Abstract
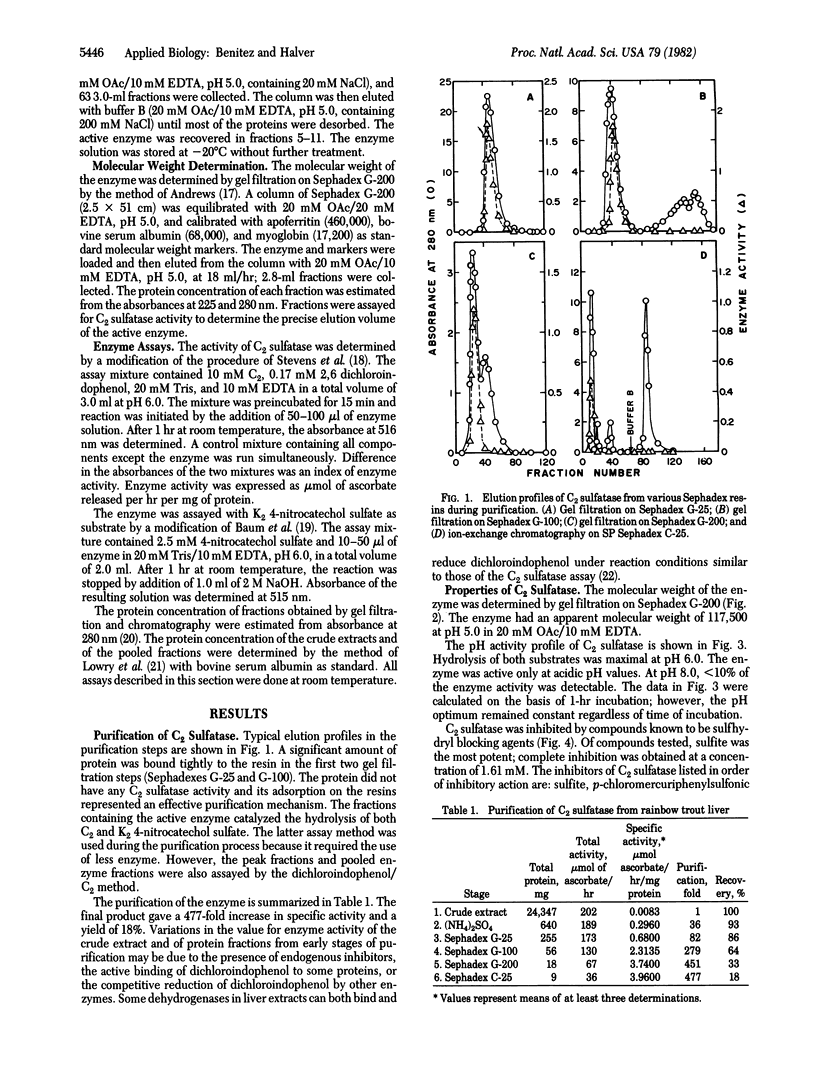
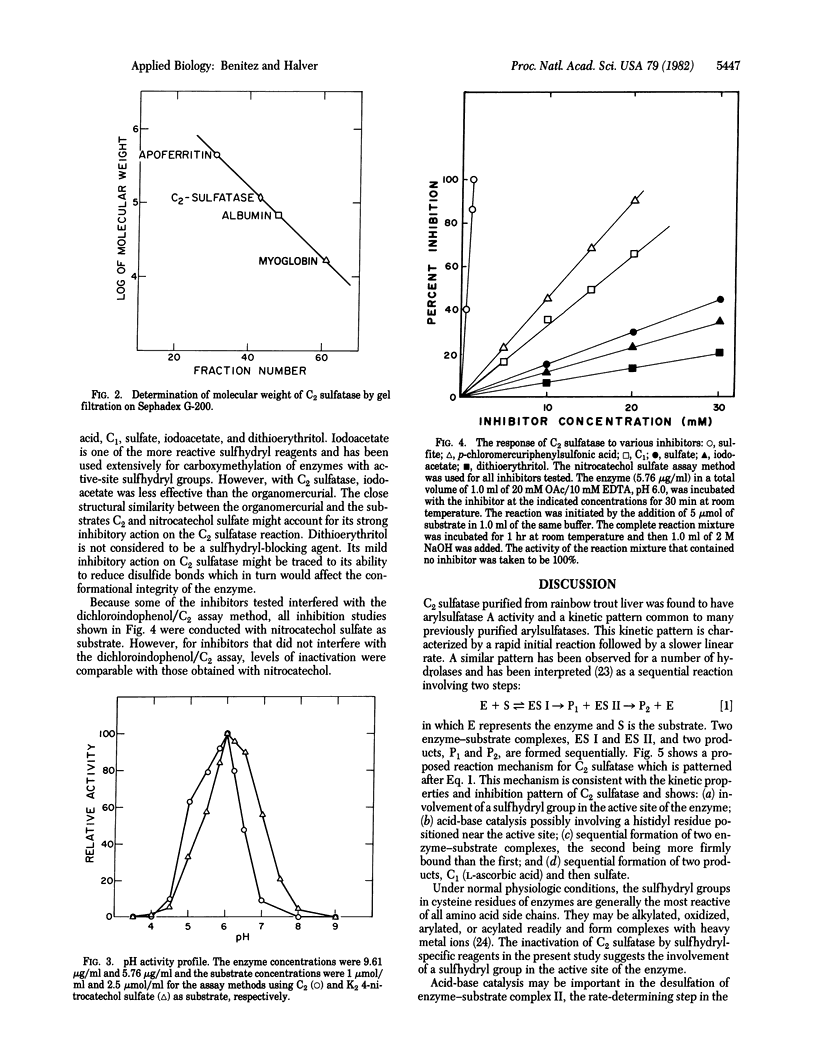
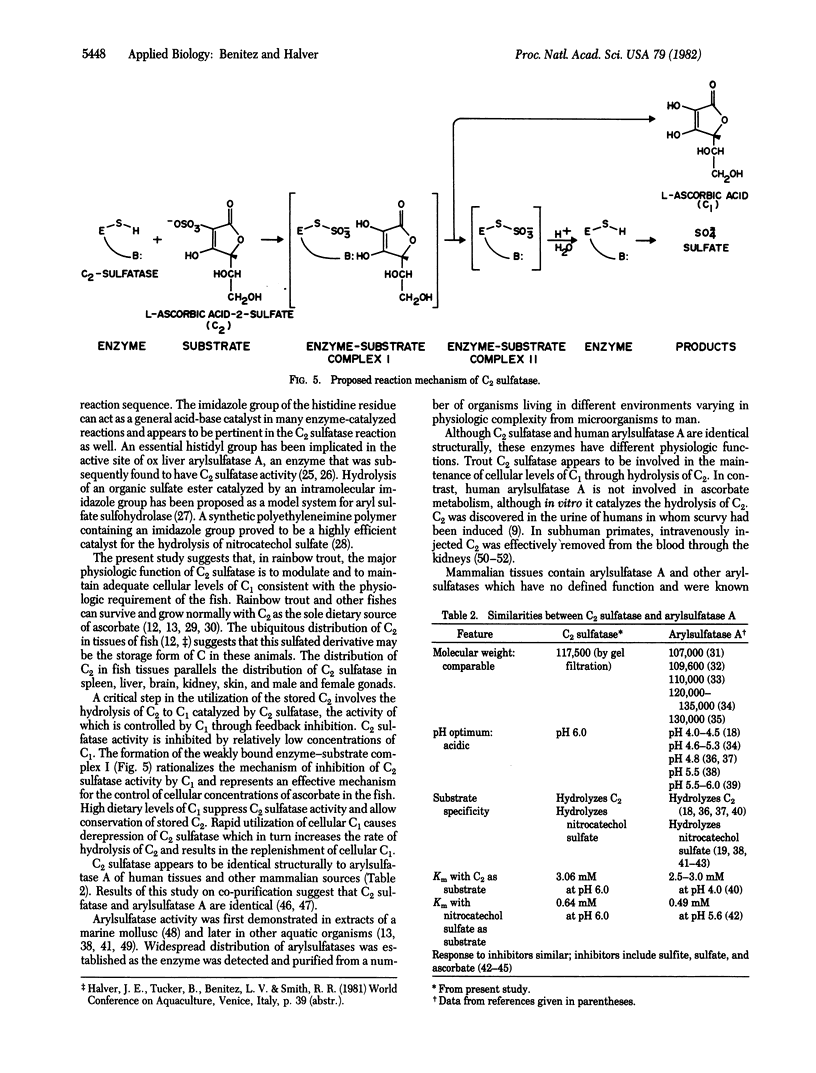
The enzyme L-ascorbic acid 2-sulfate sulfohydrolase (C2 sulfatase) was purified from rainbow trout liver. The enzyme catalyzes the hydrolysis of L-ascorbic acid 2-sulfate and has a pH optimum at 6.0. It has a molecular weight of about 117,500 at pH 5.0 and is inhibited by a number of sulfhydryl blocking agents including L-ascorbic acid. C2 sulfatase activity was observed in most metabolic organs of rainbow trout. These findings suggest that the physiologic role of the enzyme is to maintain adequate cellular concentrations of L-ascorbic acid in the fish. The activity of the enzyme is controlled by L-ascorbic acid through feedback inhibition. Comparison of kinetic constants and inhibition patterns suggests that C2 sulfatase is structurally identical to human arylsulfatase A. However, unlike C2 sulfatase, human arylsulfatase A may not be involved in ascorbate metabolism. Its physiologic substrate is reported to be cerebroside-3-sulfate, not L-ascorbic acid 2-sulfate. A scheme is proposed to account for the functional divergence of these two structurally identical enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN J. H., BALASUBRAMANIAN A. S., PATTABIRAMAN T. N., SARASWATHI S., BASU D. K., BACHHAWAT B. K. A CONTROLLED STUDY OF ENZYMIC ACTIVITIES IN THREE HUMAN DISORDERS OF GLYCOLIPID METABOLISM. J Neurochem. 1963 Dec;10:805–816. doi: 10.1111/j.1471-4159.1963.tb11905.x. [DOI] [PubMed] [Google Scholar]
- AUSTIN J. H. Metachromatic form of diffuse cerebral sclerosis. III. Significance of sulfatide and other lipid abnormalities in white matter and kidney. Neurology. 1960 May;10:470–483. doi: 10.1212/wnl.10.5.470. [DOI] [PubMed] [Google Scholar]
- AUSTIN J., MCAFEE D., SHEARER L. METACHROMATIC FORM OF DIFFUSE CEREBRAL SCLEROSIS. IV. LOW SULFATASE ACTIVITY IN THE URINE OF NINE LIVING PATIENTS WITH METACHROMATIC LEUKODYSTROPHY (MLD). Arch Neurol. 1965 May;12:447–455. doi: 10.1001/archneur.1965.00460290003001. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J., Armstrong D., Shearer L. Metachromatic form of diffuse cerebral sclerosis. V. The nature and significance of low sulfatase activity: a controlled study of brain, liver and kidney in four patients with metachromatic leukodystrophy (MLD). Arch Neurol. 1965 Dec;13(6):593–614. doi: 10.1001/archneur.1965.00470060029003. [DOI] [PubMed] [Google Scholar]
