Abstract
The tumor necrosis factor (TNF) family member APRIL (A proliferation inducing ligand) is a disease promoter in B-cell malignancies. APRIL has also been associated with a wide range of solid malignancies, including colorectal cancer (CRC). As evidence for a supportive role of APRIL in solid tumor formation was still lacking, we studied the involvement of APRIL in CRC. We observed that ectopic APRIL expression exacerbates the number and size of adenomas in ApcMin mice and in a mouse model for colitis-associated colon carcinogenesis. Furthermore, knockdown of APRIL in primary spheroid cultures of colon cancer cells and both mouse and human CRC cell lines reduced tumor clonogenicity and in vivo outgrowth. Taken together, our data therefore indicate that both tumor-derived APRIL and APRIL produced by non-tumor cells is supportive in colorectal tumorigenesis.
Keywords: APCMin, colitis-associated cancer, colorectal cancer
APRIL (A proliferation inducing ligand) is a cytokine that belongs to the tumor necrosis factor (TNF) family. It was named after its ability to enhance tumor proliferation in vitro and in vivo.1 Under physiological conditions APRIL is mainly expressed by hematopoietic cells, such as monocytes, dendritic cells, macrophages and T cells (reviewed in Hahne et al.2 and Dillon et al.3), but expression is also found in non-hematopoietic cells, including osteoclasts and epithelial cells. APRIL binds to two TNF receptor family members; B-cell maturation antigen (BCMA) and transmembrane activator and cyclophilin ligand interactor (TACI).2, 4 In addition, APRIL has high affinity for heparan sulfate proteoglycans (HSPGs).5, 6 This interaction provides a scaffold for efficient signaling and is crucial for the induction of proliferation and survival by APRIL.5, 6, 7 Besides APRIL, BCMA and TACI also interact with another member of the TNF family, B-cell activating factor belonging to the TNF family (BAFF or B-lymphocyte stimulator, BLyS).8, 9 BAFF plays an important role in B-cell homeostasis, and BAFF deficiency results in a strong reduction in mature B cells.10, 11 However, this effect is largely mediated by a unique receptor for BAFF, called BAFF-R, and not by TACI or BCMA.12, 13 In contrast to BAFF-deficient mice, APRIL deficiency revealed no gross abnormalities in lymphoid homeostasis.14, 15 Nevertheless, APRIL appears to modulate B-cell responses and class switch recombination to specific antibody subtypes16 (reviewed in Kimberley et al.17). In particular, in the intestinal tract, APRIL has been shown to affect antibody-producing cells. Upon stimulation with bacteria, intestinal epithelial cells secrete APRIL, which triggers class switch recombination in resident B cells to IgA2, a type of IgA more resistant to bacterial proteases.18
We have established mice expressing human APRIL as a transgene.19 Sera from these mice have detectable circulating APRIL levels, confirming in vitro observations that APRIL is a secreted factor and suggesting that APRIL acts systemically in the transgenic (Tg) mice.19 Young APRIL-Tg mice display no phenotypic abnormalities. However, APRIL-Tg mice have increased activation of B-1 cells, a subpopulation of B cells implicated in innate-type of humoral immune responses. Moreover, upon aging a premalignant expansion of peritoneal B-1 B cells is detected in APRIL-Tg that eventually develops into a lymphoid malignancy.19, 20 These B-1 B-cell lymphomas are highly reminiscent of human B-cell chronic lymphocytic leukemia (CLL).20 Notably, APRIL can also induce survival of human CLL and elevated APRIL serum levels were detected in CLL patients, which in a retrospective study were shown to predict prognosis.21
While a role for APRIL in B-cell malignancies is relatively established (reviewed in Kimberley et al.22), several studies have suggested an association between elevated APRIL levels and solid tumors as well.1, 23, 24, 25, 26 In fact, the first description of APRIL reported that solid tumor cell lines and primary tumor tissue express APRIL mRNA.1 Moreover, APRIL-transfected mouse fibroblasts showed an exacerbated growth in vitro and in vivo.1 Rennert and colleagues26 confirmed this tumor-supporting role for APRIL by showing that growth of APRIL-expressing carcinoma cell lines in immune-deficient mice could be prevented with soluble APRIL receptors. In later studies, immunohistochemical analysis of APRIL expression in a panel of solid tumors revealed an accumulation of APRIL in two-thirds of the tumor lesions tested.23 In line with this observation, APRIL transcripts are highly expressed in a variety of human tumors, based on microarray analyses of different tumor types.24 Taken together, the current data indicate a role for APRIL in solid malignancies, including colorectal cancer (CRC). However, direct evidence for a supportive role in tumor initiation is still largely lacking.
In this study, we describe the effect of ectopic APRIL expression in two established mouse models of intestinal cancer and provide evidence that APRIL is promoting CRC formation.
Results
Ectopic APRIL expression promotes the formation of lesions in the ApcMin mouse model
APRIL is expressed in several solid malignancies including CRC,23, 24 but its functional consequence is largely undefined. Somatic mutations in the tumor suppressor gene adenomatous polyposis coli (APC) are observed in the majority of patients with sporadic CRC, whereas germline mutations in APC form the basis of the familial adenomatous polyposis syndrome.27 The ApcMin mouse strain is a model for this syndrome and carries a heterozygous germline mutation in Apc at codon 850. In order to directly study the effect of APRIL on the formation and growth of intestinal tumors, we made use of this well-established mouse model for CRC, and crossed the APRIL-Tg mice with the C57BL/6 J-ApcMin/+ (ApcMin) mouse (reviewed in Medema and Vermeulen28).
