Abstract
We cloned a rat vascular chymase (RVCH) from smooth muscle cells (SMCs) that converts angiotensin I to II and is up-regulated in SMC from spontaneously hypertensive vs. normotensive rats. To determine whether increased activity of RVCH is sufficient to cause hypertension, transgenic mice were generated with targeted conditional expression of RVCH to SMC, with the use of the tetracycline-controlled transactivator (tTA). We confirmed conditional expression of RVCH by mRNA, protein, and chymase activity in the absence, but not in the presence, of dietary doxycycline. The systolic blood pressure (mmHg), measured by carotid artery cannulation at 10–12 weeks of age, was higher in tTA+/RVCH+ mice than in nonbinary transgenic littermates (136 ± 4 vs. 109 ± 3) (P < 0.05), as were the diastolic and mean pressures. Hypertension was completely reversed by doxycycline, suggesting a causal link with chymase expression. Medial thickening of mesenteric arteries from tTA+/RVCH+ mice vs. littermates (0.82 ± 0.1 vs. 0.42 ± 0.02) (P < 0.05) was associated with increased SMC proliferation, as judged by positive immunoreactivity, with the use of an antibody to the proliferating cell nuclear antigen. These structural changes were prevented by doxycycline. Perfusion myography of mesenteric arteries from tTA+/RVCH+ mice also revealed increased vasoconstriction in response to phenylephrine and impaired metacholine-induced vasodilatation when compared with littermate controls or with the doxycyline-treated group. Our studies suggest that up-regulation of this vascular chymase is sufficient to cause a hypertensive arteriopathy, and that RVCH may be a candidate gene and a therapeutic target in patients with high blood pressure.
Keywords: smooth muscle cells
Chymases are serine proteinases that were thought to be produced exclusively by mast cells in blood vessels and the myocardium. These enzymes generate angiotensin (Ang) II in large and resistance vessels (1–3) and in the heart (4) and also can convert big-endothelin-1 to endothelin-1 in vitro (5) and inactivate the vasodilator bradykinin (6). That chymase-dependent Ang II formation can occur in the intact animal is evident from studies in the baboon, where it was shown that Ang-converting enzyme inhibitors could not prevent the hemodynamic response to [Pro11, Da1a12]Ang I, which produces Ang II upon incubation with chymase (7). In addition to blood pressure regulation, both Ang II and endothelin-1 are involved in the stimulation of vascular smooth muscle cell (SMC) growth and proliferation, vascular remodeling, and cardiac hypertrophy (8, 9). Chymases also can influence structural remodeling through their ability to degrade extracellular matrix proteins, including fibronectin and collagen type IV, possibly through activation of matrix metalloproteinases (MMPs) (MMP-1, MMP-3, and MMP-9) (10–12). Chymases also stimulate collagen fibirillogenesis by cleavage of type I procollagen (13).
Several studies have provided compelling data to suggest that chymases could play a central role in cardiovascular diseases. For example, chymase mRNA levels and chymase-like activity are increased ≈10-fold and ≈5-fold, respectively, in the balloon injured dog carotid artery (14, 15). Tranilast (a stabilizer of mast cells) completely prevented the increase in chymase-like activity, reduced the chymase mRNA levels by ≈40%, and decreased the carotid intima/media ratio by ≈60%. Increased numbers of mast cells and enhanced chymase immunoreactivity and mRNA levels have been documented in atherosclerotic lesions (3, 16, 17). Inhibition of chymase activity also has been shown to reduce neointima formation in dog vein grafts (18). Although most previous research has suggested mast cells as the source of chymase, it is also possible that vascular SMC could produce the enzymatic activity. Our group just recently cloned a cDNA encoding a rat vascular chymase (RVCH) from SMC. We have shown that the recombinant RVCH converts Ang I to Ang II in vitro. Moreover, enhanced expression of this chymase was demonstrated in vascular SMC from spontaneously hypertensive compared with normotensive rats (19).
These studies suggest that this enzyme might be the cause of a genetic form of hypertension. To determine whether overexpression of vascular chymase (RVCH) would be sufficient to cause systemic hypertension, we generated transgenic mice with the use of an arterial SMC-restricted (SM22α promoter-driven) tetracycline-controlled transactivator (tTA) to effect conditional expression of a tTA-dependent transgene encoding RVCH. Blood pressure was elevated and was doxycycline-reversible in tTA+/RVCH+ vs. nonbinary transgenic mice. Medial thickening of mesenteric arteries was associated with SMC proliferation, and perfusion myography revealed heightened phenylephrine-induced vasoconstriction and impaired metacholine-induced vasodilatation.
Materials and Methods
Epitope Tagging of RVCH and Construction of Conditional Vectors.
To distinguish recombinant RVCH from endogenous RVCH, the hemagglutinin (HA) tag (YPYDVPDYA), a peptide from human influenza HA protein (20), was added to the C terminus of mature RVCH. A total of four PCR primers were used to add HA: primer 1, 5′-GCGGGATCCGAGAAGCTCACCCCAGGCTGC-3′; primer 2, 5′-TCAAGCGTAGTCTGGGACGTCGTATGGGTAGCTACTTTCCCTTAAGACTGT-3′; primer 3, 5′-TACCCATACGACGTCCCAGACTACGCTTGAAAAGCTTGACCTGCCTGC-3′; primer 4, 5′-CGCTCTAGAGGAAATTATGTCTTTATTGAGGTG-3′. (The HA sequence is underlined.) The final PCR product was cloned into the tetracycline-responsive (Tet-Off) vector pTRE2 (CLONTECH) at the BamHI/XbaI sites (pTRE2-RVCH-HA). The construct obtained was sequenced to verify the integrity of the reading frame and the fidelity of the sequence.
