Abstract
Background
Infection with Trichomonas vaginalis is one of the most common sexually transmitted diseases (STDs) in humans. The prevalence of infection in Iran has been reported between 2 to 8%, depending on deferent socio-cultural conditions. This study aimed to determine the prevalence of T. vaginalis in women referred to gynecologic clinics in Hamadan city, West of Iran.
Methods
This descriptive cross-sectional study was conducted on 750 women who referred to Gynecologic clinics in Hamadan from November 2010 to July 2011. Vaginal samples were obtained from them and examined by wet mount and culture methods for the detection of T. vaginalis.
Results
Sixteen out of 750 vaginal swab specimens (2.1%) were culture positive for T. vaginalis and 13 of these positive specimens (1.7%) were wet mount positive. Only 12 of 42 patients who were clinically diagnosed as having T. vaginalis infection, confirmed by culture method. Five hundred and fifty of the participants women (73.3%) had at least one of signs and symptoms of trichomoniasis. No statistical correlation was observed between clinical manifestations and parasitological results (p>0.05).
Conclusion
This study showed low prevalence of T. vaginalis infection in the study population. Since clinical signs of trichomonal vaginitis are the same of other STDs, a confirmatory laboratory diagnosis is necessary. Wet smear as well as culture are sensitive for detection of T. vaginalis.
Keywords: Trichomonasvaginalis infection, Wet mount, Culture, Iran
Introduction
Trichomonas vaginalis is a flagellated protozoan that causes trichomoniasis in human, a sexually transmitted disease (STD) with worldwide (1). Trichomoniasis is usually concomitant with other STDs, particularly gonorrhae, and indicates high-risk sexual manner (2). Presumably, prevalence of infection with T. vaginalis in nonselected population of women is 5%-20% and it is related to the target population so that the highest estimates are referred to those attending the sexual disease clinics (3). In 2001, World Health Organization (WHO) has estimated more than 170 million people to be infected annually with T. vaginalis throughout the world (4).
In the United States, prevalence of T. vaginalis among sexually active women was appraised 2 to 3 million symptomatic infection annually (5). In Iran, various studies have determined the prevalence of trichomoniasis between 2% to 8% that according to the cultural and social status can also reach over 30% (6). Trichomoniasis is associated clinically with preterm birth, low birth weight, infertility, pelvic inflammatory disease (1, 7, 8) hepatitis viruses, Mycoplasma hominis (8), incidence of cervical cancer and increased risk of Human Immunodeficiency Virus (HIV) transmission (5, 7, 9, 10). Clinical features of trichomoniasis in women vary and it may be seen from the symptomatic to asymptomatic forms (11). The prevalence of trichomoniasis and clinical features in men are poorly understood and its complications can be pointed as prostatitis, balanoposthitis, epididymitis and infertility (11, 12).
Previous studies in Hamadan City have been conducted only on symptomatic women, but the main objective of this study was to determine the prevalence of T. vaginalis infection in both the symptomatic and the asymptomatic women.
Materials and Methods
This descriptive cross-sectional study was carried out on 750 of women in Fatemieh Hospital, Hamada and 9 government and private clinics in Hamadan City, west of Iran, from November 2010 to July 2011. All of the women came to the obstetrics and gynecologic clinics for receiving health care services or treatment of disease.
The referred women who had used vaginal agents and consumption of antibiotic during the past two weeks were excluded from the study. After obtaining informed consent from all referred individuals, demographic information such as age, education, occupation, husband's occupation, pregnancy, and clinical signs and symptoms including vaginal discharge, the color and consistency of discharge, itching, dysuria, dyspareunia, and inflammation of the genital tract were collected through interview and clinical examination by the gynecologist or midwife and the data were recorded in the questionnaires. Diagnostic methods were wet mount and culture technique, which the latter method was considered as the gold standard method (2, 11). Sampling was performed by two sterile cotton tipped swabs from vagina wall and dorsal fornix. One sample swab was put in a tube containing 0.5 ml of Ringer serum and the other was placed in liquid phase of Dorset medium (13). The sample tubes containing Ringer serum were transferred immediately to the laboratory in the clinics and subjected to wet mount examination using light microscope with low (100X) and high (400X) magnifications. Dorset culture medium was sent as soon as possible to the specialized laboratory of parasitology, Hamadan University of Medical Sciences and was incubated at 37 °C. After 24 h, a drop of sediment of the culture medium was removed and examined by direct smear. The culture medium was tested daily up to 7 days until they turned positive (14).
