in lysis buffer and lysates were cleared at 10 000 × g for 15 min at 4 °C. Each assay, in quadriplicates, was performed with 15 μg of protein prepared from control or stimulated cells. Briefly, cellular extracts were then incubated in a 96-well plate, with 0.2 mM Ac-DEVD-AMC as substrates for various times at 37 °C as described previously. Caspase 3 activity is measured after emission at 460 nm (excitation at 390 nm) with or without 1 μM Ac-DEVD-CHO. Enzyme activities were expressed in arbitrary units/mg of protein.
Statistical analysis
All data are presented as mean±S.D. P-values were determined using Prism V5.0b software (GraphPad, La Jolla, CA, USA). Unless stated otherwise in the figure legend, comparisons of different groups were made with the one-way ANOVA test with Bonferroni post test. P-values <0.05 (*), <0.01 (**) and <0.001 (***) were considered statistically significant.
Acknowledgments
We are indebted to Professor Bill Earnshaw and Dr. Hiromi Ogawa for the kind gift of DT40 caspase 6+/+ and caspase 6−/− cells. This work was supported by grants from the Institut National des Cancéropoles (INCa PL2006-026 and PL2010-249) and by the Ligue Nationale contre le Cancer. GR is the recipient of fellowships from INCa and the Fondation de France. FC is supported by a grant from the Fondation pour la Recherche Médicale and by the Conseil General des Alpes-Maritimes. We acknowledge the C3M Imaging Core Facility (MICA) and genomic facility. We thank the Conseil Regional PACA and the Conseil Général des Alpes-Maritimes for their financial support to C3M.
Glossary
- STS
staurosporine
- MPP+
1-methyl-4-phenylpyridinium
- EOPD
early onset Parkinson's disease
- PARP
poly-ADP-ribose polymerase
- ROS
reactive oxygen species
- DCFDA
dichloro-dihydrofluorescein diacetate
- IP
isoelectric point
- FCS
fetal calf serum
- PVDF
polyvinylidene difluoride
- CHAPS
3-[(3–cholamidopropyl)dimethylammonio]-1-propanesulfonate
- PAGE
polyacrylamide gel electrophoresis
- Ac-DEVD-AMC
Ac-Asp-Glu-Val-Asp-AMC
- AMC
7-amino-4-methylcoumarin
- S.D.
standard deviation
- Z-VAD-fmk
benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by L Scorrano
Supplementary Material
References
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Lev N, Roncevic D, Ickowicz D, Melamed E, Offen D. Role of DJ-1 in Parkinson's disease. J Mol Neurosci. 2006;29:215–225. doi: 10.1385/jmn:29:3:215. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Gu L, Cui T, Fan C, Zhao H, Zhao C, Lu L, et al. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem Biophys Res Commun. 2009;383:469–474. doi: 10.1016/j.bbrc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, et al. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, et al. DJ-1 (PARK7), a novel gene for autosomal recessive, early onset Parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–3335. [PubMed] [Google Scholar]
- MacKeigan JP, Clements CM, Lich JD, Pope RM, Hod Y, Ting JP. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Cancer Res. 2003;63:6928–6934. [PubMed] [Google Scholar]
- Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol. 2004;24:97–105. [PubMed] [Google Scholar]
- Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 14:641–650. doi: 10.1038/sj.cdd.4402103. [DOI] [PubMed] [Google Scholar]
- Graham RK, Deng Y, Slow EJ, Haigh B, Bissada N, Lu G, et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant Huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Giaime E, Sunyach C, Druon C, Scarzello S, Robert G, Grosso S, et al. Loss of function of DJ-1 triggered by Parkinson's disease-associated mutation is due to proteolytic resistance to caspase-6. Cell Death Differ. 2010;17:158–169. doi: 10.1038/cdd.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner K, Holtorf E, Waak J, Pham TT, Vogt-Weisenhorn DM, Wurst W, et al. Structural determinants of the C-terminal helix-kink-helix motif essential for protein stability and survival promoting activity of DJ-1. J Biol Chem. 2007;282:13680–13691. doi: 10.1074/jbc.M609821200. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR, McKeown K, Trevor A, Castagnoli NE. Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology. 1988;49:17–23. doi: 10.1016/0300-483x(88)90169-2. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, et al. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- Mo JS, Kim MY, Ann EJ, Hong JA, Park HS. DJ-1 modulates UV-induced oxidative stress signaling through the suppression of MEKK1 and cell death. Cell Death Differ. 2008;15:1030–1041. doi: 10.1038/cdd.2008.26. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, DJ-1 TingJP. A cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti S, Gamas P, Belhacene N, Grosso S, Pradelli LA, Colosetti P, et al. The caspase-cleaved form of LYN mediates a psoriasis-like inflammatory syndrome in mice. EMBO J. 2009;28:2449–2460. doi: 10.1038/emboj.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M, Zachary I. Nuclear localization and apoptotic regulation of an amino-terminal domain focal adhesion kinase fragment in endothelial cells. Biochem Biophys Res Commun. 2000;276:1068–1074. doi: 10.1006/bbrc.2000.3547. [DOI] [PubMed] [Google Scholar]
- Menard I, Gervais FG, Nicholson DW, Roy S. Caspase-3 cleaves the formin-homology-domain-containing protein FHOD1 during apoptosis to generate a C-terminal fragment that is targeted to the nucleolus. Apoptosis. 2006;11:1863–1876. doi: 10.1007/s10495-006-0087-8. [DOI] [PubMed] [Google Scholar]
- Thiede B, Siejak F, Dimmler C, Rudel T. Prediction of translocation and cleavage of heterogeneous ribonuclear proteins and Rho guanine nucleotide dissociation inhibitor 2 during apoptosis by subcellular proteome analysis. Proteomics. 2002;2:996–1006. doi: 10.1002/1615-9861(200208)2:8<996::AID-PROT996>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Krippner-Heidenreich A, Walsemann G, Beyrouthy MJ, Speckgens S, Kraft R, Thole H, et al. Caspase-dependent regulation and subcellular redistribution of the transcriptional modulator YY1 during apoptosis. Mol Cell Biol. 2005;25:3704–3714. doi: 10.1128/MCB.25.9.3704-3714.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieser RJ, Eastman A. Cleavage and nuclear translocation of the caspase 3 substrate Rho GDP-dissociation inhibitor, D4-GDI, during apoptosis. Cell Death Differ. 1999;6:412–419. doi: 10.1038/sj.cdd.4400515. [DOI] [PubMed] [Google Scholar]
- Warby SC, Doty CN, Graham RK, Carroll JB, Yang YZ, Singaraja RR, et al. Activated caspase-6 and caspase-6-cleaved fragments of Huntingtin specifically colocalize in the nucleus. Hum Mol Genet. 2008;17:2390–2404. doi: 10.1093/hmg/ddn139. [DOI] [PubMed] [Google Scholar]
- Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, et al. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J Biol Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- Choi DH, Hwang O, Lee KH, Lee J, Beal F, Kim YS. DJ-1 cleavage by matrix metalloproteinase 3 mediates oxidative stress induced dopaminergic cell death. Antioxid Redox Signal. 2011;14:2137–2150. doi: 10.1089/ars.2009.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- Puissant A, Grosso S, Jacquel A, Belhacene N, Colosetti P, Cassuto JP, et al. Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines exhibit high sensitivity to the phytoalexin resveratrol. FASEB J. 2008;22:1894–1904. doi: 10.1096/fj.07-101394. [DOI] [PubMed] [Google Scholar]
- Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F, et al. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene. 2004;23:7863–7873. doi: 10.1038/sj.onc.1208069. [DOI] [PubMed] [Google Scholar]
- Jacquel A, Colosetti P, Grosso S, Belhacene N, Puissant A, Marchetti S, et al. Apoptosis and erythroid differentiation triggered by Bcr-Abl inhibitors in CML cell lines are fully distinguishable processes that exhibit different sensitivity to caspase inhibition. Oncogene. 2007;26:2445–2458. doi: 10.1038/sj.onc.1210034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.