- BALASUBRAMANIAN A. S., BACHHAWAT B. K. Purification and properties of an arylsulphatase from human brain. J Neurochem. 1963 Mar;10:201–211. doi: 10.1111/j.1471-4159.1963.tb09484.x. [DOI] [PubMed] [Google Scholar]
- BAUM H., DODGSON K. S., SPENCER B. The assay of arylsulphatases A and B in human urine. Clin Chim Acta. 1959 May;4(3):453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- Baker E. M., 3rd, Hammer D. C., March S. C., Tolbert B. M., Canham J. E. Ascorbate sulfate: a urinary metabolite of ascorbic acid in man. Science. 1971 Aug 27;173(3999):826–827. doi: 10.1126/science.173.3999.826. [DOI] [PubMed] [Google Scholar]
- Baker E. M., Halver J. E., Johnsen D. O., Joyce B. E., Knight M. K., Tolbert B. M. Metabolism of ascorbic acid and ascorbic-2-sulfate in man and the subhuman primate. Ann N Y Acad Sci. 1975 Sep 30;258:72–80. doi: 10.1111/j.1749-6632.1975.tb29269.x. [DOI] [PubMed] [Google Scholar]
- Benitez L. V., Allison W. S. The mechanism of the diaphorase reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys. 1973 Nov;159(1):89–96. doi: 10.1016/0003-9861(73)90432-3. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J., Dunikoski L. K., Jr Intramolecular catalysis of sulfate ester hydrolysis. A model for aryl sulfate sulfohydrolase. Biochemistry. 1970 Mar 17;9(6):1390–1397. doi: 10.1021/bi00808a013. [DOI] [PubMed] [Google Scholar]
- Bond A. D., McClelland B. W., Einstein J. R., Finamore F. J. Ascorbic acid-2-sulfate of the brine shrimp, Artemia salina. Arch Biochem Biophys. 1972 Nov;153(1):207–214. doi: 10.1016/0003-9861(72)90438-9. [DOI] [PubMed] [Google Scholar]
- Breslow J. L., Sloan H. R. Purification of arylsulfatase A from human urine. Biochem Biophys Res Commun. 1972 Jan 31;46(2):919–925. doi: 10.1016/s0006-291x(72)80229-8. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., LEWIS J. I., SPENCER B. Studies on sulphatases. 3. The arylsulphatase and beta-glucuronidase of marine molluscs. Biochem J. 1953 Sep;55(2):253–259. doi: 10.1042/bj0550253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Studies on sulphatases. 4. Arylsulphatase and beta-glucuronidase concentrates from limpets. Biochem J. 1953 Sep;55(2):315–320. doi: 10.1042/bj0550315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Miller R. T., Shapiro S. S., Kihara H. Ascorbic acid-2-sulfate sulfhohydrolase activity of human arylsulfatase A. Biochim Biophys Acta. 1976 Apr 8;429(2):508–516. doi: 10.1016/0005-2744(76)90298-9. [DOI] [PubMed] [Google Scholar]