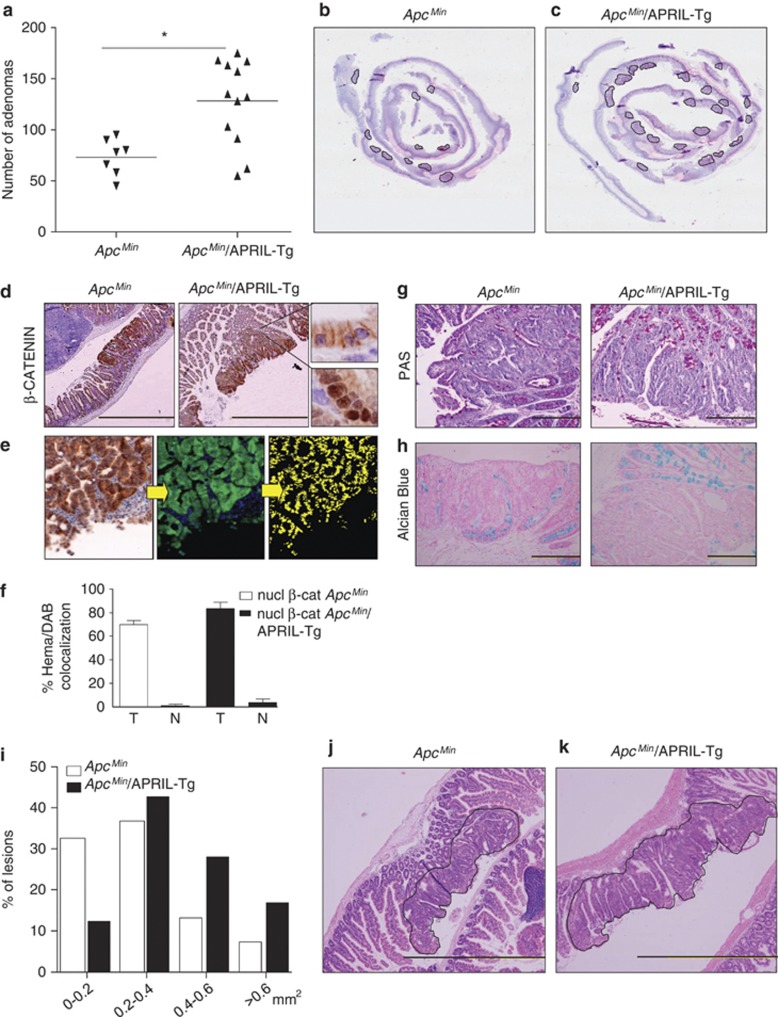
As APRIL-Tg mice are heterozygous for the APRIL transgene, the offspring of this cross allowed for a fair comparison between ApcMin littermates and ApcMin/APRIL-Tg. All ApcMin littermates develop adenomas during the period of analysis with a mean of 73 adenomas per mice. However, ApcMin/APRIL-Tg mice developed significantly more adenomas when compared with their ApcMin littermates with a mean of 128 lesions per mouse (Figures 1a–c). In the ApcMin littermates as well as in the ApcMin/APRIL-Tg mice the adenomas occurred mainly in the small intestine, in agreement with previously reported data on the location of lesions in the ApcMin model (Supplemental Figure 1).29 Adenomas display nuclear accumulation of β-catenin (Figures 1d and f) and a loss of goblet cell differentiation, as measured by periodic acid-Schiff (PAS) or Alcian Blue staining (Figures 1g and h). Notably, spectral image analysis of adenomas in both ApcMin littermates and ApcMin/APRIL-Tg mice revealed no significant difference in the nuclear localization of β-catenin, which in both cases represents around 80% of the nuclei compared with <5% in normal intestine (Figure 1f). To determine the size of the lesions, sections were analyzed and adenoma circumferences were measured. Intriguingly, an enhanced lesion size was observed in the ApcMin/APRIL-Tg mice (average 0.46 mm2) as compared with the littermates (average 0.28 mm2) (Figures 1i and j). This is even more apparent when the lesions were ranked according to the size groups (Figure 1k). The significant increase in tumor load in the ApcMin/APRIL-Tg mice demonstrates that APRIL can facilitate the intestinal adenoma formation and growth.
Figure 1.
Ectopic expression of APRIL promotes tumor lesions in ApcMin mice. ApcMin mice were crossed with APRIL-Tg mice and offspring was analyzed for intestinal adenoma formation (ApcMin n=7; APCMin/APRIL-Tg n=12). Lesions were quantified after 13 weeks. (a) ApcMin/APRIL-Tg mice display increased adenoma incidence in comparison with the ApcMin littermates. Solid lines represent the mean value for each group. Mann–Whitney test, P-value is 0.0127. (b and c) Hematoxylin and eosin staining of adenomas in ApcMin and ApcMin/APRIL-Tg mice. Representative section of ‘Swiss rolled' ileum. (d–f) β-Catenin staining of representative lesions in the ApcMin and ApcMin/APRIL-Tg. Scale bar, 500 μm. Amplifications show nuclear and membrane-bound β-catenin. (e) Nuclear β-catenin quantification by colocalization with hematoxylin using spectral imaging. IHC photomicrograph, on the first step DAB is converted into fluorescent green, and hematoxylin into fluorescent blue by CRi Nuance v2.8 software, colocalization of both is represented in yellow in the second step. (f) Quantification of colocalization in the different scanned areas (T=tumor; N=normal). (g) PAS and (h) Alcian Blue staining of representative section. Lesions are surrounded by a black line. (i and j) Hematoxylin and eosin staining of representative adenomas in ApcMin and ApcMin/APRIL-Tg mice. Lesions are surrounded by a black line. (k) APCMin/APRIL-Tg display larger lesion surface. Tumor size distribution in mm2
Tumor-infiltrating lymphocytes and monocytes can either be tumor-promoting or inhibit tumor growth. We therefore analyzed whether the effect of APRIL was due to a change in tumor-infiltrating cells. T cells and B cells were readily detected in the normal tissue, but were only very rarely detected in the adenomas themselves and no difference was observed between ApcMin and ApcMin/APRIL-Tg mice (Supplemental Figure 2). In contrast, neutrophils and macrophages were readily detected in the tumor area, but also for these cells we observed no significant changes between the two strains (Supplemental Figure 2). This therefore suggests that APRIL acts directly on the epithelial cells and not via a change in the number of infiltrating cells.
