Abstract
Background
Toxoplasma gondii can infect all warm-blooded animals. Modified agglutination test (MAT) and ELISA are widely used for the detection of T. gondii antibodies. However, there is little information on their acceptability for detecting antibodies in companion animals.
Methods
This study compared ELISA and MAT for their ability to detect T. gondii infection in naturally infected dogs and cats. Blood samples were collected from dogs and cats in different areas of Beijing, China and analyzed by ELISA and MAT. The χ2 test and κ analysis were used to evaluate their efficiency and agreement.
Results
For dogs, the seroprevalence of T. gondii antibodies detected by ELISA was 34.7%, which was significantly higher than that detected by MAT (P<0.05). There was no significant difference between ELISA and MAT for detecting T. gondii antibodies in cats. Good agreements between MAT and ELISA were seen in both dogs and cats; however, inconsistent results were demonstrated by κ analysis and in MAT titer assay.
Conclusion
Serum-based ELISA may be more satisfactory for screening test of T. gondii infection in dogs, whereas both methods could be acceptable in cats.
Keywords: Toxoplasma gondii, Dogs, Cats, Modified agglutination test, ELISA
Introduction
Toxoplasmosis is caused by Toxoplasma gondii and is a disease with a major public health impact. Contact and interaction between domestic animals and humans may lead to an increased risk of transmission of T. gondii (1). Toxoplasma gondii is a coccidia and has evolved in cats (definitive host), where sexual multiplication occurs (2). This results in dissemination of many oocysts into the environment, where they can infect all types of warm-blooded animals (wildlife, companion animals, domestic livestock), including humans (intermediate host).
Intermediate hosts can be infected by ingesting food or water contaminated with oocysts, eating undercooked meat with tissue cysts, or by transplacental infection with tachyzoites (2). Prevention and control of the prevalence of T. gondii and effective prevention of toxoplasmosis in animals and humans require good diagnostic or testing methods that are appropriate for the specific species. There are many testing methods for T. gondii detection in animals and humans (3, 4), however, serological tests have some distinct advantages that have been described elsewhere (5–7). Among the serological tests, modified agglutination test (MAT) and ELISA have been widely used both in clinical and epidemiological surveys for screening of T. gondii infection. However, evaluation of their diagnostic capability for detecting T. gondii infection in animals has focused solely on some domestic animals (4, 8–10), and few studies have investigated MAT and ELISA for detection of T. gondii antibodies in naturally infected companion animals.
In the present study, the efficiency of a commercial SPA-ELISA and MAT for detecting T. gondii antibodies in sera of naturally infected pet dogs and cats was compared, and their intrinsic agreement was also evaluated through analysis of κ statistics. Considering the lack of the information on the sensitivity and specificity of MAT and ELISA methods in dogs and cats, an MAT titer assay was also performed to evaluate the potential agreement between the methods.
Materials and Methods
Blood samples from 1876 dogs and 762 cats were collected from different localities of Beijing, China, including the animal hospital affiliated to China Agricultural University, and animal welfare shelters of Haidian district, from May 2010 to April 2011. Blood samples were obtained from the jugular or hind limb veins, and then centrifuged at 3500 rpm for 10 min for serum separation after coagulation. Separated sera were labeled and stored at –20°C for the following test. The serum samples were diluted 1:25 for the comparative analysis (MAT) and diluted in twofold serial dilutions from 1:10 to 1:640 for the MAT titer assays.
For comparison of the two testing methods, 121 serum samples from dogs and 45 from cats were randomly selected, and T. gondii antibodies were detected using ELISA and MAT. For the MAT titers assay, 417 serum samples from dogs and 263 from cats were randomly selected and detected first by ELISA, and then positive samples were tested by MAT at different titers.