Chi-squared (χ2) and Fisher exact tests were used to compare T. vaginalis infection relative to clinical manifestations and parasitological results. Analyses were performed with SPSS (version 13.5; SPSS Inc, Chicago,IL, USA) and Epi-Info software, with a probability (P) value of <0.05 were considered as statistically significant.
Results
T. vaginalis was detected in 16 out of 750 participants (2.1%) (95% CI, 1.1%-3.1%) by using culture methods whereas only 13 of 16 infected people were positive with the wet mount technique (1.7%) (Table 1).
Table 1.
Detection of Trichomonas vaginalis in vaginal secretion by parasitological method
| Method | Positive n (%) | Negative n (%) | Total |
|---|---|---|---|
| Culture | 16 (2.1) | 734 (97.9) | 750 |
| Wet mount | 13 ( 1 .7) | 737 (98.3) | 750 |
Infected individual's age ranged from 25 to 46 years old with a mean of 34. The highest infection rate was in the age group 25-34 years (10/16, 62.5%) that was statistically significant (P<0.05). Fifteen of those infected were married housewives and one was widowed. More of the infected individuals with T. vaginalis (8 /16, 50%) had primary school education (P<0.05).
Forty-two women infected with T. vaginalis were detected by clinical examination whereas 12 of them were confirmed with culture and wet mount method. Four asymptomatic infected women were not identified by clinical examination. The sensitivity, specificity and positive and negative predictive value of clinical diagnosis compared to culture method were 75%, 95%, 28% and 99%, respectively. There was no statistical correlation between clinical and parasitological diagnosis methods.
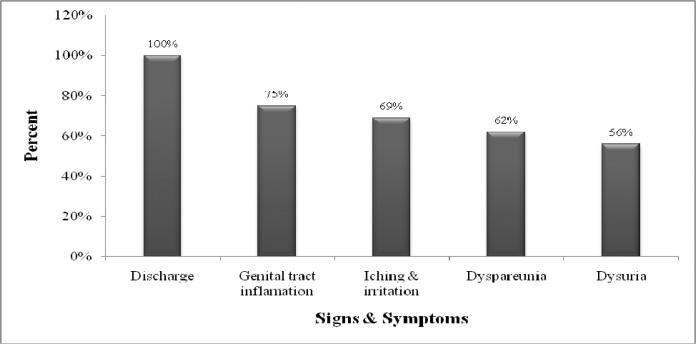
Candidiasis was observed in 113 women (15%), based on microscopic method using 10% Potassium hydroxide and gram stained smear. Five hundred and fifty (73.3%) of participants showed one or more signs and symptoms of trichomoniasis. The most common sign was vaginal discharge (71.3%). These signs and symptoms are not only specific for trichomoniasis, but also are common in all sexual transmitted disease. The most predominant signs and symptoms included vaginal discharge, itching, dysuria and inflammation of the genital tract, were observed (Fig. 1). No statistically correlation was observed between culture and wet mount for the detection of T.vaginalis infection.
Fig. 1.
The predominant clinical signs and symptoms of patients infected with Trichomonas vaginalis
Discussion
Trichomoniasis is the most common non-viral sexual infection that causes infection in genitourinary tract (15, 16). Infection is mainly transmitted through sexual activity, although non-sexual transmission is also reported (6). Almost 2%-17% of female infants may be infected by their mothers (11). In the United States, the prevalence of trichomoniasis has been estimated at 25% in those referred to STDs clinics and it is higher in certain population groups such as African American women (38%) (2). Some studies have mentioned a 25% or higher infection rate in Africa (2). For instance, the prevalence of trichomoniasis in HIV-positive women in Zaire and pregnant women in a rural area in South Africa is estimated 38% and 65%, respectively (1, 2). In Islamic countries, the prevalence of trichomoniasis ranges from 1.2% in Libya and Jordan, 3.2% in Turkey to 28.1% in Saudi Arabia (17–20).
