Abstract
Background
Geothermal waters could be suitable niches for thermophilic free living amoebae including Naegleria and Hartmannella. Ardebil Province, northwest Iran is popular for having many hot springs for recreational and health purposes activity. The present research is the first molecular based investigation regarding the presence of Naegleria and Hartmannella in the hot springs of Ardebil Province in Iran.
Methods
Overall, 30 water samples were taken from waters of thermal hot springs in Ardebil Province, Iran during 2010-2011. All collected samples were transferred to Dept. of Parasitology and Mycology, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Cultivation of concentrated water samples was performed using culture-enrichment method. Cloning of the target amoebae was obtained and morphological and molecular analysis was done using page key combined with two sets of primers, respectively. Sequence analysis and homology search was used for strains identification.
Results
Of 30 water samples, 8 (26.7%) were positive for thermotolerant Vahlkampfiids and Hartmannella based on morphological characteristics of vegetative form and double walled cysts. Cloning of the target amoebae were done successfully. Sequencing of the positive isolates revealed that the strains belonged to Naegleria (N. carteri and N. spp) and H. vermiformis.
Conclusion
The result highlights a need for improved filtration and disinfection and periodic monitoring of recreational thermal waters in order to prevent disease related to free- living amoebae. This is the first comprehensive molecular study of thermophilic Naegleria and Hartmannella in hot springs of Iran.
Keywords: Hot springs, Hartmannella, Naegleria, Iran
Introduction
Free-living amoebae (FLA) including various families which some of them are classified as potentially pathogenic organisms for human and animals (1, 2). The family Vahlkampfiidae has been found in environmental sources such as fresh water, soil, dust and clays (3). To date, the genera of Naegleria, Vahlkampfia and Paravahlkampfia introduced as potentially pathogenic amoebae for human (4–6). Indeed, recent report regarding pathogenic potential of Vahlkampfia in Iran leads to more attention regarding this free-living organism (4). The genus Naegleria spp. consists of 30 different species, two of which (N. fowleri and N. australiensis) have been described as potential pathogenic organisms and they are the causative agents of fulminant meningoencephalitis (1). These amoebae are able to tolerate extremes of temperature and thus thermal waters could be an ideal environment for Naegleria growth and survival (1, 3). It should be mentioned that nonpathogenic Naegleria could also be an important threat for human, since these avirulent amoebae could harbor pathogenic microbes and could act as Trojan horse (7). A previous research in Iran revealed the presence of vahlkampfiids in thermal waters of Sarein City (8), however this reported study were based on only morphological criteria. It is important to mention that morphological criteria can reveal the presence of vahlkampfiids, however, molecular analysis is necessary for genera identification of Vahlkampfidae family (9).
The family Hartmannellidae also includes thermotolerant amoebae such as Hartmannella vermiformis. There are recent reports regarding the pathogenic potential of this free living organism (10). A case of mixed keratitis infection has been reported due to Acanthamoeba and H. vermiformis (10).
Ardebil Province in the northwest of Iran is a famous place for having many hot springs, hot tubs, spas, and mineral waters. These thermal waters have been used for recreational and health purpose. High temperature of thermal springs is suitable factor for growth of thermotolerant amoebae such as Naegleria and Hartmannella (1). Indeed, the ability of these ubiquitous organisms to tolerate high temperatures made thermal waters favorable niches.
The present research is the first molecular based investigation regarding the presence of Naegleria and Hartmannella in hotsprings of Ardebil Province in Iran. The occurrence of these amoebae highlights the need of more monitoring of such waters and reflects periodic surveillance of recreational hot springs in Iran.
Material and Methods
Sampling
Overall, 30 water samples were taken from thermal hot springs in Ardebil Province, Iran during 2010-2011. All of the cities which contained recreational hot springs have included in the present study such as Sarein, Meshkin shahr, Nir, Ardebil and Givi. Briefly, 500 ml of surface water were collected from hot springs and transferred to the Department of Parasitology and Mycology, School of Medicine, Shahid Beheshti University of Medical Sciences, Iran. All hot springs included were used for both recreation and for health purposes.
Filtration, cultivation and cloning
Samples were filtered through cellulose nitrate membranes with a pore size of 1.2 µm. The filters were then inverted and transferred onto 1% non-nutrient agar plates covered with a thin layer of autoclaved Escherichia coli. Positive plates were screened for vahlkampfiids and Hartmannella spp. using both optical and inverted microscopes based on pages key (11). Cloning of the candidate amoebae were performed using culture replicates according to our previous study (12).