Serum IgG antibodies to T. gondii were detected using a commercial ELISA kit (Toxo SPA-ELISA kit, Haitai Bio., Shenzhen, China) according to the manufacturer's instructions. Whole T. gondii tachyzoites (as antigen) were coated on a 96-well plate. After incubation of the diluted serum sample (1:100) in the test well and subsequent washing, a horseradish-peroxidase-conjugated staphylococcal protein A was added as second antibody, which combined with the Fc fragment of IgG and had no species specificity. The plate was washed again and the chromogenic enzyme substrate was added. OD450 was read using iMark™ Microplate Absorbance Reader (Bio-Rad Laboratories, Hercules, CA, USA)
MAT was performed according to the standard procedures (11, 12). MAT was performed with a suspension of Toxoplasma tachyzoites fixed with formalin, serum samples diluted in PBS (pH 7.2), positive and negative control sera, antigen-diluting buffer containing bovine serum albumin (BSA), 2-mercaptoethanol (to deplete the serum of non-specific IgM), and Evans blue dye solution. MAT titers of 1:25 or higher were considered as positive. Positive and negative controls were incorporated in each test and tested at the same dilutions of serum samples. A negative result was when the base of the “U” bottom 96-well microtiter plates contained a blue pellet; conversely, a clear bottom indicated a positive result.
All statistical analyses were conducted using SPSS version 15.0. A χ2 test was used to analyze the difference in efficiency and P<0.05 was considered significant. κ analysis was used to evaluate the agreement between MAT and ELISA for the detection of T. gondii-specific antibodies.
Results
Paired serum samples were randomly selected and tested using the two methods. Of the 121 samples randomly selected from dogs, 42 (34.7%) were positive by ELISA and 28 (23.1%) were positive by MAT (Table 1). The χ2 test suggested that the positive rate of antibodies to T. gondii in naturally infected dogs differed significantly between the two methods (χ2=3.940, P<0.05). However, κ analysis demonstrated that the two methods had a high degree of agreement in detecting T. gondii infection in dogs (κ=0.644).
Table 1.
The inter-agreement between MAT and ELISA in the course of detecting T. gondii antibodies in pet dogs
| MAT | ELISA | Total(Rate) | |
|---|---|---|---|
| + | - | ||
| + | 26 | 2 | 28 (23.1%) |
| - | 16 | 77 | 93 (76.9%) |
| Total (Rate) | 42 (34.7%) | 79 (65.3%) | 121 |
| Weighted Kappa (κ) | 0.644 | ||
| Standard error | 0.074 | ||
| 95% CI | 0.499 to 0.789 | ||
χ2=3.940, P<0.05.
Among the 45 serum samples from cats, 16 (35.5%) were positive by ELISA and 11(24.4%) by MAT (Table 2). The χ2 test suggested that there was no significant difference between the positive rates of T. gondii antibodies detected by the two methods (χ2=1.323). The corresponding κ value decreased to 0.531, although it still demonstrated a moderate agreement between the two methods in detecting T. gondii infection in cats.
Table 2.
The inter-agreement between MAT and ELISA in the course of detecting T. gondii antibodies in pet cats
| MAT | ELISA | Total(Rate) | |
|---|---|---|---|
| + | - | ||
| + | 9 | 2 | 11 (24.4%) |
| - | 7 | 27 | 34 (75.6%) |
| Total (Rate) | 16 (35.6%) | 29 (64.4%) | 45 |
| Weighted Kappa (κ) | 0.531 | ||
| Standard error | 0.133 | ||
| 95% CI | 0.270 to 0.791 | ||
χ2=1.323, P>0.05.
Ninety-two ELISA-positive samples from 417 naturally infected dogs, and 53 from 263 cats were further tested for MAT titer (Table 3).
Table 3.
MAT titers for 92 ELISA-positive dogs and 53 ELISA-positive cats
| Samples | MAT(titer) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1:10 | 1:20 | 1:40 | 1:80 | 1:160 | 1:320 | 1:640 | |
| ELISA-positive Dogs (n=92) | 19 | 22 | 17 | 15 | 5 | 4 | 8 | 2 |
| 20.7% | 23.9% | 18.5% | 16.3% | 5.4% | 4.3% | 8.7% | 2.2% | |
| ELISA-positive Cats (n=53) | 5 | 5 | 3 | 3 | 2 | 4 | 10 | 21 |
| 9.4% | 9.4% | 5.7% | 5.7% | 3.8% | 7.5% | 18.9% | 39.6% | |
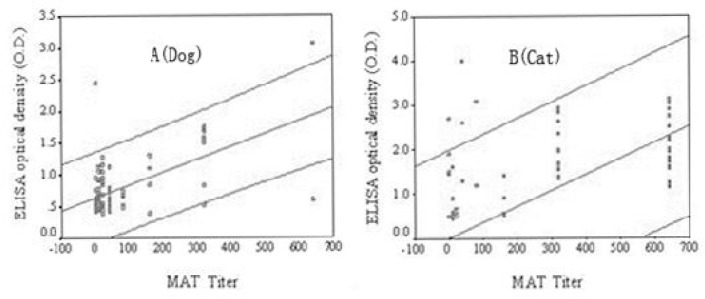
The relationship between MAT titer and ELISA OD values in dogs and cats is shown in Fig. 1, which showed a positive correlation between MAT titer and ELISA OD. Fig. 1 shows that there was a better goodness-of-fit of regression between ELISA OD value and MAT titer in dogs than in cats (95.6% versus 92.4%).