Cell Culture and Transfection.
The rat A10 SMC line was obtained from the American Type Culture Collection, and the CHO-AA8 Tet-Off cell line was purchased from CLONTECH. A10 and CHO-AA8 Tet-Off cells were cultured in DMEM and α-MEM (100 μg/ml G418), respectively, containing 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml fungizone. All cells were transiently or stably transfected with SuperFect or Effectene (Qiagen, Mississauga, ON), following the manufacturer's instructions. A10 Tet-Off cells were produced by stably integrating the pTet-Off-tTA vector into the genome after selection with G418. These cells, as well as the CHO-AA8 Tet-Off cells, were then transiently transfected with (pTRE2-RVCH-HA). Conditional expression of RVCH was assessed by Western immunoblots of lysates from cells treated with or without doxycycline (1 ng/ml) (CLONTECH). A pTRE2 vector without insert was used to transfect A10 pTet-Off -tTA and CHO-AA8 Tet-Off cells as a control.
Western Blotting.
A10 Tet-Off and CHO-AA8 Tet-Off cells were harvested after transfection, and cell lysates were prepared in 20 mM Tris⋅HCl containing 0.5 mM EDTA, 1 μg/ml leupeptin, and 0.5 μg/ml pepstatin A. Twenty micrograms of protein was resolved on a precast 8–16% polyacylaminde Tris-glycine gel (NOVEX, San Diego) and transferred to a poly(vinylidene difluoride) membrane. After blocking, membranes were incubated with high-affinity rat monoclonal antibody against HA (clone 3F10, 1:1,000; Roche Molecular Biochemicals). The membranes were washed and incubated with anti-rat IgG secondary antibody conjugated with peroxidase (1:10,000). The target protein was detected and visualized by enhanced chemiluminescence (ECL kit; Amersham Pharmacia) according to the manufacturer's instructions.
Generation of Transgenic Mice and Genotyping.
Two transgenic mouse lines were generated. The first transgenic line was produced in an SJL/B6 background (Charles River Breeding Laboratories) by a standard technique for microinjection of pTRE2-RVCH-HA. The second transgenic mouse line was produced in a C57/B6 background with the use of microinjection of tTA under the control of a 3-kb SM22α promoter (a kind gift of Eric N. Olson, University of Texas Southwestern Medical Center, Dallas) (ref. 21 and Fig. 1). The pTRE2-RVCH-HA transgenic mouse line was then back-crossed with C57/B6, and the resulting F3 offspring were bred with the SM22a-tTA mouse line to specifically activate expression of RVCH in SMC. Thus, RVCH expression is expected only in binary animals (tTA+/RVCH+) that have both tTA and RVCH, but not in single animals that have only tTA or RVCH. To suppress RVCH expression, doxycycline was given at 200 μg/ml in drinking water bottles containing 2.5% sucrose and wrapped with aluminum foil. The water containing doxycycline was changed every 3 days to avoid precipitation. Genotyping of transgenic mice was initially carried out with the use of Southern blot and dot blot. These analyses correlated well with PCR, which subsequently was used to genotype the mice. Genomic DNA was extracted from the tail with phenol/chloroform. For Southern blot analysis, 8 μg of DNA was digested with BamHI. The digested DNA was separated on a 0.8% agarose gel, transferred to a Hybond-N+ membrane, and hybridized with the 32P-labeled 3-kb pTRE2-RVCH-HA construct. Undigested DNA was used for dot blot analysis with the same construct. Hybridization was performed with QuickHyb (Stratagene) according to the manufacturer's instructions. Specific primers were designed for amplification of RVCH and tTA by PCR. To amplify RVCH, the forward primer was 5′-GAGGCCTGTAAAATCTATAGAC-3′ and the reverse primer was 5′-TGTGTATCTTTGAGAGCCTCAA-3′. To amplify tTA, the forward primer was 5′-GCTGCTTAATGAGGTCGG-3′, and the reverse primer was 5′-CTCTGCACCTTGGTGATC-3′. The conditions used for PCR included denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 45 s, 57°C for 45 s, and 72°C for 1 min.
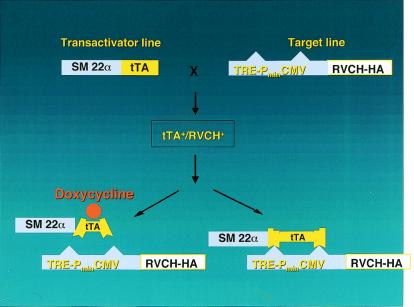
Figure 1.
To archive conditional and targeted expression of RVCH in SMC of transgenic mice, we used the Tet-Off binary system. A transgenic mouse line was made in which the RVCH-HA construct is controlled by a compound promoter (TRE-PminCMV) that is silent without binding of the tetracycline transactivator (tTA). A second transgenic mouse line was engineered to express the tTA under the control of an arterial SMC-specific promoter, SM22α. When the two lines are bred, binary transgenic mice, tTA+/RVCH+, are generated in which RVCH is only expressed in arterial SMC. To conditionally turn off RVCH expression, doxycyline, which changes the conformation of tTA, was administered to the mice in their drinking water.