- Halver J. E., Smith R. R., Tolbert B. M., Baker E. M. Utilization of ascorbic acid in fish. Ann N Y Acad Sci. 1975 Sep 30;258:81–102. doi: 10.1111/j.1749-6632.1975.tb29270.x. [DOI] [PubMed] [Google Scholar]
- Harinath B. C., Robins E. Arylsulphatases in human brain: assay, some properties, and distribution. J Neurochem. 1971 Feb;18(2):237–244. doi: 10.1111/j.1471-4159.1971.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Harinath B. C., Robins E. Arylsulphatases in human brain: separation, purification, and certain properties of the two soluble arylsulphatases. J Neurochem. 1971 Feb;18(2):245–257. doi: 10.1111/j.1471-4159.1971.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Harzer K., Benz H. U. Deficiency of lactosyl sulfatide sulfatase in metachromatic leucodystrophy (sulfatidosis). Hoppe Seylers Z Physiol Chem. 1974 Jun;355(6):744–748. [PubMed] [Google Scholar]
- Hatanaka H., Ogawa Y., Egami F. Copurification of L-ascorbate-2-sulfate sulfohydrolase and arylsulfatase activities from the liver of a marine gastropod, Charonia lampas. J Biochem. 1975 Feb;77(2):353–359. doi: 10.1093/oxfordjournals.jbchem.a130732. [DOI] [PubMed] [Google Scholar]
- Hatanaka H., Ogawa Y., Egami F. L-ascorbic acid sulfate esters with special reference to enzymatic hydrolysis. J Biochem. 1974 Apr;75(4):861–866. doi: 10.1093/oxfordjournals.jbchem.a130458. [DOI] [PubMed] [Google Scholar]
- Hornig D., Gallo-Torres H. E., Weiser H. A biliary metabolite of ascorbic acid in normal and hypophysectomized rats. Biochim Biophys Acta. 1973 Oct 5;320(3):549–556. doi: 10.1016/0304-4165(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Maru A., Imai Y., Makita A., Tsuji I. A variant form of arylsulfatase A in human urine derived directly from the renal pelvis: kinetic and immunological characterization. Biochim Biophys Acta. 1980 Dec 4;616(2):218–227. doi: 10.1016/0005-2744(80)90140-0. [DOI] [PubMed] [Google Scholar]
- JATZKEWITZ H. [Leukodystrophy, Scholz' type, (metachromatic form of diffuse sclerosis) with sphinolipoidosis (cerebroside-sulfuric acid ester storage disease)]. Hoppe Seylers Z Physiol Chem. 1960 May 31;318:265–277. doi: 10.1515/bchm2.1960.318.1.265. [DOI] [PubMed] [Google Scholar]