Fig. 1.
Relationship between MAT titer and ELISA OD for 92 dogs and 53 cats. Data shown for individual dogs and cats were fitted to a linear regression. A and B show the agreement analysis through straight line regression between MAT titer and ELISA OD detected in dogs and cats, respectively. The middle line is the regression line, and the whole area between the outer lines is the prediction interval
Such a result further demonstrated that there was a better agreement between ELISA and MAT in detecting of T. gondii antibodies in dogs. Table 3 indicates that cats had a higher titer than dogs. Twenty-one (39.6%) of the 53 serum
samples from cats reached a titer of 1:640, while only two (2.2%) of the 92 serum samples from dogs reached this titer. If we use a titer of 1:10 as a cut-off value for MAT, among the ELISA-positive serum samples, 73 (79.3%) from dogs and 44 (83.0%) from cats were seropositive for T. gondii antibodies. However, if we use 1:20 as a positive cut-off value for MAT, the corresponding seropositivity rate decreased to 55.4% for dogs and 81.2% for cats.
Discussion
Diagnosis of toxoplasmosis by demonstration of T. gondii in tissue is too difficult. Cat/ mouse-based bioassays are not suitable for use in the field or for rapid detection, due to the length of time required to obtain a result, or their applicability and reliability, and other reasons that have been described elsewhere (5–7). Serological tests are still considered useful for epidemiological surveys and for estimating infection rate of T. gondii, which helps to prevent and control its transmission through improving management and reducing potential human exposure, because there is still no suitable human vaccine (7).
There are many references to serological tests for T. gondii in the literature, including indirect hemagglutination test (IHAT), indirect fluorescent antibody test (IFAT), latex agglutination test (LAT), ELISA and MAT, which have been widely used to detect T. gondii antibodies in sera of animals and humans (4, 7, 13). The sensitivity and specificity of the various tests differ according to sampling methods and reference populations, and even when the same tests are used in different species (13–15). Several problems associated with the available methods for serodiagnosis of T. gondii infection have been an impetus to search for alternative methods.
ELISA and MAT are the most widely used methods for T. gondii detection worldwide (3). However, most of the data on MAT and ELISA have been obtained from parasites isolated from naturally and experimentally infected domestic animals (3, 9), with few tests based on companion animals. When a gold standard is not available, at least two different tests should be performed (16); however, it is difficult and impractical to conduct at least two diagnostic tests in practice, especially for epidemiological surveys or high-throughput screening. In the present study, we compared an available commercial ELISA with MAT for detection of T. gondii antibodies in sera of naturally infected dogs and cats.
In this study, we found a significant difference between the two tests for detection of T. gondii antibodies in sera from dogs. A high seroprevalence of T. gondii antibodies in dogs (34.7%) was detected using ELISA, but MAT showed that only 23.1% of the sera were seropositive at a cut-off titer of 1:25, which was higher than that detected previously using MAT (10.81%) in Lanzhou, China (17). This result was consistent with a previous study in domestic pigs (9), which also has suggested that ELISA may be a good tool for epidemiological studies of T. gondii infection in pigs. However, the opposite conclusion was reached in another study in naturally infected sheep (10). There was no significant difference between the corresponding seropositivity rates in pet cats detected by ELISA and MAT. This suggests that both ELISA and MAT are suitable for testing the seroprevalence of T. gondii antibodies in cats. However, κ analysis demonstrated that there was a better agreement between ELISA and MAT for the detection of T. gondii antibodies in dogs than in cats. Taken together, our results suggest that, for accurate diagnosis of T. gondii in pet animals, ELISA should be recommended for primary screening, but the screening result may need to be adjusted by MAT.