Rat Vascular Chymase Expression by RT-PCR.
To assess whether RVCH expression was confined to arteries, RT-PCR was carried out on different tissues in addition to the aorta, including heart, lung, kidney, bladder, stomach, and intestine, from 10- to 12-week-old tTA/RVCH mice and nonbinary littermates. A subgroup of binary mice were on doxycycline (200 μg/ml) for 1 week before RNA extraction. Total RNA was extracted with the use of Trizol (GIBCO/BRL), according to the manufacturer's instructions. First-strand cDNA was synthesized with the SuperScript Preamplification system (GIBCO), followed by PCR amplification of RVCH. The PCR conditions were the same as those described above.
Immunoprecipitation of RVCH.
A total of 10 pooled aortae from tTA+/RVCH+ or control mice of age 10–12 weeks or a subgroup of age-matched binary mice on doxycycline (200 μg/ml) for 1 week were homogenized with a Pellet pestle motor (VWR Canlab, Mississauga, ON) in 20 mM Tris⋅HCl containing 0.5 mM EDTA, 1 μg/ml leupeptin, and 0.5 μg/ml pepstatin A. HA-tagged RVCH was immunoprecipitated with the use of a kit from Roche Molecular Biochemicals, according to the manufacturer's instruction. A high-affinity anti-HA mAb (2 μg/ml) was incubated with the tissue homogenates. Protein G agarose was then added, and immune complexes were collected by centrifugation and subjected to Western blot analysis.
Explant of Aorta and Chymase Activity Assay.
Smooth muscle cells were obtained from explants of the aorta derived from 10-week-old mice. Culture conditions were similar to those described for A10 cells, and cells were assayed at passage 2 for chymase activity. To test the conditional regulation of RVCH activity, cells were cultured in the absence or presence of 1 ng/ml doxycycline. The SMC were disrupted in 20 mM Tris⋅HCl buffer (pH 8.0) containing 0.5 mM EDTA, 1 μg/ml leupeptin, and 0.5 μg/ml pepstatin A. A synthetic fluorescent substrate, Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin, was used to measure chymase activity in the lysates, defined as fluorescent units/min per mg protein (22).
Blood Pressure Measurement.
At age 10–12 weeks, transgenic mice from binary or single groups were randomized for blood pressure measurement, to avoid bias due to circadian variation. Equal numbers of male and female mice were included in each group. Briefly, mice were anesthetized with ketamine and xylazine (100 mg/kg and 10 mg/kg, i.p.) and placed in a supine position. The right carotid artery was prepared for catheterization with a 1.4-F high-fidelity micromanometer Millar catheter (model SPR-671; Millar Instruments, Houston, TX). The catheter was advanced into the carotid artery, and systemic blood pressure was recorded when a stable reading was achieved (generally within 5–10 min). Then the catheter was further advanced into the left ventricular cavity to record left ventricular pressures for the calculation of ±dP/dtmax. In the doxycycline treatment group, doxycycline (200 μg/ml) was administered in the drinking water beginning at 6 weeks of age and continued until blood pressure measurement was performed.
Morphometric Assessment.
Mice were fixed in situ by the injection of 4% paraformaldehyde through the left ventricle. The mesenteric artery was harvested and fixed in 4% paraformaldehyde and subsequently embedded in paraffin. Ten-micrometer sections were stained by Movat's pentachrome method. Medial and lumen area were measured with the use of the image pro-plus program (Media Cybernetics, Silver Spring, MD).
Immunohistochemical Staining.
The mesenteric arteries from 10- to 12-week-old tTA+/RVCH+ and control mice were fixed with 4% paraformaldehyde and embedded in paraffin for immunohistochemical staining. After blocking with normal serum, tissue sections were then incubated with a mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (1:100; Amersham Pharmacia). Sections were stained by an immunoperoxidase method with the use of a Vectastain ABC kit (Vector Laboratories) according to the manufacturer's instructions. Sections were counterstained with eosin. To ensure the specificity of the staining, normal IgG was used as a negative control, and the primary antibody was preincubated with the antigen.
Pressure Myography.
Mesenteric arteries were dissected and placed immediately in ice-cold physiological salt solution (PSS) containing (in mmol/liter) 130 NaCl, 14.9 NaHCO3, 10.0 glucose, 4.70 KCl, 1.17 MgSO4, 1.18 KH2PO4, 1.60 CaCl2, and 0.027 EDTA (pH 7.4) and aerated with 12% O2, 5% CO2, and 83% N2. Mesenteric arteries were mounted in a pressure myograph (Living Systems Instrumentation, Burlington, VT), and their diameters were measured by video microscopy. All experiments were performed at 37°C. Mesenteric arteries were pressurized to 60 mm Hg and allowed to equilibrate for 30 min, with the physiological salt solution refreshed every 10 min. After equilibration, a basal diameter was obtained. To assess contractile responses in mesenteric arteries, a cumulative dose–response curve for phenylephrine (1 nmol/liter to 30 μmol/liter) was plotted. To assess metacholine-mediated relaxation, mesenteric arteries were submaximally precontracted with phenylephrine (1 μmol/liter), and a cumulative dose–response curve for metacholine (1 nmol/liter to 10 μmol/liter) was plotted. There was a 3- to 5-min equilibration period between doses.