PCR amplification and gel electrophoresis
Amoebae were harvested from plates and washed using phosphate-buffered saline (PBS pH 7). DNA Extraction was performed using the Instagene matrix (Chelex; Biorad). Briefly, approximately 1000 cells were incubated with 50 µl Chelex. Incubation was done at 56°C for 20 min. Additional incubation were performed for 10 min using boiling water. DNA pellet was obtained by centrifuging the samples at 10 000 g for 5 min and the supernatant was used as the DNA template for PCR reaction. Modified phenol-chloroform methods were performed for DNA extraction of cysts according to our previous study (12).
The PCR reaction was performed in 30 µl Ampliqone (Taq DNA Polymerase Master Mix Red, Denmark) as a readymade mixture. Briefly, 25 µl of master mix with 5 ng DNA templates and 20 pmol primers were combined to achieve a volume of 30 µl. Two sets of primers were used for identification of vahlkampfiids and Hartmannella spp. The first set was ITS primers which were able to detect Naegleria spp. and they are designed to obtain a 400-430 bp PCR product (13). The sequences of ITS primers were: forward 5'GAACCTGCGTAGGGATCATTT 3’ and reverse primer ITS2 5’ TTTCTTTTCCTCCCCTTATTA 3’. The second set was a primers which could amplify a fragment of 18s rRNA gene of Hartmannella (12, 14). The sequences were: forward 5'GCT CCA ATA GCG TAT ATT AA 3’ and revers 5’ AGA AAG AGC TAT CAA TCT GT 3’. Each PCR cycling condition included 35 cycles of denaturation at 94°C for 1 min, followed by 35 repetition cycles at 94°C for 35 s, annealing at 56°C for 45 s, and extension at 72°C for 1 min.
Gel electrophoresis were performed to detect PCR products using 1.5% agarose gel stained with a solution of ethidium bromide (25 mg ml-1) and examined under UV illumination.
Sequencing of the PCR products
PCR-products were submitted to sequencing using an ABI 3130X automatic sequencer at the Research Center for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran. Homolgy analysis of the obtained sequences with genes in the gene data bank was done using BLAST software from the National Center for Biotechnology Information (NCBI) site. The highest homology and query overage was the base of strains identification.
Results
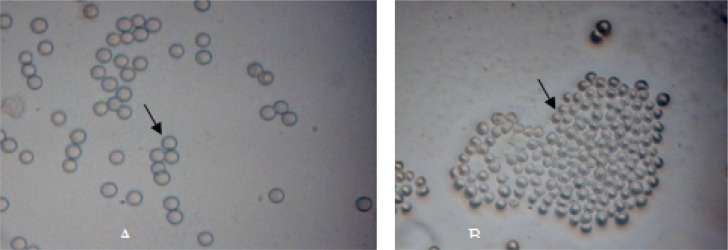
Overall, out of 30 water samples, 8 (26.7%) were positive for vahlkampfiids and Hartmannellidae family based on morphology characteristics. Temperatures and pH of thermal springs were ranged from 41-53° C and 4.90-7, respectively (Table 1). Vahlkmapfiids identification was based on spherical double wall cysts and temporarily branched trophozoites (Fig. 1). Hartmannella were also characterized using morphological criteria including small spherical or ovoid cyst shape and outer wall of some were separated (Fig. 1). The trophozoite form was limax containing one small nucleolus. Cloning of the candidate amoebae were done successfully after 2 months.
Table 1.
Location and distribution of Free- living Naegleria and Hartmannella in hot springs of Ardebil province, Iran
| Code | Locality | Water type | pH | Tem (°C) | Genus | Accession number |
|---|---|---|---|---|---|---|
| HSS1 | Sarein | bicarbonate spring | 6.15 | 46 | Naegleria (N. carteri) | JQ023595 |
| HSS5 | Sarein | bicarbonate spring | 6.28 | 41 | Hartmannella vermiformis | JQ023592 |
| HSS9 | Sarein | bicarbonate spring | 7.00 | 42.0 | Hartmannella vermiformis | JQ023591 |
| HSS3 | Sarein | bicarbonate spring | 6.07 | 43.50 | Hartmannella vermiformis | JQ023590 |
| HSM1 | Meshkin shahr | Sulfur spring | 4.90 | 45.1 | Hartmannella vermiformis | JQ023593 |
| HSM1 | Meshkin shahr | Sulfur spring | 4.90 | 45.1 | Naegleria spp. | JQ023596 |
| HSM2 | Meshkin shahr | bicarbonate spring | 6.39 | 48.2 | Hartmannella vermiformis | JQ023594 |
| HSN1 | Nir | Sodium chloride | 6.18 | 53 | Naegleria spp. | JQ023597 |
HSS: Hot springs Sarein/HSM: Hot spring Meshkin shahr/HSN: Hot spring Nir
Fig. 1.