- Jatzkewitz H., Mehl E. Cerebroside-sulphatase and arylsulphatase A deficiency in metachromatic leukodystrophy (ML). J Neurochem. 1969 Jan;16(1):19–28. doi: 10.1111/j.1471-4159.1969.tb10339.x. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B. The sulphatase of ox liver. XII. The effect of tyrosine and histidine reagents on the activity of sulphatase A. Biochim Biophys Acta. 1969 Mar;175(2):355–364. doi: 10.1016/0005-2795(69)90013-0. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B. The sulphatase of ox liver. XVII. An essential histidyl residue in sulphatase A. Biochim Biophys Acta. 1974 Nov 5;371(1):76–88. doi: 10.1016/0005-2795(74)90157-3. [DOI] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Kenyon G. L., Bruice T. W. Novel sulfhydryl reagents. Methods Enzymol. 1977;47:407–430. doi: 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]
- Kiefer H. C., Congdon W. I., Scarpa I. S., Klotz I. M. Catalytic accelerations of 10-fold by an enzyme-like synthetic polymer. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2155–2159. doi: 10.1073/pnas.69.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovell R. T. Essentiality of vitamin C in feeds for intensively fed caged channel catfish. J Nutr. 1973 Jan;103(1):134–138. doi: 10.1093/jn/103.1.134. [DOI] [PubMed] [Google Scholar]
- Mead C. G., Finamore F. J. The occurrence of ascorbic acid sulfate in the brine shrimp, Artemia salina. Biochemistry. 1969 Jun;8(6):2652–2655. doi: 10.1021/bi00834a060. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Cerebroside 3-sulfate as a physiological substrate of arylsulfatase A. Biochim Biophys Acta. 1968 Mar 25;151(3):619–627. doi: 10.1016/0005-2744(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Eine Cerebrosidsulfatase aus Schweineniere. Hoppe Seylers Z Physiol Chem. 1964;339(1):260–276. [PubMed] [Google Scholar]
- Mumma R. O., Verlangieri A. J. Isolation of ascorbic acid 2-sulfate from selected rat organs. Biochim Biophys Acta. 1972 Jul 19;273(2):249–253. doi: 10.1016/0304-4165(72)90214-0. [DOI] [PubMed] [Google Scholar]
- Mumma R. O., Verlangieri A. J., Weber W. W., 2nd L-Ascorbic acid 3-sulfate. Preparation and characterization. Carbohydr Res. 1971 Aug;19(1):127–132. doi: 10.1016/s0008-6215(00)80321-3. [DOI] [PubMed] [Google Scholar]