Ectopic APRIL overexpression promotes colitis-associated colon carcinogenesis
Although the ApcMin mice represent a well-established mouse model for human CRC, the adenomas mainly occur in the small intestine as opposed to the colon. We therefore investigated a second mouse model that mimics colitis-associated colon carcinogenesis (CAC), which is, in contrast to the ApcMin model, characterized by the development of lesions in the colon. This model depends on administration of the mutagene azoxymethane (AOM) and the subsequent induction of inflammation with dextrane sodium sulfate (DSS). DSS is toxic to mucosal epithelial cells in the colon, and the eventual destruction of the mucosal barrier leads to inflammation.30
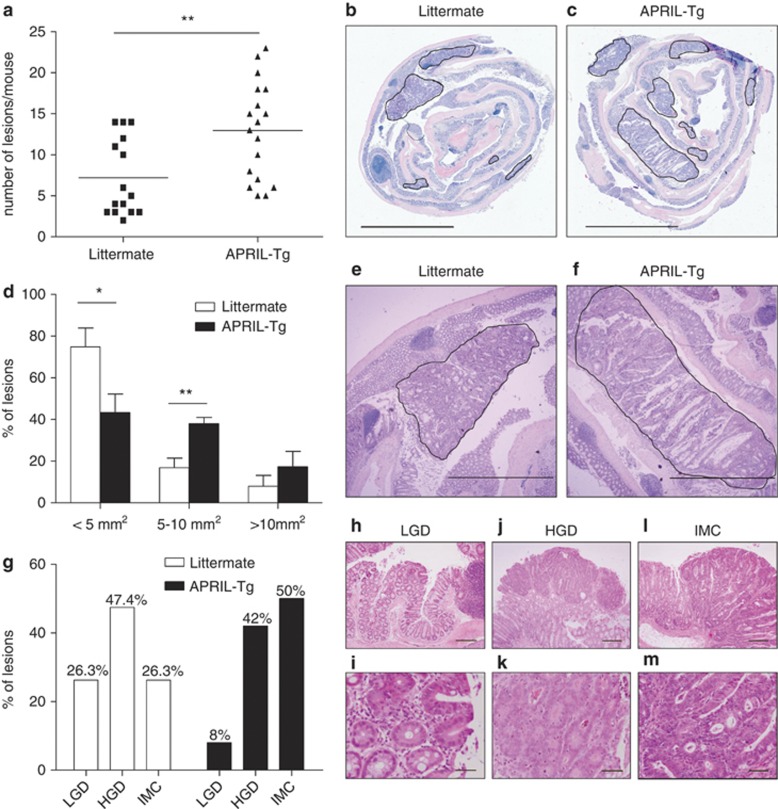
To determine the effect of APRIL on CAC, APRIL-Tg mice and littermates were injected with AOM followed by three cycles of DSS administration in the drinking water. All wild-type mice exposed to this AOM/DSS treatment developed colorectal adenomas within 60 days (Figure 2a). However, we observed a significantly elevated incidence in APRIL-Tg mice (Figures 2a-c). Additionally, adenomas in APRIL-Tg mice had a higher average size (Figures 2d-f). More importantly, histopathological grading of tumors in APRIL-Tg mice revealed a greater proportion of high-grade lesions in APRIL-Tg mice as compared with control animals (Figure 2g). Representative pictures of low-grade dysplasia, high-grade dysplasia and intramucosal carcinoma are shown in (Figures 2h-m). These data show that APRIL can significantly enhance the growth and progression of colitis-associated neoplasia, which provides further prove that APRIL can participate to CRC.
Figure 2.
APRIL-Tg mice display increased tumor incidence and enlarged adenocarcinomas in a colitis-associated cancer model. Colorectal tumors were induced in 6- to 8-week-old littermate (n=15) and APRIL-Tg mice (n=18) treated with AOM/DSS. (a) After 60 days, tumor incidence per mouse was determined. Solid lines represent the mean value for each group. The statistical significance of the data was determined using Mann–Whitney test, P-value 0.0033. (b, c and e, f) Representative sections of ‘Swiss rolled' colons. Hematoxylin and eosin stainings of adenomas in littermates (b and e) or APRIL-Tg (c and f). Tumors are surrounded by a black line. (d) Tumor size distribution in littermate (n=15) and APRIL-Tg mice (n=18) treated with AOM/DSS. Data shown are mean±S.E.M. P-value 0.0221 for <5 mm2 lesions, P-value 0.0047 for 5–10 mm2 lesions. (g) APRIL-Tg mice induced with AOM/DSS display a reduced amount of low-grade dysplasia (LGD) and increased numbers of intramucosal carcinoma (IMC) as compared with their littermates; HGD, high-grade dysplasia are similar. (h–m) Representative images visualizing the three dysplastic categories of adenomas scored: (h and i) photomicrograph of an incipient low-grade lesion located on the tip of a mucosal fold. Inset shows basally oriented, small nuclei and diminished goblet cell differentiation (original magnification, × 64 (h); × 320 (i). (j and k) High-grade lesion displaying back-to-back glands with pseudostratified nuclei with open chromatin and frequent mitoses (original magnification, × 40 (j); × 200 (k). (l and m) Large lesion displaying irregular glandular arrangements with complete loss of apicobasal orientation, many apoptotic bodies and intraglandular necrotic debris, consistent with intramucosal carcinoma (original magnification, × 40 (l); × 200 (m)
APRIL is expressed by human primary colon cancer cell cultures and CRC cell lines
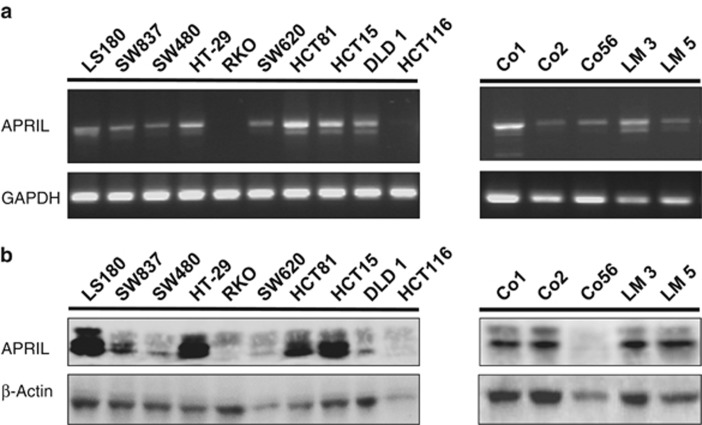
The results described above indicate that elevated APRIL levels, as a result of Tg expression, enhance tumorigenesis of intestinal neoplasia in two independent mouse models of CRC. To study the effect of APRIL on human colon tumors, we analyzed CRC cell lines as well as several primary spheroid cultures of colon cancer cells that were previously established in our laboratory.31, 32 We observed expression of APRIL at both mRNA and protein levels in the majority of the CRC cell lines (Figures 3a and b). RT-PCR analysis indicated two bands expressed at different ratios, which we confirmed by sequencing to represent APRIL splice variants (results not shown). Importantly, APRIL was also detected in the primary colon cancer spheroid cultures at the mRNA and protein level, indicating that APRIL is expressed in primary colorectal tumor samples as well (Figures 3a and b, right panels).
Figure 3.
