Abstract
Background
Plasmodium vivax is the predominant species causes of malaria with about 90% total annual reported malaria in Iran. This study conducted to determine the susceptibility of Plasmodium vivax isolates to chloroquine in Sistan and Balochistan Province, southeastern Iran.
Methods
A total 270 subjects with symptomatic malaria and confirmed P. vivax infection completed the designed 28-day in vivo study. The thick and thin film blood smears were screened for malaria parasites by microscopy. The nested PCR was applied using the Plasmodium 18 subunit ribosomal ribonucleic (Ssr RNA) genes for detecting mixed infections and diagnosis of parasites in the samples with low parasite on days 0, 5, 6, 7, and 28.
Results
P. vivax was cleared in 15%, 50%, 95%, and 100% of patients on days 1, 2, 3, 4 respectively by microscopy assessment. Six patients were exhibited specific P. vivax band in nested PCR on day 5. No recurrence was observed on days 7, 14 and 28. Mean (±standard deviation) parasite clearance time was 2.41 (±0.8) days.
Conclusion
P. vivax is still susceptible to chloroquine in Southeatern Iran. This finding is compatible with results of neighboring countries Pakistan and Afghanistan.
Keywords: Malaria, Plasmodium vivax, Chloroquine, Iran
Introduction
Plasmodium vivax (P. vivax) is the main cause of malaria in Asian, Central and South American countries (1). Meanwhile it is actually coming back to Europe with recent report from Greece (2). About 70-80 million symptomatic malaria cases due to P. vivax occur in the world annually (3). Since six decades ago, chloroquine has been used for treatment of human malaria in the world. Resistance to chloroquine occurred in P. falciparum very soon after extensive use of this agent in endemic areas around the world. Thereafter, the emergence of chloroquine resistant P. vivax was reported for the first time in Australian soldiers returning from Papua New Guinea (4). Consequently; chloroquine resistant P. vivax was observed in Myanmar (5), southeastern Turkey (6), Ethiopia (7, 3), Republic Korea (8), Indonesia (9, 10), India (11) and with reports also from geographical regions in Southern and Central America (4, 12). These reports underline again the serious concerns about emerging resistance in P. vivax isolates to chloroquine in new regions of the world. However, chloroquine seems still a suitable drug in Afghanistan (13), Azerbaijan (14) and Thailand (15). P.vivax is the predominant causative species of malaria with about 90% of the total annual reported cases of malaria in Iran and both were P.falciparum and P.vivax have been reported since 1921 (16).
Plasmodium falciparum resistant to chloroquine and sulfadoxin premethamin (S/P) has been reported in Iran (17, 18). Furthermore, S/P and artosonate combination is currently administered for treatment of this infection. While the decreased susceptibility of P. vivax to chloroquine has been reported in clinical studies in south of Iran (17, 19, 20), chloroquine has been the main drug of choice for treatment of patients infected by P. vivax. Therefore, assessment of susceptibility of P.vivax to chloroquine is an important public health priority in endemic countries such as Iran.
In recent years, a decrease in reported cases of malaria has been occurred in Iran. The statistics related to years 2008 and 2009 are 11,460 and 6,122 malaria patients, respectively (21). Immigration from, Afghanistan and Pakistan, two malaria endemic neighbor countries, resistance of vectors to insecticides, socioeconomic situation and emergence of P. falciparum species which are resistant to some anti-malaria drugs have impeded malaria control in this part of Iran (22, 23).
In order to ensure good patient care and to support the malaria control efforts of the Health Ministry in Iran, we performed a susceptibility study for P. vivax in the southeast of Iran following clinical reports of resistance from neighboring countries.
Materials and Methods
Study area
Sistan and Baluchistan Province is located in southeast of Iran and possess common borders with Pakistan and Afghanistan. The highest numbers of malaria infection in Iran have been reported from this province during the past decade. Anopheles (A) stephancy, A. culcificis, A. fluviatalis, A. superpictus and A. pulcherrimus are the main malaria vectors in the area (24). P. falciparum resistance to chloroquine was reported for the first time in this province in 1983 (25). The malaria transmission is unstable with two peaks from May to August and from October to November. The study sampling was carried out between May and November 2010 at the health centers in Sistan and Baluchistan province. These centers provide malaria diagnosis and treatment services free of charge. Both autochthonous and imported malaria have been reported in the area. Imported malaria originated from Pakistan and Afghanistan.