Statistical Analysis.
All data are expressed as means ± SEM. The number of animals in each group for each measurement is given in the figure legends. The differences between groups were compared with the use of one-way ANOVA followed by a Bonferroni analysis. A P value < 0.05 was considered statistically significant.
Results
Targeted and Conditional Expression of Vascular Chymase in Cultured Cell Lines.
We first tested the construct for conditional expression of RVCH in A10 Tet-Off and CHO-AA8 Tet-Off cells. Western blot analysis, with high-affinity rat monoclonal anti-HA antibody, detected a band at the predicted molecular mass of 31 kDa in both A10 and CHO Tet-Off cells (data not shown). We confirmed that the addition of the HA tag dose did not affect chymase activity (data not shown). Doxycycline completely abolished the expression of chymase in these cells, indicating successful conditional regulation of expression. No HA-tagged chymase protein was detectable in transfected A10 cells without the tTA, indicating that there is no leak of RVCH expression.
Generation and Cross-Breeding of Transgenic Mice.
As illustrated in Fig. 1, we bred heterozygous RVCH with heterozygous tTA mice and observed the anticipated Mendelian inheritance pattern; i.e., 25% of the offspring (61/244 tested) were binary transgenic tTA+/RVCH+. This result also indicates lack of fetal toxicity related to the chymase transgene. The litter size of offspring was also normal and similar in all groups (8–10 pups). The body weight of tTA+/RVCH+ mice was similar to that of littermates, further indicating that chymase expression did not outwardly impede fetal development and growth.
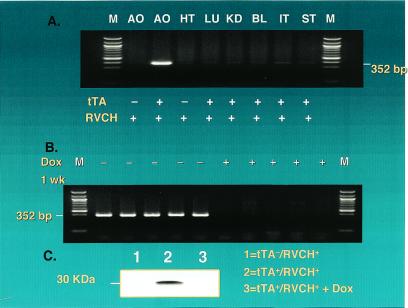
To determine whether RVCH expression was selectively targeted to vascular SMC, RT-PCR was performed with the use of RNA from different tissues. RVCH transcripts were detected only in aorta from tTA+/RVCH+ transgenic mice, but not in heart, lung, kidney, bladder, intestine, and stomach (Fig. 2A). The expression of RVCH was completely turned off when it was assessed 1 week after doxycycline treatment was started in tTA+/RVCH+ mice, indicating conditional regulation of RVCH (Fig. 2B). This conditional regulation of chymase was further confirmed at the protein level by immunoprecipitation of RVCH with the use of a high-affinity antibody against the HA tag (Fig. 2C). Furthermore, SMC from tTA+/RVCH+ mice showed elevated chymase activity compared with nonbinary control mice, which was also conditionally regulated by doxycycline (Fig. 3).
Figure 2.
Conditional and targeted expression of RVCH. (A) Targeted expression of RVCH was assessed by RT-PCR as described in Materials and Methods. RVCH mRNA was found mainly in aorta (AO) from tTA+/RVCH+ mice but not in AO from littermate controls. RVCH mRNA is below the level of detection in heart (HT), lung (LU), kidney (KD), bladder (BL), intestine (IT), and stomach (ST). (B) To confirm the conditional expression of RVCH in transgenic mice, we used RT-PCR to show that RVCH mRNA expression was repressed by feeding the transgenic mice doxycycline (Dox) (200 μg/ml) in the drinking water for 1 week. (C) A total of 10 aortae from each group were pooled and subjected to immunoprecipitation, with the use of high-affinity rat mAb against the HA-tagged RVCH. RVCH protein was detected in tTA+/RVCH+ mice but not in tTA−/RVCH+ mice. RVCH expression was completely abolished in Dox-treated tTA+/RVCH+ mice.
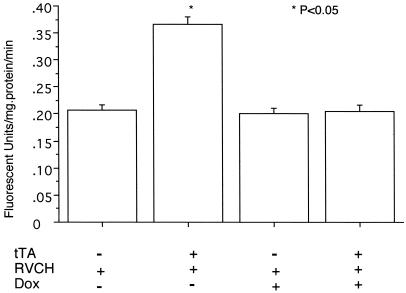
Figure 3.
Conditional regulation of RVCH enzyme activity in SMC. Chymase activity was measured using a specific fluorogenic substrate, Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin. Chymase activity was significantly higher in SMC from chymase-overexpressing mice (tTA+/RVCH+) when compared with littermate controls (tTA−/RVCH+). Doxycycline (Dox) (200 μg/ml) completely reversed the increase in chymase activity in tTA+/RVCH+ mice and had no effect on chymase activity in SMC from tTA−/RVCH+ mice. The data are depicted as means ± SEM of six independent experiments. *, P < 0.05 vs. all other groups.
Blood Pressure and Heart Function.