APRIL is expressed in a panel of colorectal carcinoma cell lines and primary colon cancer spheroid cultures. (a) RT-PCR analysis for APRIL expression in CRC cell lines (left panel) and in primary colon cancer cells (right panel). The upper band at 750 represents the isoforms-α and -γ, whereas the lower band at 700 represents the β-form. GAPDH was used as loading control. (b) Western blot analysis for APRIL on lysates of CRC cell lines (left panel) and primary colon cancer cells (right panel). β-Actin is used as loading control
APRIL inducible knockdown affects tumor growth in vitro and in vivo
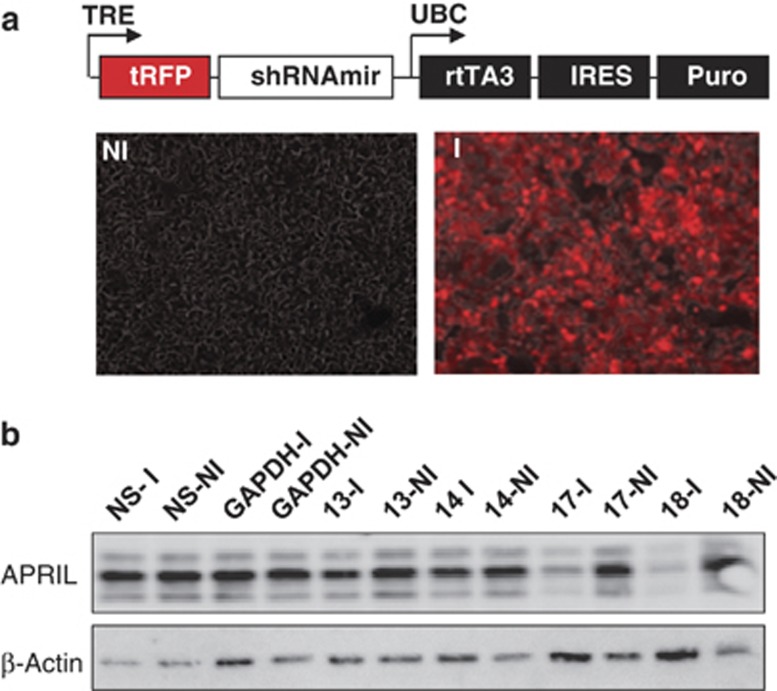
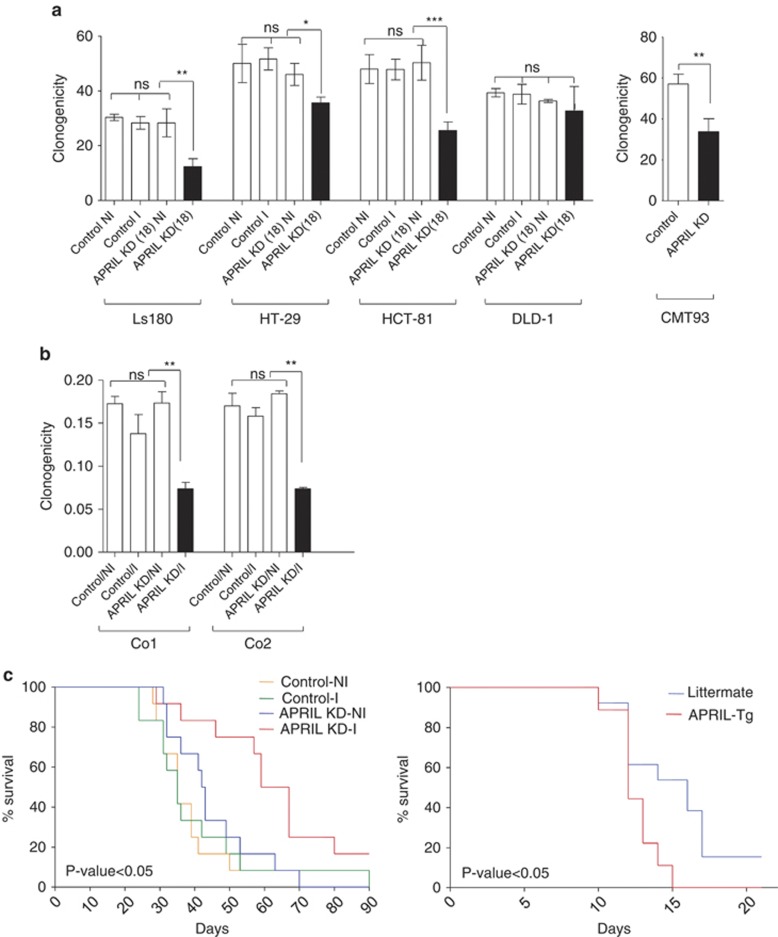
In order to directly address the role of APRIL produced by tumor cells, we used inducible shRNA expression to knockdown APRIL in CRC cell lines and colon cancer spheroid cultures. First we evaluated the efficacy of a series of shRNAs on APRIL expression in the CRC cell line LS180. shRNA expression is induced by doxycycline and is monitored by the expression of red fluorescent protein (RFP) from the same promoter (Figure 4a). Two constructs, 17 and 18, effectively knocked down APRIL protein levels in a time-dependent manner (Figure 4c, Supplemental Figure 3) and were selected for further study. APRIL-induced proliferation was previously shown to be most effective under conditions where growth factors are limiting.1 We therefore knocked down APRIL in serum-starved colon cancer lines and observed that both shRNA resulted in a clear decrease in clonogenicity in three independent colon cancer cell lines, LS180, HT-29 and HCT-81, while a nonspecific shRNA had no effect (Figure 5a, Supplemental Figure 4A). Similarly, silencing of APRIL in the mouse CRC cell line CMT93 also resulted in a clear decrease in clonogenicity (Figure 5a, Supplemental Figure 5). Notably, knockdown of APRIL in the colon cancer cell line DLD-1 had no significant effect on clonogenicity (Figure 5a), which corresponds well with the very low expression of APRIL in this line (Figure 3). This indicates that the effect of APRIL on clonogenicity of colon cancer cell lines is a general phenomenon in the lines that express APRIL. In agreement, two independent spheroid colon cancer stem cell (CSC) cultures derived from a primary resection specimen also showed a significant decrease in clonogenic potential (two to threefold), confirming the effect of APRIL on primary tumor cells as well (Figure 5b). Importantly, the decrease in clonogenicity was reversed by the addition of recombinant APRIL, while an APRIL mutant deficient for binding to HSPGs failed to do so (Supplemental Figure 4B).
Figure 4.