Reliability and agreement are basic quality requirements of diagnostic tests; however, the way in which reliability and agreement studies are conducted, reported and interpreted seems not to be straightforward (18). Here, the concern is with how well these tests agree, and not with their relationship with the gold standard or true diagnosis. Reliability here is the extent to which the results of different test methods agree, and not merely the extent to which the results are associated or correlated. In such a context, an agreement test should be used.
The κ test remains the most commonly used measure (19, 20), mainly because it provides a measure of agreement beyond that which would be expected by chance, as estimated by the observed data. In the present study, κ analysis was used to determine the potential agreement between ELISA and MAT in detecting T. gondii antibodies in pet dogs and cats. The result showed that there was a good agreement between the two test methods in both species, although there was a slightly better agreement in dogs (0.644 versus 0.531).
Due to the lack of references to the sensitivity and specificity of the two methods for detecting T. gondii antibodies in sera from pet dogs and cats, in this study, we used the MAT titers to analyze the seropositivity rate of T. gondii antibodies, which had been detected by ELISA. We found that there was a higher MAT titer in the sera from ELISA-positive cats; in contrast, the number of ELISA-positive dogs with high MAT titer was small. In this regard, such results may display a higher degree of agreement between the two methods in cats than in dogs, which was not consistent with the κ analysis. Notably, agreement studies should not be confused with studies of accuracy in which measures of sensitivity and specificity are commonplace for comparisons when a reference or gold standard exists.
In the present study, the differences in agreement between the two methods in dogs and cats might be attributed to the different sensitivity and specificity between ELISA and MAT in detecting T. gondii antibodies, and differences in immune status, age, living conditions, or species susceptibility. An apparent difference in the agreement obtained through κ analysis and our MAT titers assay was also noted. This could have resulted from the intrinsic difference between the two methods, such as there being no default paired samples in MAT titer analysis. It could also have been due to the difference in immune status or species susceptibility, for example, a lower MAT titer was found in dogs than in cats, which has also been found in other studies (17, 21).
The advantages of ELISA over other diagnostic methods are that it can be semi-automated, the procedures can easily be conducted, and the results can be read objectively without the need for experienced personnel. This means that it can be used for screening many samples rapidly and is ideal for epidemiological surveys worldwide. For MAT, although it does not require species-specific conjugates, the length of time needed and the subjective nature of results interpretation may render it impractical for widespread application in clinical diagnosis or epidemiological surveys.
As far as we are aware, the present study is the first to compare MAT and ELISA for detection of T. gondii antibodies in serum samples from naturally infected cats and dogs from Beijing, China. We demonstrated that ELISA performed better than MAT for detecting T. gondii serum antibodies in dogs, whereas there was no significant difference between the seropositivity rates for T. gondii antibodies detected by the two methods in cats. At the same time, κ analysis showed that the two methods could be considered to have good agreement for detection of T. gondii antibodies in dogs and cats, although a slightly better agreement was found in dogs. We think that the variation between the results of the two serological tests might have been due to differences in sensitivity and specificity, and to differences between the animal species. We assumed that the reliability of SPA-ELISA and MAT was similar between the two groups of animals; however, we did not select them according to their biological factors such as age, sex, living conditions, physiological status, stage of disease, and species susceptibility, which might also have influenced the sensitivity and specificity of the methods.
In conclusion, our results demonstrated good agreement between ELISA and MAT for the detection of T. gondii antibodies in both dogs and cats. However, a serum-based ELISA would be more satisfactory for epidemiological survey of toxoplasmosis in dogs, whereas for cats, both methods could be used. For accurate diagnosis, the screening result may need to be adjusted by MAT.
Acknowledgements
The authors would like to thank Dr. Zhaoxia Zhang for her technical assistance and Weiwei Zhang for her assistance in collecting samples. We also thank Professor Xingquan Zhu for his kindness to supply the sera of positive control. This work was supported by the special fund for agro-scientific research in the public interest (Grant No. 200903036—03). The authors declare that there is no conflict of interest.