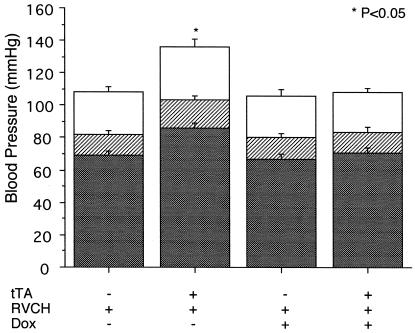
Systolic, diastolic, and mean arterial blood pressures were measured in ketamine/zylazine-anesthetized mice by right carotid artery cannulation with a Millar catheter. Mice from the four different groups were randomized, and blood pressure was measured by a single experienced investigator. Systolic blood pressure was increased by an average of 27 mmHg in tTA+/RVCH+ mice compared with littermate controls. Similarly, a 24% increase in diastolic blood pressure was found in binary vs. single transgenic mice (Fig. 4). Mean arterial pressure was increased from 82 mmHg in nonbinary control mice to 103 mmHg in tTA+/RVCH+ mice. Blood pressure was similar in single mice compared with nontransgenic littermates, suggesting that the hypertension in the binary mice is not caused by insertion of RVCH or tTA. Doxycycline treatment had no effect on blood pressure in single mice, but it completely reversed blood pressure to normal levels in tTA+/RVCH+ mice, indicating a causal link between chymase expression and hypertension. There were no significant differences in +dP/dtmax (6,495 ± 721 vs. 6,363 ± 975 mmHg/s) or in −dP/dt (6,026 ± 700 vs. 6,070 ± 665 mmHg/s) in tTA+/RVCH+ mice when compared with littermate controls. The heart rate was also similar in tTA+/RVCH+ mice (330 ± 17 beats per min) when compared with littermate controls (329 ± 16 beats per min). A modest increase in the left ventricle to body weight ratio was observed in chymase-overexpressing mice (3.53 ± 0.04) when compared with littermate controls (3.31 ± 0.02) (P < 0.05).
Figure 4.
Blood pressure (mmHg) was measured with the use of Millar catheter cannulation of the right carotid artery of transgenic mice at age 10–12 weeks. Systolic blood pressure is shown as the open bar, diastolic blood pressure as the solid bar, and mean arterial pressure as the striped bar. Elevation in all three values was observed in the tTA+/RVCH+ group relative to the control littermates. In the subgroup of tTA+/RVCH+ mice given doxycycline (Dox) in the drinking water at 200 μg/ml from 6–12 weeks of age, values were indistinguishable from those for control littermates ± Dox. Values are depicted as means ± SEM of 8–20 independent experiments. *, P < 0.05 vs. all other groups.
Medial Thickening of Mesenteric Artery.
We next determined whether the elevated blood pressure attributed to chymase expression was associated with structural changes in the blood vessel wall, as described in Materials and Methods. The medial to lumen ratio was increased by 93% in tTA+/RVCH+ binary mice compared with that of single mice, e.g., tTA−/RVCH+ (Fig. 5). We also found that there was abundant expression of PCNA-positive cells in the medial layer of the mesenteric artery from tTA+/RVCH+ mice, indicating that proliferation of SMC was likely contributing to the medial thickening (Fig. 6b). The increase in the media to lumen ratio, as well as the PCNA positivity, was completely reversed by doxycycline treatment, indicating that these structural abnormalities are the result of chymase expression. We also examined the aortae from these mice and determined that there was a similar increase in the media to lumen ratio in the binary transgenic compared with the single mice, but the numbers of elastic laminae were similar (data not shown).
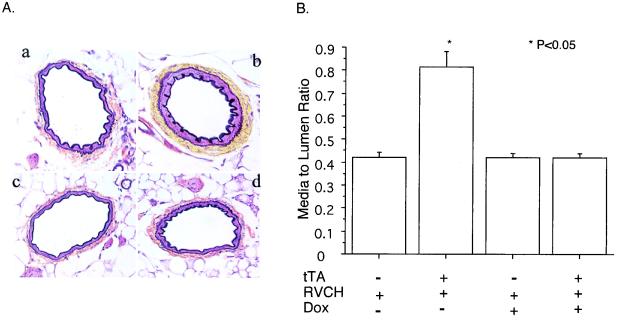
Figure 5.
Medial thickening in the mesenteric artery of 10- to 12-week-old mice. (A) A representative Movat's pentachrome staining shows medial thickening of the mesenteric artery from tTA+/RVCH+ mice. (a) tTA−/RVCH+; (b) tTA+/RVCH+; (c) tTA−/RVCH+ with doxycycline (Dox);. (d) tTA+/RVCH+ with Dox. (B) Quantification of media to lumen ratio revealed a 93% increase in the mesenteric artery from chymase-overexpressing mice compared with littermate controls. Medial thickening of the mesenteric artery was completely prevented by feeding Dox to chymase-overexpressing mice at 200 μg/ml in drinking water from 6–10 weeks of age. Dox had no effect on the media to lumen ratio in littermate controls. The data are depicted as means ± SEM of 8–10 independent experiments. *, P < 0.05 vs. all other groups.
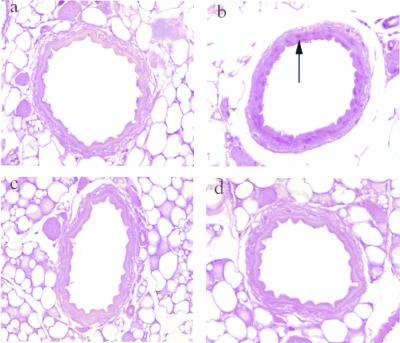
Figure 6.
Immunohistochemical staining with the use of an antibody against proliferating cell nuclear antigen (PCNA). (a) tTA−/RVCH+. (b) tTA+/RVCH+. (c) tTA−/RVCH+ with doxycycline (Dox). (d) tTA+/RVCH+ with Dox. We found abundant PCNA-positive cells (arrow) in the medial layer of the mesenteric artery from chymase-overexpressing mice (tTA+/RVCH+), whereas PCNA positivity was rare in the control littermates, or in either group after Dox from 6–12 weeks of age.