APRIL inducible knockdown. (a) Map of the inducible-lentiviral vector used to transduce CRC cell lines. (b) Representative images of LS180 cells transduced with an inducible shRNA vector treated with (right panel, I=induced) and without (left panel, NI=not induced) doxycycline. Pictures show overlay between red fluorescence (RFP) and brightfield microscopy. (c) Western blot analysis for APRIL expression in LS180 lines transduced with different shRNA targeting APRIL and cultured in the presence (I=induced) or absence (NI=not induced) of doxycycline. Constructs 17 and 18 show significant reduction of APRIL expression levels. β-actin is used as loading control
Figure 5.
APRIL knockdown affects clonogenic growth of human and mice colorectal cancer cells and in vivo tumor outgrowth. (a) LS180, HT29, HCT-81, DLD-1 cells were sorted and plated at a density of 100 cells per well. After 10 days, cells in the colonies were counted and the plating efficiency was calculated. The data shown represent the mean±S.D. Samples are compared by one-way ANOVA analysis and Tukey's multiple comparison post-test. Right panel: CMT93 cell lines were transduced with shRNA targeting APRIL or control shRNA. 100 cells were sorted per well of a six-well plate. After 5 days, cells are fixed and stained with a crystal violet/glutaraldehyde solution. The amount of colonies was counted and the plating efficiency was calculated. P-value 0.011. (b) The shRNA-transduced primary colon cancer spheroid culture Co2 and Co1 were sorted based on RFP expression (shRNA expression), and limiting-dilution assay was performed as described previously.31 The clonal frequency and statistical significance are represented. The data shown represent the mean±S.D; **P<0.01. (c) Human LS180 cells (left panel) and mouse C26 (right panel) cells transduced with the different shRNA constructs (control is empty vector; APRIL-KD is APRIL-knockdown) were injected subcutaneously into nude mice. Tumor growth and survival was measured over time and used to generate a survival curve as depicted in the Kaplan–Meier curve. Log-rank test P-value 0.012 for LS180 and P-value 0.016 for C26
To directly investigate which receptor is involved in the induction of growth by APRIL, we first determined the expression of the three known binding partners of APRIL, TACI, BCMA and HSPGs. Although TACI and BCMA can be detected by qPCR, their expression is around a 1000-fold lower than in splenocytes (Supplemental Figure 6). We have not been able to detect TACI or BCMA receptor expression on the cell surface (data not shown), whether the detected transcript levels of these receptors are therefore translated into significant amounts of protein in these epithelial cells is questionable. HSPGs are however consistently expressed by both mouse and human colorectal cell lines (Supplemental Figure 7). Combined with the observation that the APRIL HSPG mutant did not reinduce clonogenicity points to the possibility that HSPGs mediate this effect.
To further analyze the consequences of APRIL-knockdown in vivo, LS180 cells were injected subcutaneously into T-cell-deficient nude mice and tumor growth was measured in the absence or presence of doxycycline in the drinking water. Survival curves of the mice indicated that LS180 cells with silenced APRIL have a significant delay in tumor growth (Figure 5, left panel), indicating that tumor-produced APRIL has a clear tumor-promoting effect. In the reverse set up, injection of the mouse colon cancer cell line C26 into wild-type and APRIL-Tg mice revealed that also in a completely syngeneic setting that exogenous APRIL is capable of enhancing colon cancer growth (Figure 5, right panel). Combined with our data, therefore, indicate that APRIL supports colon cancer growth.
Discussion
Previously, we have shown that 1-year-old APRIL-Tg mice develop B1 cell-associated neoplasia resembling human CLL.20 A retrospective study revealed that APRIL serum levels are elevated in CLL patients compared with the healthy subjects, and inversely correlate with survival probability suggesting that APRIL could serve as a prognostic factor.21 Reports on the role of APRIL in B-cell neoplasm have been manifold since.22 In this study, we analyzed the effect of APRIL on CRC growth in vivo. By using two etiologically independent mouse models of colorectal carcinogenesis we demonstrate a role for APRIL in enhancing intestinal tumorigenesis. Moreover, we showed that APRIL depletion in colon cancer cells, which endogenously express APRIL, decreased their clonogenic capacity and diminished their in vivo outgrowth. Our in vivo analyses using knockdown corroborates earlier findings describing the effect of BCMA-Fc on tumor growth of a lung and colon carcinoma cell line.26 In both cases, reduced tumor outgrowth was noted, indicating that these colon cancer cell lines appear to require APRIL for their in vivo growth.
Reports on APRIL expression in colon carcinoma samples are somewhat controversial. For example, several groups including our own described mRNA expression of APRIL in colon carcinoma tumors and cell lines.1, 18, 26, 33 An analysis using two different microarray databases, however, revealed overexpression of APRIL in various solid tumors, but not in colon carcinoma.24 Similarly, Mhawech-Fauceglia and co-workers23, 24 investigated APRIL expression in an immunohistochemical study on a large panel of solid tumors, and detected APRIL protein in the majority of tumor tissues analyzed. Notably, they concluded that not the solid tumors themselves, but rather tumor-infiltrating neutrophils present in the stoma constitute the main source of APRIL.23 The authors postulated that the retention of APRIL in the lesions occurs by binding to HSPGs. This is in contrast to an immunohistochemical study by Petty et al.,34 who detected APRIL expression in tumor cells in more than half of the 234 CRC samples tested, but not in normal colon tissue. The later study is consistent with the observation that APRIL transcripts are elevated in primary human colon carcinoma tissue compared with peritumoral tissue.1 In addition, APRIL expression was found to increase upon chemotherapeutic treatment of patients with 5-fluorouracil.34
It is not clear what the underlying reason for these discrepancies is, but possible explanations include quality of the analyzed samples, the use of different tools for the detection of APRIL as well as differences in the composition of the patient cohorts analyzed. In fact, we have observed that APRIL serum levels are rather heterogeneous among CRC patients and our retrospective analysis points to a correlation between high APRIL serum levels and decreased survival probability, emphasizing the clinical relevance of the tumor-supporting role of APRIL (manuscript in preparation).
We clearly detect APRIL by both PCR and western blot in the majority of CRC cell lines and importantly also in several primary colon cancer spheroid cultures, confirming the expression of APRIL by epithelial cancer cells. This indicates that CRC cells do express APRIL, but obviously does not exclude a role for tumor-infiltrating cells producing APRIL as well. As a matter of fact, an association between non-tumor cell—produced APRIL and patient prognosis was observed in a set of CRC patients.34 In addition, the mouse models used in this study depend on paracrine-produced APRIL and clearly show that this is sufficient to enhance intestinal tumorigenesis. This would suggest that exogenous APRIL serves as the main source of APRIL in tumor lesions. However, we also show that selective APRIL knockdown in epithelial tumor cells significantly reduces in vivo tumor growth in T-cell-deficient nude mice, which otherwise have an intact immune cell compartment. Tumor-infiltrating immune cells such as neutrophils and macrophages, potential sources of APRIL, therefore appear unable to compensate for the deletion of APRIL expression by CRC cells, demonstrating that APRIL produced by CRC cells is enhancing tumor growth.