(A) Light micrograph of Naegleria cysts in non-nutrient agar x400 (B) Light micrograph of Hartmannella vermiformis cysts in non-nutrient agar x400
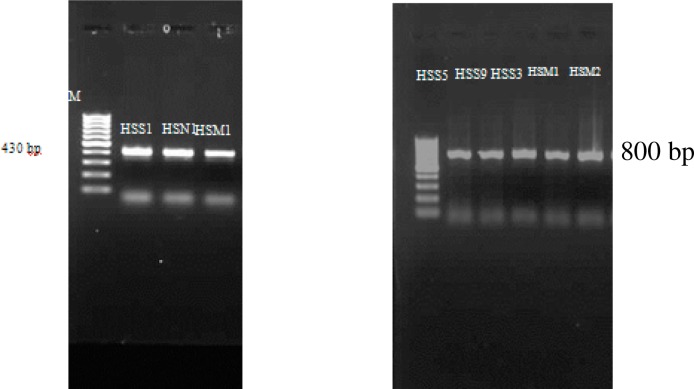
A 400 bp and 800 bp PCR products were obtained for vahlkampfiids and Hartmannella, respectively (Fig. 2). Sequence analysis of the PCR products revealed that the three vahlkampfiid amoebae belonged to the Naegleria genera (Isolates: HSS1, HSN1, HSM1). Basic local alignment search tool (BLAST) showed that one of strains had a high homology to N. carteri (Accession number: AM167887.1). Moreover, all of the Hartmannella isolates detected in the present study were identified as H. vermiformis.
Fig. 2.
PCR products of the isolated strains (Hartmannella spp. and Naegleria spp.) from hot springs of Ardebil province, Iran
Nucleotide sequence accession numbers were deposited in the GenBank database (accession number: JQ023590-JQ023597).
Discussion
The present study is the first molecular identification of FLA belonging to the Naegleria spp. and H. vermiformis in hot springs of Iran. Presence of potentially pathogenic FLA could be a serious hazard for people using such waters. It is important to mention that some of thermal waters in this region are used for recovery of eye trauma. Therefore, the occurrence of H. vermiformis in thermal waters could lead to the exposure of high risk people to thermotolerant amoebae. Recent reports reflect the pathogenic potential of H. vermiformis for human cornea (10, 15). This is in agreement with Lorenzo et al. (2007) study who revealed the presence of mixed keratitis infection due to Acanthamoeba and Hartmannella (10). Hartmannella amoebae are also considered as suitable hosts for pathogenic microorganisms including Legionella pneumophila and Pseudomonas (16). Previous researches stated that Hartmannellid amoebae are an important growth factor for L. pneumophila (16). Indeed, various factors including the ability of Hartmannella to grow at temperatures above 40 0C and the isolation of this thermotolerant amoebae from amoebic keratitis patients emphasize that Hartmannella could be a potential pathogen for human (10). Moreover, according to previous researches it has been found that Hartmannella isolated from keratitis patient could lead to cytotoxicity on epithelial corneal cells (10). To this end, presence of Hartmannella in hot springs should be considered as health hazard.
The present study also reports the occurrence of Naegleria sp. based on molecular approaches. This is the first report of N. carteri in Iran. It is important to note that although the identified Naegleria are non-pathogenic and they have not isolated from clinical cases yet, but they could be a suitable host for pathogenic microorganisms (17). A previous research reported that N. pagei could coexict with pathogenic L. pneumophila (17). Indeed, non-pathogenic free living amoebae must consider as a carrier of pathogenic microbes (18). It should be noted that in one springs we have identified mixed (isolates: HSM1) amoebae belonging to Hartmannella and Naegleria. Filtration of this contaminated spring was not adequate for decontamination of water.