Functional Analysis of the Mesenteric Artery.
To determine whether the structurally abnormal vessels from the binary transgenic mice were also functionally abnormal, perfusion myography was used to study the response to phenylephrine and metacholine in the isolated mesenteric artery (100–200 μm). Phenylephrine-mediated constriction was significantly enhanced in mesenteric arteries obtained from tTA+/RVCH+ mice, as compared with single mice, e.g., tTA−/RVCH+ (Fig. 7). The phenylephrine EC50 concentrations for tTA+/RVCH+ and tTA−/RVCH+ mice were 36 ± 8 and 131 ± 19 nM, respectively. This enhanced phenylephrine-mediated constriction in tTA+/RVCH+ mice was not observed after a 6-week doxycycline treatment, and the EC50 for phenylephrine in doxycycline-treated tTA+/RVCH+ mice was 183 ± 21 nM. To assess endothelium-dependent relaxation, mesenteric arteries from all four groups were similarly submaximally preconstricted with phenylephrine (1 μM). Metacholine-mediated relaxation was impaired in mesenteric arteries from tTA+/RVCH+ compared with tTA−/RVCH+ mice (Fig. 8). This impairment in metacholine-mediated relaxation was abolished in tTA+/RVCH+ mice receiving doxycycline. This impairment in metacholine-mediated relaxation appears to be endothelial-specific, inasmuch as a single dose of sodium nitroprusside (10 μM) mediated similar complete relaxation in tTA+/RVCH+, tTA−/RVCH+, and doxycycline-treated tTA+/RVCH+ mice (106 ± 3%, 97 ± 6%, and 96 ± 3%, respectively).
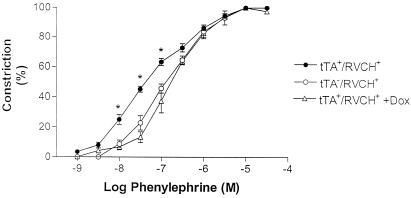
Figure 7.
Phenylephrine-induced constriction in isolated mesenteric arteries from transgenic mice. Dose-dependent phenylephrine-induced vasoconstriction was observed in tTA−/RVCH+ mice. Heightened reactivity in response to phenylephrine is observed in chymase-overexpressing mice (tTA+/RVCH+) and is completely prevented by doxycycline (Dox) treatment (200 μg/ml). The data are depicted as means ± SEM of 4–5 independent experiments. *, P < 0.05 vs. all other groups.
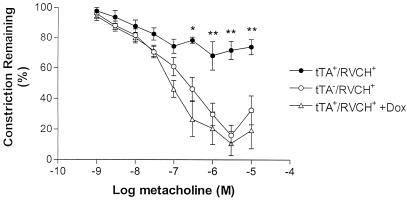
Figure 8.
Metacholine-mediated relaxation in phenylephrine-preconstricted mesenteric arteries in transgenic mice. Metacholine induced dose-dependent relaxation in the mesenteric artery from control tTA−/RVCH+ mice. Impaired vasodilatation was observed in the mesenteric artery from chymase-overexpressing mice (tTA+/RVCH+) when compared with littermate controls. Doxycycline (Dox) (200 μg/ml) completely abrogated the impaired vasodilatation response of tTA+/RVCH+ mice. The data are depicted as means ± SEM of 3–5 independent experiments. *, P < 0.05 and **, P < 0.001 vs. all other groups.
Discussion
Hypertension remains a major risk factor for coronary artery disease and stroke, and yet there is little information regarding the molecular basis for this relatively common condition (23). Furthermore, in a large proportion of patients, high blood pressure remains difficult to control with currently available therapies. Chymases are enzymes that are known to convert Ang I to II and big endothelin-1 to endothelin-1, and these enzymes have other properties related to vascular remodeling, such as enhanced matrix metalloproteinase activity, which may also be relevant to hypertension (24). Our group has recently cloned a chymase (RVCH) from rat vascular SMC, which is elevated in the vessels of spontaneously hypertensive vs. normotensive rats (19). To determine whether overexpression of this chymase is sufficient to cause hypertension, we generated transgenic mice in which we targeted conditional expression of RVCH to SMC, with the use of tTA driven by the SM22α promoter. We confirmed conditional expression of RVCH in SMC by RT-PCR, immunoprecipitation, and chymase activity in tTA+/RVCH+ mice. Blood pressure was elevated and was doxcycline-reversible in tTA+/RVCH+ vs. nonbinary transgenic mice, suggesting a causal link with chymase expression. Medial thickening of mesenteric arteries was associated with SMC proliferation, and functional abnormalities were also observed, i.e., enhanced phenylephrine-induced vasoconstriction and reduced metacholine-induced vasodilatation. These studies not only provide an animal model for hypertension, but also a link between a gene and an important pathological condition.