Study samples
A total of randomly 285 subjects with symptomatic malaria and confirmed P. vivax infections participated in the study. All of them were referred to the health centers of Chabahar distinct located in Siststan and Baluchistan Province to receive required treatments. However, 15 patients were lost to follow up in various stages of the study mostly due to traveling or mix (P. vivax and P. falciparum) infection.
Age of the patients ranged between 2 and 60 years. Chloroquine was administrated to all subjects during 3 days (10mg/kg on days 0 and 1and 5mg/kg on day 2). According to the Iran national drug policy, primaquine (PQ) is given during 8 weeks for preventing relapses. Since half-life time of chloroquine extends nearly 30 days and during this time it can eliminate emergent parasites (Baird 2009, Naing 2010), thus PQ administration was postponed to day 28. Inclusion criteria for the registered malaria patients were patients who aged more than 1 year old, positive for P. vivax mono infection with parasite density above 250/µl, and fever or history of fever during 48 hours prior to the time of recruitment. Patients with the following conditions were excluded from the study: presence of clinical condition requiring hospitalization, presence of severe malnutrition, pregnancy, chronic infectious diseases, and concomitant febrile illnesses. All patients were followed up for 28 days according to the WHO in vivo test protocol (26). The patients were visited on days 1, 2, 3, 4, 5, 6, 7, 14, 28. Auxiliary temperature was measured to detect fever and thick and thin blood slides were collected on the above-mentioned days.
The treatment failure was defined as clinical deterioration due to P. vivax, which required hospitalization in presence of parasitemia, presence of parasitemia and auxiliary temperature of ≥ 37.5 at any time between days 3 and 28, and presence of parasitemia between days 7 and 28 irrespective of clinical conditions. Blood samples were taken on days 0, 5, 6, 7, 28 and stored at -20°C until DNA extracting for nested PCR. The study protocol was approved by the Tehran University of Medical Sciences, Iran. The consent obtained from all participants or their parents.
Laboratory investigation
Thick and thin film blood smears were prepared and stained with 3% Giemsa. Parasite density was determined for every positive thick film by counting the number of parasites against 200 white blood cells and was multiplied by 40 to obtain the number of parasites per mm3 of blood. Genomic DNA was extracted from the 200µL blood samples using a QIA quick PCR purification kit (Quigen, Germany) according to the manufacturer’s protocol. The nested PCR was applied using the Plasmodium 18 sub-unit ribosomal ribonucleic (Ssr RNA) genes for detecting mix infections and diagnosis of parasites in the samples with low parasite (less 10 P/µl). Primers and detail PCR amplifying method have previously been described (27). Negative and positive controls were used in each set of experiments. The amplified products were run in 2% agarose gel electrophoresis and stained by ethidium bromide for visual detection bands by ultraviolet transillumination.
Results
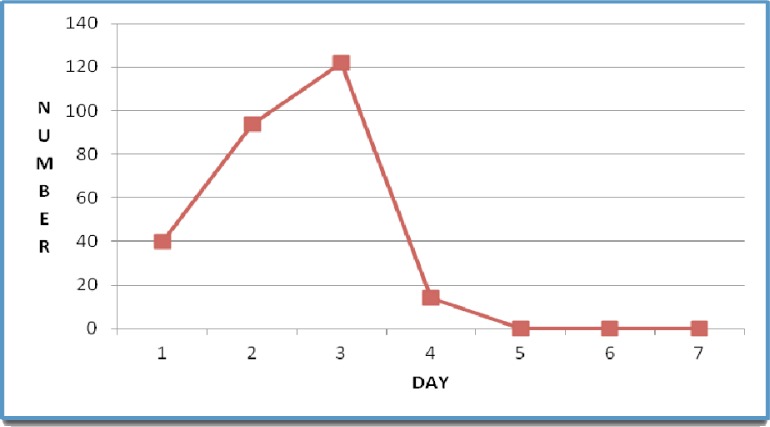
Out of 270 patients who completed the 28-day follow-up, 180 individuals (66.6%) were male. chloroquine was well tolerated and no patient reported vomiting within 1h of drug administration. Fever resolved on the first day in all subjects. According microscopy findings P. vivax was cleared in 15%, 50%, 95%, and 100% of patients on days 1, 2, 3 and 4, respectively (Fig. 1). No recurrent infection was observed on days 7, 14 and 28.
Fig. 1.
