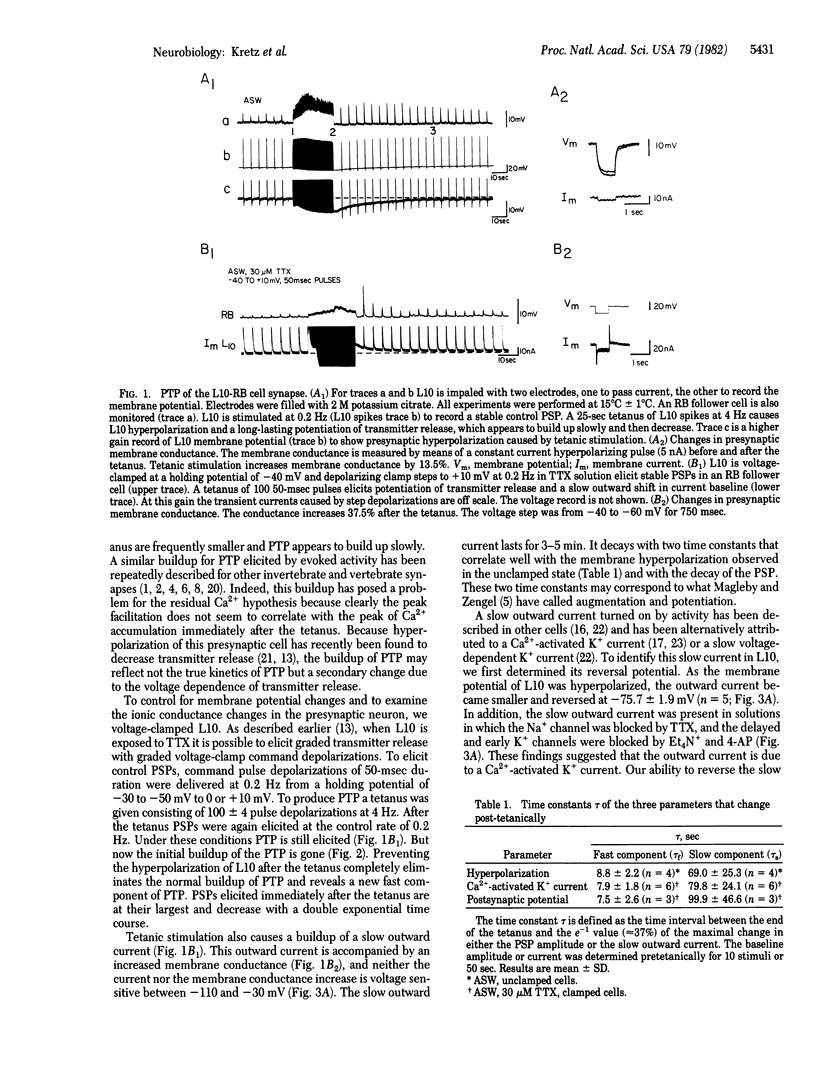
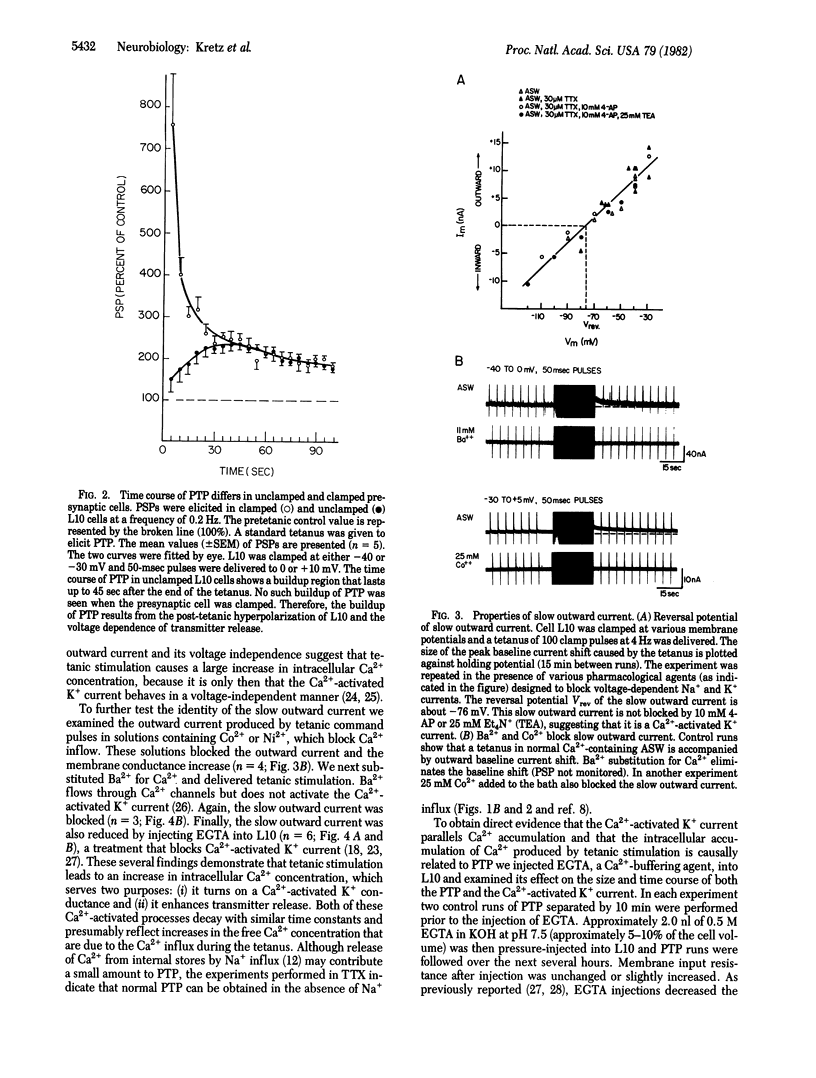
Abstract
We have examined the presynaptic changes underlying post-tetanic potentiation (PTP) in Aplysia by using voltage-clamp techniques combined with specific pharmacological blocking agents. The amplitude and time course of PTP parallel a slow outward clamp current that we have identified as a Ca2+-activated K+ current. Because this current is proportional to intracellular Ca2+ concentration our findings provide evidence for the "residual Ca2+ hypothesis," according to which PTP is caused by the accumulation of intracellular Ca2+ after tetanus. To obtain further evidence for this mechanism we injected EGTA intracellularly and found that it decreased the duration of both PTP and the Ca2+ -activated K+ current.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Shapiro E., Dieringer N., Koester J. Biophysical mechanisms contributing to inking behavior in Aplysia. J Neurophysiol. 1979 Sep;42(5):1233–1250. doi: 10.1152/jn.1979.42.5.1233. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J Physiol. 1978 May;278:501–511. doi: 10.1113/jphysiol.1978.sp012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–375. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C. Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol. 1949 Nov;33(2):147–170. doi: 10.1085/jgp.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Stevens C. F. A mechanism for spike frequency adaptation. J Physiol. 1976 Apr;256(2):315–332. doi: 10.1113/jphysiol.1976.sp011327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapfer W. T., Tremblay J. P., Woodson P. B., Barondes S. H. Frequency facilitation and post-tetanic potentiation of a unitary synaptic potential in Aplysia californica are limited by different processes. Brain Res. 1976 Jun 4;109(1):1–20. doi: 10.1016/0006-8993(76)90377-2. [DOI] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic inhibition in Aplysia involves a decrease in the Ca2+ current of the presynaptic neuron. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1185–1189. doi: 10.1073/pnas.77.2.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waziri R., Kandel E. R., Frazier W. T. Organization of inhibition in abdominal ganglion of Aplysia. II. Posttetanic potentiation, heterosynaptic depression, and increments in frequency of inhibitory postsynaptic potentials. J Neurophysiol. 1969 Jul;32(4):509–519. doi: 10.1152/jn.1969.32.4.509. [DOI] [PubMed] [Google Scholar]
- Waziri R. Presynaptic electrical coupling in Aplysia: effects on postsynaptic chemical transmission. Science. 1977 Feb 25;195(4280):790–792. doi: 10.1126/science.189390. [DOI] [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]