Like other parts of the world, the reports of trichomoniasis in Iran are different. For example, the prevalence of trichomoniasis in some areas of Iran include: 9.2% in Tabriz (21), 3.2% in Tehran (22), 4% in Babol (23), 2% in Yazd (24), 5.6% in Mashhad, 1.37% in Charmahal Bakhtiari province, 17% in Zahedan and 10.7% in Bandar Abbas (25). In this study, prevalence of trichomoniasis was 2.1% by using the culture method as the gold standard. The result of this study is similar to two other studies conducted in Hamadan in 2005 (3%) (26) and 2007 (2.2%) (25), but is different from the study in 2006 (18.1%) (27). Difference in results may be due to the selection of different population groups. Therefore, it is necessary to pay attention to special population sub-groups in certain communities, because of the wide variety in prevalence rates of trichomoniasis in the world. Diagnosis of trichomoniasis based on only clinical symptoms should not be done due to the two reasons. First, clinical symptoms of trichomoniasis may be similar to those of other STDs. Second, clinical symptoms such as strawberry cervix and spumy discharge are seen in 2% and 12% of T. vaginalis infected patients, respectively (11). Some studies have indicated that diagnosis based solely on clinical examination show 88% false negative and 29% false positive (11). As the most important point of this study, 73.3% of participants had clinical signs and symptoms. Forty-two of them were clinically diagnosed infected with T. vaginalis, in which only 12 were confirmed by culture technique. Also 4 of the infected patients were asymptomatic, which were not diagnosed by clinical examination. In this study, positive predictive value for clinical diagnosis of T. vaginalis infection was 28%, so the use of the laboratory diagnosis methods is necessary. The most common method for diagnosis of T. vaginalis is wet mount, but its sensitivity has been reported between 38% and 82%. Molecular method based on PCR is an accurate method with a sensitivity of 80% to 100%, but it is not routine in all laboratories (2, 11, 28).
In conclusion, the results of this study shows the prevalence of T. vaginalis infection in the study population is relatively low and other causes of vaginitis such as bacterial and fungal infections should be more consideration. Since clinical signs of trichomonal vaginitis are the same of other STDs, a confirmatory laboratory diagnosis is necessary. Further studies in different population groups are needed to determine other aspects of epidemiology of this infection in Iran.
Acknowledgments
This investigation was financially supported by Tehran University of Medical Sciences (Project no: 90-02-27-11738). The authors thank all the clinicians and technicians of Hamadan University of Medical Sciences for helping with physical examination and the preparation of vaginal samples. The authors declare that they have no conflicts of interest.
References
- 1.Ali V, Nozaki T. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin Microbiol Rev. 2007;20(1):164–87. doi: 10.1128/CMR.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17(4):794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess DE. Trichomonas infection. Topley & Wilsone′s Microbiology & Microbial Infection, Parasitology. In: Cox FEG, Gillespie SH, Despommier DD, editors. London: Hodder Arnold; 2005. p. 261. [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: WHO; 2001. Global prevalence and incidence of selected curable sexually transmitted infections. [Google Scholar]
- 5.Jhon DT, Petri WA. 9th ed. St.Louis: Sanders; 2006. Markell and Voge′s Medical Parasitology. [Google Scholar]
- 6.Edrissian GH, Rezaeian M, Ghorbani M, Keshavarz H, Mohebali M. 1st ed. Tehran University of Medical Sciences; 2007. Medical Protozoology. [Google Scholar]
- 7.Wright JM, Dunn LA, Kazimierczuk Z, Burgess AG, Krauer KG, Upcroft P, Upcroft JA. Susceptibility in vitro of clinically met-ronidazole- resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2- fluoro-2'-deoxyadenosine. Parasitol Res. 2010;107(4):847–53. doi: 10.1007/s00436-010-1938-3. [DOI] [PubMed] [Google Scholar]