With the use of β-galactosidase as a reporter gene, previous research found that expression of a transgene controlled by a 3-kb SM22α promoter was restricted to vascular SMC in arteries but not SMC from visceral organs (21). With the use of RT-PCR, we detected RVCH mRNA expression in aorta but not heart, lung, liver, kidney, bladder, intestine, and stomach. Although immunostaining of the aortae for HA in the binary transgenic mice was unsuccessful, suggesting a low level of expression, we were able to detect RVCH-HA in aortic tissue by immunoprecipitation, starting with a relatively large sample (10 aortae). These results are comparable, however, to those recently obtained in studies in which Tet-Off was used successfully to express another transgene in a target organ (25). Despite a relatively low level of expression, we were able to show that a phenotype could be produced when a gene of interest was conditionally expressed in vascular SMC.
We found that the blood pressure is increased in both male and female tTA+/RVCH+ mice at 10–12 weeks of age. The magnitude of blood pressure elevation in the current study is comparable to that reported in transgenic mice with overexpression of human renin and angiotensinogen (26). The severity of hypertension is also similar to that observed in mice lacking either endothelial nitric oxide synthase (27) or the calcium-activated potassium channel (28). We successfully established a causal link between the expression of chymase and hypertension by treating tTA+/RVCH+ mice with doxycycline from 6 weeks of age. We chose this time point because it has been reported that blood pressure is elevated beginning at 5–6 weeks of age in spontaneously hypertensive rats (29). The suppression of RVCH was evident after only 1 week of doxycycline, by both RT-PCR and immunoprecipitation. As both systolic and diastolic blood pressure had returned completely to normal in doxycycline-treated tTA+/RVCH+ mice, we confirmed that overexpression of RVCH is the cause of the hypertension and that doxycycline had no direct effect on blood pressure.
In genetic forms of systemic hypertension, structural remodeling of the vessels, as well as proliferation of SMC, is thought to underlie the development of the hypertension (30). In this model, we found that hypertension was associated with a significant increase in medial thickening of the large (aorta) and resistance (mesenteric) arteries. This observation suggests that chymase overexpression could cause hypertension as a consequence of induced structural remodeling. In fact, initiation of doxycycline at 6 weeks was sufficient to abrogate both the remodeling as well as the hypertension observed at 10–12 weeks. The factors that might up-regulate chymase expression and lead to remodeling and hypertension are not known, but we have observed the induction of RVCH associated with the proliferation of SMC (19) in pulmonary arteries in which hypertrophy and hypertension are caused by injection of the endothelial toxin monocrotaline (31).
It has been documented that hypertension is associated with both structural and functional abnormalities of resistance arteries (32). Heightened responsiveness of the mesenteric artery to phenylephrine-induced vasoconstriction is perhaps not surprising and could be a consequence of the increased muscularity of the artery in tTA+/RVCH+ vs. single mice. It is quite intriguing, however, that we also found impaired metacholine-mediated vasodilatation in tTA+/RVCH+ mice with a normal vasodilator response to sodium nitroprusside (10 μM), suggesting endothelial dysfunction. Although we do not know why expression of RVCH in SMC causes endothelial dysfunction, it is reasonable to speculate that RVCH-expressing SMCs could suppress the endothelial NO signaling pathway, inasmuch as it has been reported that SMCs regulate endothelial nitric oxide synthase expression (33). In addition, endothelial cell dysfunction may also be secondary to hypertension-induced endothelial injury, which is well documented in the literature (34).
We have shown that RVCH can convert Ang I to II, but other potential properties of this chymase could also influence blood pressure and structural remodeling. In preliminary studies, we have found increased Ang II and endothelin-1 immunoreactivity in the mesenteric artery of the binary compared with single mice. Increased expression of both Ang II and endothelin-1 could be generated by RVCH in keeping with the properties of mast cell chymases. In addition, chymases can activate MMPs, which could facilitate SMC proliferation (35, 36). Furthermore, cells stably transfected with RVCH have elevated collagen production (unpublished data).
We found no significant changes in heart rate, cardiac systolic function (dP/dtmax), or diastolic function in tTA+/RVCH+ mice when compared with littermate controls (data not shown) and only a modest increase in the heart weight to body weight ratio in tTA+/RVCH+ mice. Thus, neither changes in heart rate nor cardiac function is responsible for a reflex elevation in blood pressure, nor is the hypertension of the severity that could be expected to result in abnormal cardiac function. It is possible, however, that a more severe phenotype could result from stress or exercise.
In conclusion, we have created a model of systemic hypertension that could be useful in further addressing pathophysiology and in studying the contribution of this risk factor to other variables leading to cardiovascular disease in normal and genetically engineered experimental animals. These studies also encourage the pursuit of elevated chymase activity as a feature of clinical systemic hypertension and the development of chymase inhibitors as an antihypertensive therapeutic strategy.
Acknowledgments
We are grateful to Eric N. Olson (University of Texas Southwestern Medical Center, Dallas) for providing the SM22α promoter. We are also grateful to Judy Edwards, Olga Manuk, Judy Matthews, and Mingda Shi for secretarial and technical assistance. We thank the transgenic core facility at the Hospital for Sick Children for the generation of transgenic mice. M.R. is a Research Endowed Chair of the Heart and Stroke Foundation of Ontario and a Distinguished Scientist of the Canadian Institute of Health Research. H.J., R.G., and X.Y. are Research Fellows of the Canadian Institute of Health Research and the Heart and Stroke Foundation of Canada. This research was supported by a grant (T6.T4144) from the Heart and Stroke Foundation of Canada.