The mechanism by which APRIL enhances epithelial tumor growth remains ill-defined. Both TACI and BCMA are expressed mainly on B cells, and their expression on epithelial cells has not been described so far.26 Although it is possible that APRIL-receptor expression is induced in vivo on tumor cells by external factors, such as inflammation or stress, our in vitro data clearly indicate that CRC cells can respond to APRIL without any further need for stimulation, as they are triggered directly by APRIL in their clonogenic growth. Whether this is a result of undetectable expression levels of TACI and BCMA protein or whether HSPGs are involved remains to be defined. We have not been able to detect TACI or BCMA on the colon cancer cells that are stimulated by APRIL, but we and others have observed that HSPGs form a binding partner for APRIL on these cells.5, 6, 7, 35 It has been shown that this HSPG–APRIL interaction can directly stimulate B cells independent of TACI or BCMA.35 This indicates that HSPGs could represent a third receptor for APRIL. In agreement, we did show that the HSPG interaction with APRIL is crucial for the observed effect, which either indicates a direct signaling through HSPGs or alternatively that HSPGs provide a platform for a yet to be determined receptor. These observations are consistent with our previous findings that revealed that heparin could prevent APRIL-induced tumor proliferation,5 but still do not provide conclusive evidence for HSPGs as the direct receptor.
Although the in vitro stimulation of APRIL on tumor cells is direct, it is unclear whether the stimulation exerted by APRIL in the mouse models or in cancer patients also directly acts on the epithelial tumor cells. Our data do not exclude that in an in vivo setting non-tumor cells are also stimulated by APRIL and provide tumor support in a paracrine manner. In this light, it is interesting to note that polymorphisms have been described for BCMA, which are associated with ulcerative colitis and irritable bowel syndrome.36 Chronic inflammation as in ulcerative colitis is suggested to prime the tumor microenvironment and is a clear determinant in the onset and/or progression of CRC (reviewed in Medema and Vermeulen28; Terzic et al.37; Grivennikov et al.38). The interplay among tumor cells and stromal cells, including infiltrating cells, involves cytokines, chemokines and growth factors. TNF family members are involved in this crosstalk influencing either tumor support or clearance. Our data indicate that the TNF ligand APRIL has a protumorigenic role, which could be mediated directly or via the stroma, but is a determinant in tumor progression. The effects, however, do not appear to result from enhanced infiltration, but could still be due to a change in the activation of the infiltrating cells or their secreted cytokines. Future studies should reveal how APRIL modulates tumor growth and whether APRIL antagonism is a therapeutic possibility to limit tumor growth and/or to sensitize tumor cells to therapy.
Materials and Methods
Statistics
Unless indicated, P-values were calculated using Mann–Whitney test for comparing medians. One-way ANOVA followed by Bonferroni post-tests were calculated using PASW Statistics 18 software. Asteriks indicates P<0.05, i.e. statistically significant; two asteriks indicate P<0.01, i.e. statistically very significant and three asteriks indicate P<0.0001, i.e. statistically extremely significant. The survival rates in mice were calculated using the Kaplan–Meier method, and differences in survival curves were analyzed by log-rank test.
Cells
CRC cell lines were obtained from (ATCC) and cultured in IMDM (Lonza, Verviers, Belgium) containing 8% FCS, 2mM L-glutamine, Pen/Strep and maintained at 37 °C with 5% CO2. Colorectal CSCs were isolated as described.39 Primary samples were named Co, and liver metastases were named LM, both followed by a number representing the isolation. CSCs were cultured in modified neurobasal A medium containing N2 supplement (Invitrogen, Breda, NL, USA), lipid mixture-1 (Sigma-Aldrich, St. Louis, MO, USA), basic fibroblast growth factor (20 ng/ml, R&D Systems, Abingdon, UK) and epidermal growth factor (50 ng/ml, R&D Systems). Colon CSC cultures were derived as described previously in the study by Vermeulen et al.31 Mouse colorectal cell lines C26 and CMT-96 were in IMDM containing 8% FCS, 2mM L-glutamine, Pen/Strep supplemented with β-mercaptoethanol.
Colonic epithelial cell isolation
Colonic epithelial cells were isolated by flushing of the intestine with PBS, and crypts were subsequently isolated by incubation in PBS/50 mM EDTA. Immune cells were separated by low-speed centrifugation at 250 × g, which allows for pelleting of the crypts. RNA was subsequently isolated from the epithelial cells, which were checked for purity after trypsinization by FACS staining for Epcam and CD45. Being 95–98% EPCAM+exposant CD45−exposant positive.
Mice and in vivo models
C57BL/6J-ApcMin/+ (ApcMin) mice were purchased from The Jackson laboratory (Bar Harbor, Maine, USA). ApcMin and APRIL-Tg were bred at the AMC and CNRS in accordance with the rules of ethical committees of the institutes, and further animal experiments were performed in compliance with national and institutional guidelines and accepted by the respective ethical committees. The generation of APRIL heterozygous Tg mouse line has been described.19 APRIL-Tg C57BL/6 mice were crossed with the ApcMin mice. The offspring, containing both ApcMin littermates and ApcMin/APRIL, was killed at 13 weeks, intestines were removed and opened longitudinally, the different segments of the intestines; that is, the duodenum, jejunum, ileum and colon were analyzed individually. Lesions were quantified under a Nikon (Brussels, Belgium) SMZ-10 stereozoom microscope. For the DSS/AOM model, APRIL-Tg mice were backcrossed on a BALB/c background for a minimum of 10 generations. Six- to eight-week-old APRIL-Tg BALB/c mice and littermates were injected with a single dose of AOM (12.5 mg/kg) followed by three cycles of DSS administration (cycle 1: 2.5%, 5 days; cycle 2: 2.5%, 5 days; and cycle 3: 2%, 5 days) in the drinking water. In between the cycles the mice received no treatment for 2 weeks. Colons were removed and the area of macroscopic tumors was determined using a thickness gage. For histological analysis, the entire colon was prepared according to the Swiss roll procedure, fixed with formaldehyde and embedded in paraffin. Four-μm sections were deparaffinized and stained with hematoxylin and eosin and analyzed for adenomas.