In conclusion, the result of the present study highlights an urgent need for improved filtration and disinfection and periodic monitoring of thermal waters in order to prevent disease related to thermotolerant free living amoebae.
Acknowledgments
The corresponding author was supported by a Research Grant from the National Elites Foundation for distinguished Young Assistant Professors. The present research was funded by the project # 89-01-91-7574 from the Shahid Beheshti University of Medical Sciences, Tehran. The authors declare that they have no conflicts of interest.
References
- 1.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free living amoebae: Acanthamoeba spp, Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea . FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Rezaeian M, Niyyati M. Pathogenic Free Living Amebas In Human. 1st ed. Tehran: TUMS Publication; 2010. [Google Scholar]
- 4.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Visvesvara GS, Sriram R, Qvarnstorm Y, Bandyopadhyay K, Silva A, Pina NJ, Cabral GA. Paravahlkampfia francinae n. sp. Masquerading as an agent of primary amoebic meningoencephalitis. J Eukaryot Microbiol. 2009;56:357–366. doi: 10.1111/j.1550-7408.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Marciano-Cabral F, Jamerson M, Kaneshiro ES. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010;8(1):71–82. doi: 10.2166/wh.2009.129. [DOI] [PubMed] [Google Scholar]
- 7.Jamerson M, Remmers K, Cabral G, Marciano-Cabral F. Survey for the presence of Naegleria fowleri amebae in lake water used to cool reactors at a nuclear power generating plant. Parasitol Res. 2009;104:969–978. doi: 10.1007/s00436-008-1275-y. [DOI] [PubMed] [Google Scholar]
- 8.De Jonckheere JF. Molecular definition and the ubiquity of species in the genus Naegleria . Protist. 2004;155:89–103. doi: 10.1078/1434461000167. [DOI] [PubMed] [Google Scholar]
- 9.Badirzadeh A, Niyyati M, Babaei Z, Amini H, Badirzadeh H, Rezaeian M. Isolation of Free-Living Amoebae from Sarein Hot Springs in Ardebil Province, Iran. Iranian J Parasitol. 2011;6(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzo-Morales J, Martínez-Carretero E, Batista N, Alvarez-Marín J, Bahaya Y, Walochnik J, Valladares B. Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella . Parasitol Res. 2007;102(1):167–9. doi: 10.1007/s00436-007-0754-x. [DOI] [PubMed] [Google Scholar]
- 11.Page FC. A New Key to Freshwater and Soil Gymnamoebae; Ambleside, UK: Freshwater Biological Association; 1988. [Google Scholar]
- 12.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Mohebali M, Maghsood AH, Motevalli-haghi A, Martín-Navarro CM, Farnia SH, Valladares B, Rezaeian M. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121(3):242–245. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Pelandakis M, Pernin P. Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment. Appl Environ Microbiol. 2002;68:2061–2065. doi: 10.1128/AEM.68.4.2061-2065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasjerdi Z, Niyyati M, Haghighi A, Shahabi S, Biderouni FT, Taghipour N, Eftekhar M, Nazemalhosseini Mojarad E. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res. 2011;109(3):575–80. doi: 10.1007/s00436-011-2288-5. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy SM, Devine P, Hurley C, Ooi YS, Collum LMT. Corneal infection with Hartmannella vermiformis in contact-lens wearer. Lancet. 1995;346:637–638. [PubMed] [Google Scholar]
- 16.Centeno M, Rivera F, Cerva L, Tsutsumi V, Gallegos E, Calderon A, Ortiz R, Bonilla P, Ramirez E, Suarez G. Hartmannella vermiformis isolated from the cerebrospinal fluid of a young male patient with meningoencephalitis and bronchopneumonia. Arch Med Res. 1996;27:579–586. [PubMed] [Google Scholar]
- 17.Huang SW, Hsu BM. Survey of Naegleria and its resisting bacteria-Legionella in hot spring water of Taiwan using molecular method. Parasitol Res. 2010;106:1395–1402. doi: 10.1007/s00436-010-1815-0. [DOI] [PubMed] [Google Scholar]
- 18.Huang SW, Hsu BM, Chen NH, Huang CC, Huang KH, Chen JS, Kao PM. Isolation and identification of Legionella and their host amoebae from weak alkaline carbonate spring water using a culture method combined with PCR. Parasitol Res. 2011 doi: 10.1007/s00436-011-2366-8. [DOI] [PubMed] [Google Scholar]