- 8.Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, Song LY, Zhong HM, Lun ZR. Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol Res. 2006;100(1):123–30. doi: 10.1007/s00436-006-0215-y. [DOI] [PubMed] [Google Scholar]
- 9.Meri T, Jokiranta TS, Suhonen L, Meri S. Resistance of Trichomonas vaginalis to metronidazole: report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J Clin Microbiol. 2000;38(2):763–7. doi: 10.1128/jcm.38.2.763-767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowell AL, Sanders-Lewis KA, Secor WE. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis . Antimicrob Agents Chemother. 2003;47(4):1407–9. doi: 10.1128/AAC.47.4.1407-1409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis . Clin Microbiol Rev. 1998;11(2):300–17. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP, Garber GE. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis . Clin Microbiol Rev. 2004;17(4):783–93. doi: 10.1128/CMR.17.4.783-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashirybod H. 1st ed. Tehran, Iran: Tehran University of Medical Science; 1988. Human Parasitic Infection. [Google Scholar]
- 14.World Health Organization. 1st ed. Geneva: World Health Organization; 1991. Basic laboratory methods in medical parasitology. [Google Scholar]
- 15.Khan NA. Emerging Protozoan Pathogenes. 1st ed. Taylor & Francis group; 2008. [Google Scholar]
- 16.Conrad M, Zubacova Z, Dunn LA, Upcroft J, Sullivan SA, Tachezy J, Carlton JM. Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol. 2011;175:30–38. doi: 10.1016/j.molbiopara.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassem HH, Majoud OA. Trichomoniasis among women with vaginal discharge in Benghazi city, Libya. J Egypt Soc Parasitol. 2006;36(3):1007–16. [PubMed] [Google Scholar]
- 18.Mahafzah AM, Al-Ramahi MQ, Asa'd AM, El-Khateeb MS. Prevalence of sexually transmitted infections among sexually active Jordanian females. Sex Transm Dis. 2008;35(6):607–10. doi: 10.1097/OLQ.0b013e3181676bbd. [DOI] [PubMed] [Google Scholar]
- 19.Selvitopu A, Ozcelik S, Degerli S. The incidence of Trichomonas vaginalis in vaginal specimens from gynecologic patients. Turkiye Parazitol Derg. 2006;30(3):175–7. [PubMed] [Google Scholar]
- 20.Madani TA. Sexually transmitted infections in Saudi Arabia. BMC Infect Dis. 2006;6:3. doi: 10.1186/1471-2334-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazloumi Gavgani A-S, Namazi A, Ghazanchaei A, Alizadeh S, Sehhati F, Rostamzadeh S, Dolatkhah A. Prevalence and risk factors of trichomoniasis among women in Tabriz. Iranian Journal of Clinical Infectious Diseases. 2008;3(2):67–71. [Google Scholar]
- 22.Rezaeian M, Vatanshenassan M, Rezaie S, Mohebal M, Niromand N, Niyyati M, Farnia S, Babaei Z. Prevalence of Trichomonas vaginalis Using Parasitological Methods in Tehran. Iranian J Parasitol. 2009;4(4):43–47. [Google Scholar]
- 23.Bakhtiari A, Hajian-Tilaki K, Pasha H. Genital infection by Trichomonas Vaginalis in women referring to Babol health centers: prevalence and risk factors. IRCMJ. 2008;10(1):16–21. [Google Scholar]
- 24.Fattahi Bafghi A, Aflatoonian A, Barzegar K, Ghafourzadeh M, Nabipour S. Frequency distribution of trichomoniasis in pregnant women referred to health centers of Ardakan, Meibod and Yazd, Iran. Jundishapur Journal of Microbiology. 2009;2(4):132–139. [Google Scholar]
- 25.Rabieea S, Fallahb M, Zahabic F. Frequency of Trichomoniasis in Patients Admitted To Outpatient Clinics in Hamadan (2007) and relationship between Clinical Diagnosis and Laboratory Findings. JRHS. 2010;10(1):31–35. [PubMed] [Google Scholar]
- 26.Habibi-Pour R, Amir-Khani A, Matin-Nia N. Study of Trichomonas vaginalis infection in women attending Tamin-Ejtemaee hospital in Hamadan infection in women attending Tamin-Ejtemaee hospital in Hamadan. Sciences. 2006;8(4):9–15. [Google Scholar]
- 27.Shobeiri F, Nazari M. A prospective study of genital infections in Hamadan,Iran. Southeast Asian J Trop Med Public Health. 2006;37(3):174–177. [PubMed] [Google Scholar]
- 28.Vatanshenassan M, Rezaie S, Mohebali M, Niromand N, Kazemi B, Babaei Z, Rezaeian M. Trichomonas vaginalis: investigation of a novel diagnostic method in urine samples using cysteine proteinase 4 gene and PCR technique. Exp Parasitol. 2010;126(2):187–90. doi: 10.1016/j.exppara.2010.04.021. [DOI] [PubMed] [Google Scholar]