Immunohistochemistry
For histological analysis the entire small intestine and colon were prepared according to the Swiss roll procedure. Freshly isolated intestines were first flushed with cold PBS and subsequently with formalin, rolled and fixed with formalin overnight at room temperature. The intestines were then transferred to a tissue cassette and dehydrated by serial immersion in volumes of 70, 80, 90, 96 and 100% EtOH for 1 h each at RT. Excess of ethanol was removed by incubation in xylene for 1 h at RT. The cassettes were subsequently immersed in liquid paraffin (56 °C) overnight and embedded in paraffin. Four-μm sections were generated, deparaffinized and stained. β-Catenin (anti-β-catenin 1 : 200 dilution; BD Transduction Labs, Breda, NL, USA) staining was performed using ARK Peroxidase Kit, as described by the manufacturer (Dako, Heverlee, Belgium). Multispectral data sets from slides stained for β-catenin were acquired using a Nuance camera system (Caliper Life Science/CRi, Hopkinton, MA, USA) from 420 to 720 nm at intervals of 20 nm. After loading the spectra hematoxylin and DAB obtained from single-staining slides, data sets were spectrally unmixed allowing for exclusive visualization of colocalization only. To analyze the frequency of hematoxylin-b-catenin colocalization, spectral images, we used Inform 1.2 software (Caliper Life Science/CRi). A training set defining the categories of tumor, normal and stroma was created. The software was trained on these areas using the spectra of hematoxylin and DAB and tested to determine how accurate it could differentiate between the three categories. This process was repeated until further iterations no longer improved accuracy. Then the β-catenin images were analyzed using the nuclear algorithm scoring the colocalization in nuclei of DAB and hematoxylin.40,41 Alcian Blue staining was performed with Alcian Blue 8GX (Sigma-Aldrich, Zwijndrecht, The Netherlands) and counterstained with Nuclear fast Red (LabVision Inc., Duiven, The Netherlands). Hematoxylin and eosin staining was performed with Ehrlich HE solution (Sigma-Aldrich) on paraffin-embedded sections. For PAS staining, standard histological techniques were used. For the analysis of infiltration, we stained for CD3 (clone SP7, dilution 1 : 1000, Neomarkers, Fremont, CA, USA); B220 (MCA1258 GT/RAT9-6B2 dilution 1 : 15 000, Serotec, Puchheim, Germany); Ly6-FITC (clone RB68C5, 1 : 1000 dilution, BD Bioscience, Breda, NL, USA), F4/80 ( MCA497GA/A3-1, 1 : 500 dilution, Serotec).
Lentiviral constructs and shRNA used
To knockdown human APRIL, we used the inducible shRNA lentiviral system p-TRIPZ (Open Biosystems, Breda, The Netherlands), and we analyzed clones ID V2THS_17313, V2THS_17314, V2THS_17317 and V2THS_17318. As negative control, we used non-silencing-TRIPZ lentiviral inducible (RHS4743). As a positive control for the p-TRIPZ system, we used GAPDH (glyceraldehyde 3-phosphate dehydrogenase; RHS4744). LS180 cells were transduced with the lentiviral constructs according to the manufacturer's instructions. Transfectants were selected with puromycin at 2 μg/ml (Sigma-Aldrich) 48 h after transduction. To induce shRNA expression, we used 1 μg/ml of doxycycline (Sigma-Aldrich). To knockdown murine APRIL in mouse cell line CMT93, we used the RNAi pRetroSUPER vector system (kind gift from R Bernards) using the following siRNA sequence 5′-GGGAGAAGAGAAACTCTAT-3′, and empty vector as control.
PCR and western blot
For the APRIL expression PCR, we used the primers (Fw 5′-CCAGCCTCATCTCCTTTCTTGC-3′, Rv 5′-TCACAGTTTCACAAACCCCAGG-3′) and as loading control we used GAPDH (Fw 5′-AAGGTGAAGGTCGGAGTCAAC-3′, Rv 5′-TGGAAGATGGTGATGGGATT-3′). To analyze BCMA and TACI expression levels in mice, we used the primers (TACI Fw 5′-GTGTGGCCACTTCTGTGAGA-3′, Rv 5′-CTGGTGCCTTCCTGAGTTGT-3′ BCMA Fw 5′-ATCTTCTTGGGGCTGACCTT-3′, Rv 5′-CTTTGAGGCTGGTCCTTCAG-3′, using as housekeeping gene 18S seq Fw 5′-CCGATAACGAACGAGACTCTGG-3′, Rv 5′-TAGGGTAGGCACACGCTGAGCC-3′). For human TACI Fw 5′-GCTGAAGCTGAGTGCAGATCA-3′, Rv 5′-CCCTCTTCTTGAGGAAGCAGG-3′ BCMA Fw 5′-GCATCAAGAGCAAACCGAAGG-3′, Rv 5′-TCTATCTCCGTAGCACTCAAAGC-3′ and using GAPDH as housekeeping control Fw 5′-AAGGTGAAGGTCGGAGTCAAC-3′, Rv 5′-TGGAAGATGGTGATGGGATT-3′. For the study of APRIL protein expression, western blots were performed as follows. Protein lysates were separated on a 10% acrylamide reducing gel and transferred to a Inmovilon-P PVDF (Hybond ECL membrane, Amersham Biosciences, Munich, Germany), which was subsequently blocked with 5% skim milk (Sigma-Aldrich) and incubated with anti-APRIL antibody (Aprilyer-5; Alexis Biochemicals, Zandhoven, Belgium). HRP-conjugated goat anti-mouse antibody (SouthernBiotech, Uithoorn, NL, USA) was used as secondary antibody and detected with ECL Plus Western Blot Detection Reagents (Amersham Biosciences) using a LAS3000 (FUJIFILM, Dusseldorf, Germany). Actin was used as loading control (anti-actin (1–19) antibody (Santa Cruz, Heidelberg, Germany) and detected with HRP-conjugated goat anti-rabbit (Southern Biotech) secondary antibody. All antibodies were diluted in 2.5% milk on PBS-Tween 0.2%.
Clonogenic assays and limiting-dilution assay
Transduced LS180, HT29, HCT-81 and DLD-1 lines were serum starved for 24 h. Using FACSorting, 100 cells were platted in a well of a six-well plate either in induced or non-induced media conditions (± doxycycline). Ten days later, colonies are fixed and stained with a crystal violet/glutaraldehyde solution. The colonies were counted and the clonogenicity indicates the amount of colonies per 100 cells platted. Transduced Co1 and Co2 CSCs were sorted based on RFP-positivity and deposited in a limiting-dilution scheme (1, 2, 4, 6, 8, 12, 16, 20 and 24 cells per well). The occurrence of new spheres was determined after 2 weeks, and clonal frequency and statistical significance were evaluated with the Extreme Limiting-Dilution Analysis ‘limdil' function (http://bioinf.wehi.edu.au/software/elda/index.html).