Parasite clearance time during the 28 days of patients follow up after chloroquine treatment in infected patients with Plasmodium vivax in southeast of Iran
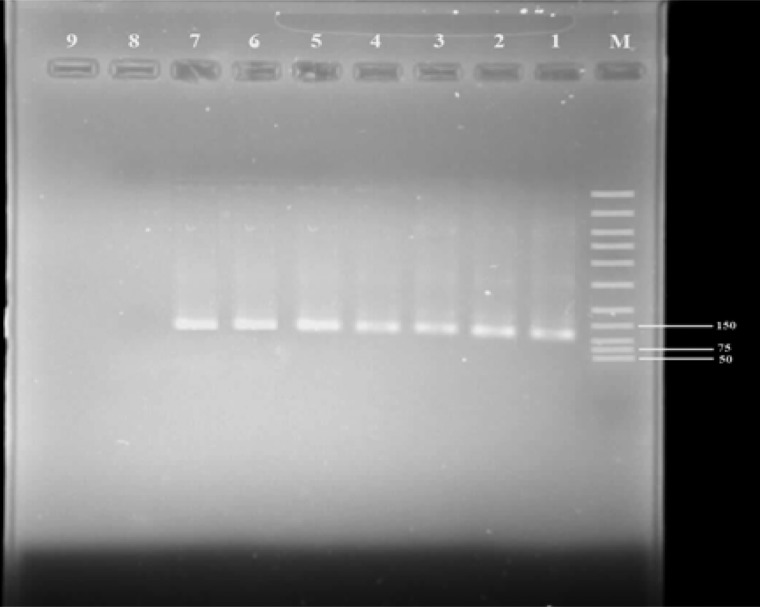
The PCR product was consider positive when a band of size of ∼1200 Bp was observed for Plasmodium genus on day 0. All 270 subjects showed ∼120 Bp band in the nested PCR, which is indicative of P. vivax malaria (Fig. 2). Six (2.2%) patients demonstrated 120 bp bands in nested PCR on day 5. On days 7 and 28 no band was illustrated for P. vivax. Two patients with vivax malaria by microscopy assay showed mixed infection with P. falciparum that was removed from the study. Mean parasite clearance time (MPCT) and SD were 2.43 (95% confidence interval 2.31-2.5) and 0.8 days respectively. MPCT based on sex was 2.39 and 2.44 days in males and females, respectively. There was no significant difference regarding MPCT between males and females (T = 0.5, P = 0.59, df= 268). The number of parasite ranged from 1,000 to 35,000 P/ µl blood with geometric mean of 7,500 P/µl.
Fig. 2.
Gel photograph showing nested PCR amplified products from different Plasmodium vivax infected isolates in southeast of Iran on day 5. The DNA marker is shown on the right (Lane 1). Lane2 to 7 are Patients Plasmodium vivax isolates. Lane 8 and 9 are Plasmodium falciparum and negative controls respectively
Discussion
Emergence of chloroquine resistant P. vivax isolates in many countries especially in those near Iran and spread of chloroquine resistant P. falciparum in various areas of Iran mad it important to monitor closely of susceptibility of P. vivax to this drug in the country.
No genetic marker has been determined yet for chloroquine resistant P. vivax in contrary to P. falciparum, in vivo study, so, was performed to determine any resistance to chloroquine. The nested PCR could well detect low parasitemia so that, six patients exhibited P. vivax infection in contrary microscopy results on day 5. The findings of this study showed susceptibility of P. vivax to chloroquine in the region. This result is compatible with former studies in Iran (17, 20). It seems that decreased use of chloroquine in the area due to administration of S/P and ACT for treating P. falciparum as well as substantially decreased numbers of malaria infection play important roles in the observed susceptibility of P. vivax to chloroquine in this region.
Our results are in agreement with conducted studies in Pakistan (28) and Afghanistan (13), where P. vivax is susceptible to chloroquine. It seems there may be a regional pattern in P. vivax susceptibility to chloroquine due to traveling of people between this part of Iran and the neighbor countries. The nested PCR on day zero showed malaria infection in all samples, therefore the use of this diagnosis method could be applied to in vivo studies. In addition, parasitological and molecular investigations indicated no recurrent infection on days 7 and 28. In the study conducted in Turkey by PCR, 9.5% resistance in P. vivax isolates to chloroquine was reported (6), while no case of resistance has been observed in Iran by this method. In two previous studies performed in southeast of Iran, mean parasite clearance time has been reported as 1.95 and 2.56 days, respectively (17, 20). Our findings indicate MPCT was 2.4 days, which is higher than that of Edrissian (1999) study but is compatible with Nateghpour (2007) study. It seems the presence of various haplotypes of P. vivax in the area exhibited different parasite clearance time in our study. Otherwise, the patients may have been infected by haplotypes with different susceptibility to chloroquine. No association was determined between the level of parasitemia, sex, and age with parasite clearance time in the patients.