Abbreviations
- RVCH
rat vascular chymase
- Ang
angiotensin
- SMC
smooth muscle cell
- tTA
tetracycline-controlled transactivator
- MMPs
matrix metalloproteinases
- HA
hemagglutinin
- PCNA
proliferating cell nuclear antigen
- RT-PCR
reverse transcription–PCR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Takai S, Shiota N, Sakaguchi M, Muraguchi H, Matsumura E, Miyazaki M. Clin Chim Acta. 1997;265:13–20. doi: 10.1016/s0009-8981(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan N, Jardine A G, McGrath J C, Connell J M. Circulation. 1999;99:2914–2920. doi: 10.1161/01.cir.99.22.2914. [DOI] [PubMed] [Google Scholar]
- 3.Takai S, Shiota N, Kobayashi S, Matsumura E, Miyazaki M. FEBS Lett. 1997;412:86–90. doi: 10.1016/s0014-5793(97)00752-7. [DOI] [PubMed] [Google Scholar]
- 4.Urata H, Kinoshita A, Misono K S, Bumpus F M, Husain A. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 5.Wypij D M, Nichols J S, Novak P J, Stacy D L, Berman J, Wiseman J S. Biochem Pharmacol. 1992;43:845–853. doi: 10.1016/0006-2952(92)90252-e. [DOI] [PubMed] [Google Scholar]
- 6.Reilly C F, Schechter N B, Travis J. Biochem Biophys Res Commun. 1985;127:443–449. doi: 10.1016/s0006-291x(85)80180-7. [DOI] [PubMed] [Google Scholar]
- 7.Hoit B D, Shao Y, Kinoshita A, Gabel M, Husain A, Walsh R A. J Clin Invest. 1995;95:1519–1527. doi: 10.1172/JCI117824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Iwao H. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 9.Sakai S, Miyauchi T, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Nature (London) 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 10.Fang K C, Raymond W W, Blount J L, Caughey G H. J Biol Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Lees M, Newlands G F, Nagase H, Woolley D E. Biochem J. 1995;305:301–306. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson J L, Jackson C L, Angelini G D, George S J. Arterioscler Thromb Vasc Biol. 1998;18:1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 13.Kofford M W, Schwartz L B, Schechter N M, Yager D R, Diegelmann R F, Graham M F. J Biol Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 14.Shiota N, Okunishi H, Fukamizu A, Sakonjo H, Kikumori M, Nishimura T, Nakagawa T, Murakami K, Miyazaki M. FEBS Lett. 1993;323:239–242. doi: 10.1016/0014-5793(93)81348-4. [DOI] [PubMed] [Google Scholar]
- 15.Shiota N, Okunishi H, Takai S, Mikoshiba I, Sakonjo H, Shibata N, Miyazaki M. Circulation. 1999;99:1084–1090. doi: 10.1161/01.cir.99.8.1084. [DOI] [PubMed] [Google Scholar]
- 16.Kaartinen M, Penttila A, Kovanen P T. Arterioscler Thromb. 1994;14:966–972. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 17.Jeziorska M, McCollum C, Woolley D E. J Pathol. 1997;182:115–122. doi: 10.1002/(SICI)1096-9896(199705)182:1<115::AID-PATH806>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Takai S, Yuda A, Jin D, Nishimoto M, Sakagichi M, Sasaki S, Miyazaki M. FEBS Lett. 2000;467:141–144. doi: 10.1016/s0014-5793(00)01125-x. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Ju H, Leung D, Massaeli H, Shi M, Rabinovitch M. J Clin Invest. 2001;107:703–715. doi: 10.1172/JCI9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Miano J M, Mercer B, Olson E N. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kido H, Fukusen N, Katunuma N. Arch Biochem Biophys. 1985;239:436–443. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- 23.Garbers D L, Dubois S K. Annu Rev Biochem. 1999;68:127–155. doi: 10.1146/annurev.biochem.68.1.127. [DOI] [PubMed] [Google Scholar]
- 24.O'Callaghan C J, Williams B. Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 25.Redfern C H, Coward P, Degtyarev M Y, Lee E K, Kwa A T, Hennighausen L, Bujard H, Fishman G I, Conklin B R. Nat Biotechnol. 1999;17:165–169. doi: 10.1038/6165. [DOI] [PubMed] [Google Scholar]
- 26.Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo M S, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- 27.Huang P L, Huang Z, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 28.Brenner R, Perez G J, Bonev A D, Eckman D M, Kosek J C, Wiler S W, Patterson A J, Nelson M T, Aldrich R W. Nature (London) 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 29.Yamori Y, Tomimoto K, Ooshima A, Hazama F, Okamoto K. Jpn Heart J. 1974;15:209–210. doi: 10.1536/ihj.15.209. [DOI] [PubMed] [Google Scholar]
- 30.Touyz R M. Can J Cardiol. 2000;16:1137–1146. [PubMed] [Google Scholar]
- 31.Cowan K N, Jones P L, Rabinovitch M. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intengan H D, Schiffrin E L. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 33.Di L G, Bhargava J, Powell R J. J Vasc Surg. 2000;31:781–789. doi: 10.1067/mva.2000.103788. [DOI] [PubMed] [Google Scholar]
- 34.Boulanger C M. J Mol Cell Cardiol. 1999;31:39–49. doi: 10.1006/jmcc.1998.0842. [DOI] [PubMed] [Google Scholar]
- 35.Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari S T, Clowes A W. Circ Res. 1996;79:812–820. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 36.George S J, Lloyd C T, Angelini G D, Newby A C, Baker A H. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]