Tumor growth of LS180-transduced cells in immune-deficient mice
APRIL shRNA-transduced LS180 cells (250 000 cells) were left untreated or induced for 24 h with 1 μg/ml of doxycycline in vitro. Subsequently, cells were harvested and suspended in 200 μl PBS-BSA (0.5%) and injected subcutaneously into the flank of BALB/c nude mice (Harlan Nederland, Zeist, The Netherlands). Tumor growth was measured along a period of 100 days. When tumors reach 1 cm3, mice were killed in accordance with ethical approval.
Tumor growth of mice cell lines into BALB/c mice
C26 mouse colorectal cells (7.5 × 105) were subcutaneously injected into APRIL-Tg and control mice. Tumor growth was measured and mice were killed.
Acknowledgments
We thank the animal caretakers and Berend Hooibrink and Toni van Capel for excellent assistance with FACSorting experiments and the RHEM histology facility in Montpellier. In addition, we thank Irmgard Corten and Nan van Geloven for the help with statistical calculations. JPM is supported by a VICI grant (NWO) and KWF grants (2007–3750 and 2009–4416). MH was supported by the Ligue Nationale Contre Le Cancer and the Fondation de France, and LF by the Fondation pour la Recherché Médicale en France.
Glossary
- APC
adenomatous polyposis coli
- APRIL
a proliferation inducing ligand
- AOM
azoxymethane
- BAFF
B-cell activating factor
- BCMA
B-cell maturation antigen
- CAC
colitis-associated colon carcinogenesis
- CSCs
cancer stem cells
- CRC
colorectal cancer
- DSS
dextrane sodium sulfate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HSPG
heparan sulfate proteoglycan
- RFP
red fluorescent protein
- TACI
transmembrane activator and cyclophilin ligand interactor
- Tg
transgenic
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by SJ Martin
Supplementary Material
References
- Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Gross JA, Ansell SM, Novak AJ, An APRIL. to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- Medema JP, Planelles-Carazo L, Hardenberg G, Hahne M. The uncertain glory of APRIL. Cell Death Differ. 2003;10:1121–5. doi: 10.1038/sj.cdd.4401291. [DOI] [PubMed] [Google Scholar]
- Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks J, Planelles L, Jong-Odding J, Hardenberg G, Pals ST, Hahne M, et al. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M, et al. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J. 2009;23:1584–1595. doi: 10.1096/fj.08-124669. [DOI] [PubMed] [Google Scholar]
- Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D, et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardenberg G, van Bostelen L, Hahne M, Medema JP. Thymus-independent class switch recombination is affected by APRIL. Immunol Cell Biol. 2008;86:530–534. doi: 10.1038/icb.2008.17. [DOI] [PubMed] [Google Scholar]
- Kimberley FC, Medema JP, Hahne M. APRIL in B-cell malignancies and autoimmunity. Results Probl Cell Differ. 2009;49:161–182. doi: 10.1007/400_2008_19. [DOI] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Stein JV, Lopez-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodriguez D, Gomez-Caro R, et al. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles L, Carvalho-Pinto CE, Hardenberg G, Smaniotto S, Savino W, Gomez-Caro R, et al. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 2004;6:399–408. doi: 10.1016/j.ccr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Planelles L, Castillo-Gutierrez S, Medema JP, Morales-Luque A, Merle-Beral H, Hahne M. APRIL but not BLyS serum levels are increased in chronic lymphocytic leukemia: prognostic relevance of APRIL for survival. Haematologica. 2007;92:1284–1285. doi: 10.3324/haematol.10317. [DOI] [PubMed] [Google Scholar]
- Kimberley FC, Hahne M, Medema JP. APRIL hath put a spring of youth in everything': Relevance of APRIL for survival. J Cell Physiol. 2009;218:1–8. doi: 10.1002/jcp.21561. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Kaya G, Sauter G, McKee T, Donze O, Schwaller J, et al. The source of APRIL up-regulation in human solid tumor lesions. J Leukoc Biol. 2006;80:697–704. doi: 10.1189/jlb.1105655. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Veyrune JL, De Vos J, Klein B. APRIL is overexpressed in cancer: link with tumor progression. BMC Cancer. 2009;9:83. doi: 10.1186/1471-2407-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Allal A, Odunsi K, Andrews C, Herrmann FR, Huard B. Role of the tumour necrosis family ligand APRIL in solid tumour development: retrospective studies in bladder, ovarian and head and neck carcinomas. Eur J Cancer. 2008;44:2097–2100. doi: 10.1016/j.ejca.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Rennert P, Schneider P, Cachero TG, Thompson J, Trabach L, Hertig S, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000;192:1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi AK. Hereditary gastrointestinal polyposis and nonpolyposis syndromes. N Engl J Med. 1994;331:1694–1702. doi: 10.1056/NEJM199412223312507. [DOI] [PubMed] [Google Scholar]
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–26. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Melo DeSousaE, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, de Sousa MF, Sprick MR, Kemper K, Perez Alea M, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Ju S, Jiang S, Zhu L, Wang Y, Wang H. Reduced APRIL expression induces cellular senescence via a HSPG-dependent pathway. Pathol Oncol Res. 2009;15:693–701. doi: 10.1007/s12253-009-9172-y. [DOI] [PubMed] [Google Scholar]
- Petty RD, Samuel LM, Murray GI, MacDonald G, O'Kelly T, Loudon M, et al. APRIL is a novel clinical chemo-resistance biomarker in colorectal adenocarcinoma identified by gene expression profiling. BMC Cancer. 2009;9:434. doi: 10.1186/1471-2407-9-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007;109:2961–2967. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- Chae SC, Yu JI, Oh GJ, Choi CS, Choi SC, Yang YS, et al. Identification of single nucleotide polymorphisms in the TNFRSF17 gene and their association with gastrointestinal disorders. Mol Cells. 2010;29:21–28. doi: 10.1007/s10059-010-0002-6. [DOI] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield JR. Cellular context in epigenetics: quantitative multicolor imaging and automated per-cell analysis of miRNAs and their putative targets. Methods. 2010;52:271–280. doi: 10.1016/j.ymeth.2010.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.