In conclusion, P. vivax still has suitable susceptibility to chloroquine in southeast of Iran and chloroquine can be administrated for treatment of the vivax malaria patients at this region. Nested PCR was also suitable assay to determine exact malaria parasite clearance time in our in vivo study.
Acknowledgement
We would like to thank those individuals from the malaria endemic region of Iran, who kindly contributed to this study and are grateful to Mr Sakeni, Mr. Gorgigh for their assistance to sampling. This study was supported financially by the Ministry of Health treatment and Medial education. The authors declare that there is no conflict of interests.
References
- 1.Danis K, Baka A, Lenglet A, Van Bortel W, Terzaki I, Tseroni M, Detsis M, Papanikolaou E, Balaska A, Gewehr S, Dougas G, Sideroglou T, Economopoulou A, Vakalis N, Tsiodras S, Bonovas S, Kremastinou J. Autochthonous Plasmodium vivax malaria in Greece. Euro Surviell. 2011;16:1–5. [PubMed] [Google Scholar]
- 2.Yeshwondim AK, Tekle AH, Dengela DO, Yohannes AM. Therapeutic efficacy of chloroquine and chloroquine plus primaquine for the treatment of Plasmodium vivax in Ethiopia. Acta Trop. 2010;113:105–13. doi: 10.1016/j.actatropica.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Riecmann KH, Davis DR. Plasmodium vivax resistance to chloroquine? Lancet. 1989;II:1183–4. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 4.Myat-Phone-Kyaw, Myin-Oo, Myint-Lwin, Thaw-Zin, Kyin-Hla-Aye, New-New-Yin Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma) Trans R SOC Trop Med Hyg. 1993;87:678. doi: 10.1016/0035-9203(93)90294-z. [DOI] [PubMed] [Google Scholar]
- 5.Dilmec F, Kurcer MA, Akkafa F, Simsek Z. Monitoring of failure of chloroquine treatment for Plasmodium vivax using polymerase chain reaction in Sanliurfa province, Turkey. Parasitol Res. 2010;106:783–8. doi: 10.1007/s00436-009-1710-8. [DOI] [PubMed] [Google Scholar]
- 6.Yohannes AM, Teklehaimanot A, Bergqvist Y, Ringwald P. Confirmed vivax resistance to chloroquine and effectiveness of artemether-lumefantrine for the treatment of vivax malaria in Ethiopia. Am J Trop Med Hyg. 2011;84:137–40. doi: 10.4269/ajtmh.2011.09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chotivanich K, Sattabongkot J, Choi YK, Park JS, Sritabal J, Lim CS, Udomsangpetch R, White NJ, Lee WJ. Antimalarial drug susceptibility of Plasmodium vivax in the Republic of Korea. Am J Trop Med Hyg. 2009;80(Suppl 6):902–4. [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz LK, Lackritz EM, Patchen LC. Chloroquine resistant Plasmodium vivax from Indonesia. NEJM. 1991;324:927–33. doi: 10.1056/NEJM199103283241317. [DOI] [PubMed] [Google Scholar]
- 9.Sutanto I, Suprijanto S, Nurhayati , Manoempil P, Baird JK. Resistance to chloroquine by Plasmodium vivax at Alor in the Lesser Sundas Archipelago in eastern Indonesia. Am J Trop Med Hyg. 2009;81:338–42. [PubMed] [Google Scholar]
- 10.Garg M, Gopinathan N, Bodhe P, Kshirsagar NA. vivax malaria resistant to Chloroquine case report from Bombay. Trans R Soc Trop Med Hyg. 1995;89:656–7. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 11.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg. 2011;65:90–3. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- 12.Awab GR, Pukrittayakamee S, Immwong M, Dondorp AM, Woodrow CL, Lee SJ, Day NPG, Singhasivanon P, White NJ, Kaker F. Dihydroartemisinin-Piperaquine versus Chloroquine to treat vivax malaria in Afghanistan: an open randomized, non –inferiority, trial. Malar J. 2010;9:105. doi: 10.1186/1475-2875-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valibayov A, Abdullayev F, Mammadov S, Gasimov E, Sabatinelli G, Kondrachine AV, Ringwald D. Clinical efficacy of chloroquine followed by primaquine for Plasmodium vivax treatment in Azerbaijan. Acta Trop. 2003;88:99–103. doi: 10.1016/s0001-706x(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 14.Muhamad P, Ruengweerayut R, Chacharoenkul W, Rungsihirunrat K, Na-Bangchang K. Monitoring of clinical efficacy and in vitro sensitivity of Plasmodium vivax to chloroquine in area along Thai Myanmar border during 2009-2010. Malar J. 2011;10:44. doi: 10.1186/1475-2875-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latichev LN. Cited by povlovsky En in: Epidemic parasitology mission to Iran and Parasitological surveys. Acad Sci USSR. 1984:235–8. [Google Scholar]
- 16.Edrissian GH, Nateghpoor M, Afshar A, Sayedzadeh A, Mohsseni GH, Satvat MT, Emadi AM. Monitoring the response of Plasmodium falciparum and P.vivax to antimalarial drugs in the malarious areas in south-east Iran. Arch Iran Med. 1999;2:61–6. [Google Scholar]
- 17.Heidari A, Dittrich S, Jelinek T, Kheirandish A, Banihashemi K, Keshavarz H. Genotypes and in vivo resistance of Plasmedium falciparum isolates in an endemic region of Iran. Parasitol Res. 2007;100:589–92. doi: 10.1007/s00436-006-0291-z. [DOI] [PubMed] [Google Scholar]
- 18.Hamedi Y, Nateghpour M, Tan-ariya P, Tieensuwan M, Silachmroon U, Loo-areesuwan S. Plasmodium vivax malaria in southeast Iran in 1999-2001:Estabilishing the response to chloroquine in vitro and in vivo. Southeast Asian J Trop Med Public Health. 2002;33:512–8. [PubMed] [Google Scholar]
- 19.Nateghpour M, Sayedzadeh SA, Edrissian GH, Raeisi A, Jahantigh A, Motevalli-Haghi A, Mohseni GH, Rahimi A. Evaluation of sensitivity of Plasmodium vivax to chloroquine. Iranian J Publ Health. 2007;36:60–3. [Google Scholar]
- 20.WHO. Malaria profile of Iran. 2010. available: www.who.int/malaria/publications/country-profiles/profile_irn_en.pdf.
- 21.Heidari A, Keshavarz H, Dittrich S, Jelinec T. Allelic Dimorphism of the Plasmodium falciparum Erythrocyte Binding Antigen-175 (EBA-175) Gene in the southeast of Iran. Iranian J Parasitol. 2009;4(2):17–22. [Google Scholar]
- 22.Mardani M, Keshavarz H, Heidari A, Hajaran H, Raeisi A, Khorramizadeh MR. Genetic polymorphism at the C-terminal domain (Region III) of knob-associated histidine-rich protein (KAHRP) of Plasmodium falciparum in isolates from Iran. Parasitol Res. 2011;109(6):1647–52. doi: 10.1007/s00436-011-2437-x. [DOI] [PubMed] [Google Scholar]
- 23.Zakeri S, Razavi S, Djadid ND. Genetic diversity of transmission blocking vaccine candidate (Pvs25 and Pvs28) antigen in Plasmodium vivax clinical isolates from Iran. Acta Trop. 2009;109:176–80. doi: 10.1016/j.actatropica.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Edrissian GhH. Malaria in Iran: Past and Present situation. Iranian J Parasitol. 2006;1:1–14. [Google Scholar]
- 25.WHO. Monitoring Anti-Malarial Drug Resistance; Report of a WHO Consultation; Geneva, Switzerland: 2002. [Google Scholar]
- 26.Snounon G, Viriyyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested Polymerase chain reaction. Mol Biochem Parasitol. 1993;61:313–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 27.Leslie T, Mayan I, Hasan MA, Safi MH, Klinkenberg E. Sulfadoxine-pyrimethamine, chlorproguanil-dapsone, or chloroquine for the treatment of Plasmodium vivax malaria in Afghanistan and Pakistan: A randomised control trial. JAMA. 2007;297:2201–9. doi: 10.1001/jama.297.20.2201. [DOI] [PubMed] [Google Scholar]