References
- 1.Barbosa IR, Holanda C, De Andrade-Neto VF. Toxoplasmosis screening and risk factors amongst pregnant females in Natal, northeastern Brazil. Trans Roy Soc Trop Med Hyg. 2009;103(4):377–82. doi: 10.1016/j.trstmh.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Hill DE, Chirukandoth S, Dubey JP, Lunney JK, Gamble HR. Comparison of detection methods for Toxoplasma gondii in naturally and experimentally infected swine. Vet Parasitol. 2006;141(1-2):9–17. doi: 10.1016/j.vetpar.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Moghazy EI, Kandil FM, Shaapan RM. Toxoplasma gondii: comparison of some serological tests for antibody detection in sera of naturally infected pigs. World J Zoology. 2011;6(2):204–8. [Google Scholar]
- 5.Dubey JP, Rajapakse RP, Wijesundera RR, Sundar N, Velmurugan GV, Kwok OCH, Su C. Prevalence of Toxoplasma gondii in dogs from Sri Lanka and genetic characterization of the parasite isolates. Vet Parasitol. 2007;146(3-4):341–6. doi: 10.1016/j.vetpar.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Dubey JP. Toxoplasma gondii Infections in Chickens (Gallus domesticus): Prevalence, Clinical Disease, Diagnosis and Public Health Significance. Zoonoses Public Health. 2010;57(1):60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 7.Dubey JP. The History of Toxoplasma gondii-The First 100 Years. J Eukaryot Microbiol. 2008;55(6):467–75. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubey JP, Fair PA, Bossart GD, Hill D, Fayer R, Sreekumar C, Kwok OCH, Thulliez P. A comparison of several serologic tests to detect antibodies to Toxoplasma gondii in naturally exposed bottlenose dolphins (Tursiops truncatus) J Parasitol. 2005;91(5):1074–81. doi: 10.1645/GE-582R.1. [DOI] [PubMed] [Google Scholar]
- 9.Gamble HR, Dubey JP, Lambillotte DN. Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma infection in the domestic pig. Vet Parasitol. 2005;128(3-4):177–81. doi: 10.1016/j.vetpar.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Shaapan RM, El-Nawawi FA, Tawfik MAA. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet Parasitol. 2008;153(3-4):359–62. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Desmonts G, Remington JS. Direct agglutination-test for diagnosis of Toxoplasma infection – method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11(6):562–8. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey JP, Emond JP, Desmonts G, Anderson WR. Serodiagnosis of postnatally and prenatally induced toxoplasmosis in sheep. Am J Vet Res. 1987;48(8):1239–43. [PubMed] [Google Scholar]
- 13.Greiner M, Gardner IA. Application of diagnostic tests in veterinary epidemiologic studies. Prev Vet Med. 2000;45(1-2):43–59. doi: 10.1016/s0167-5877(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 14.Mainar-Jaime RC, Barberan M. Evaluation of the diagnostic accuracy of the modified agglutination test (MAT) and an indirect ELISA for the detection of serum antibodies against Toxoplasma gondii in sheep through Bayesian approaches. Vet Parasitol. 2007;148(2):122–9. doi: 10.1016/j.vetpar.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JL, Navarro IT, Vidotto O, Gennari SM, Machado RZ, Pereira BD, Sinhorini IL. Toxoplasma gondii: Comparison of a rhoptry-ELISA with IFAT and MAT for antibody detection in sera of experimentally infected pigs. Exp Parasitol. 2006;113(2):100–5. doi: 10.1016/j.exppara.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Enoe C, Georgiadis MP, Johnson WO. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev Vet Med. 2000;45(1-2):61–81. doi: 10.1016/s0167-5877(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu SM, Huang SY, Fu BQ, Liu GY, Chen JX, Chen MX, Yuan ZG, Zhou DH, Weng YB, Zhu XQ, Ye DH. Seroprevalence of Toxoplasma gondii infection in pet dogs in Lanzhou, Northwest China. Parasites and Vectors. 2011;4(64) doi: 10.1186/1756-3305-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM. Evaluation of diagnostic tests when there is no gold standard-A review of methods. Health Technol Asses. 2007;11(50):48–51. doi: 10.3310/hta11500. [DOI] [PubMed] [Google Scholar]
- 19.Sim J, Wright CC. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–68. [PubMed] [Google Scholar]
- 20.Kottner J, Audige L, Brorson S, Donner A, Gajewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64(1):96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Dubey JP, Zhu XQ, Sundar N, Zhang H, Kwok OCH, Su C. Genetic and biologic characterization of Toxoplasma gondii isolates of cats from China. Vet Parasitol. 2007;145(3-4):352–6. doi: 10.1016/j.vetpar.2006.12.016. [DOI] [PubMed] [Google Scholar]