- Murai T., Andrews J. W., Bauernfeind J. C. Use of -l-ascorbic acid, ethocel coated ascorbic acid and ascorbate 2-sulfate in diets for channel catfish, Ictalurus punctatus. J Nutr. 1978 Nov;108(11):1761–1766. doi: 10.1093/jn/108.11.1761. [DOI] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. II. The purification and properties of sulphatase A. Biochem J. 1953 Nov;55(4):653–661. doi: 10.1042/bj0550653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi M. C., Marks J. S., Davidson R. G. Electrophoresis of arylsulfatase from normal individuals and patients with metachromatic leukodystrophy. Am J Hum Genet. 1973 May;25(3):310–316. [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Carmack C., Haverback B. J. The determination of arylsulfatases in biological fluids. Clin Chim Acta. 1970 Sep;29(3):481–491. doi: 10.1016/0009-8981(70)90019-7. [DOI] [PubMed] [Google Scholar]
- Roy A. B., Jerfy A. The sulphatase of ox liver. XIV. The subunit structure of sulphatase A. Biochim Biophys Acta. 1970 Apr 28;207(1):156–164. doi: 10.1016/0005-2795(70)90147-9. [DOI] [PubMed] [Google Scholar]
- Roy A. B. L-ascorbic acid 2-sulphate. A substrate for mammalian arylsulphatases. Biochim Biophys Acta. 1975 Feb 19;377(2):356–363. doi: 10.1016/0005-2744(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Roy A. B. Sulphatases, lysosomes and disease. Aust J Exp Biol Med Sci. 1976 Apr;54(2):111–135. doi: 10.1038/icb.1976.13. [DOI] [PubMed] [Google Scholar]
- Roy A. B. The hydrolysis of ascorbate 2-sulfate by sulfatase A. Methods Enzymol. 1979;62:42–47. doi: 10.1016/0076-6879(79)62187-0. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Fluharty A. L., Shapiro S. S., Miller R. T., Davis L. L., Kihara H. Assay procedures for ascorbic acid 2-sulfate sulfohydrolase. Anal Biochem. 1977 May 1;79(1-2):23–32. doi: 10.1016/0003-2697(77)90374-8. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Fluharty A. L., Skokut M. H., Kihara H. Purification and properties of arylsulfatase. A from human urine. J Biol Chem. 1975 Apr 10;250(7):2495–2501. [PubMed] [Google Scholar]
- Tolbert B. M., Downing M., Carlson R. W., Knight M. K., Baker E. M. Chemistry and metabolism of ascorbic acid and ascorbate sulfate. Ann N Y Acad Sci. 1975 Sep 30;258:48–69. doi: 10.1111/j.1749-6632.1975.tb29267.x. [DOI] [PubMed] [Google Scholar]


