Abstract
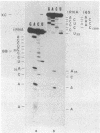
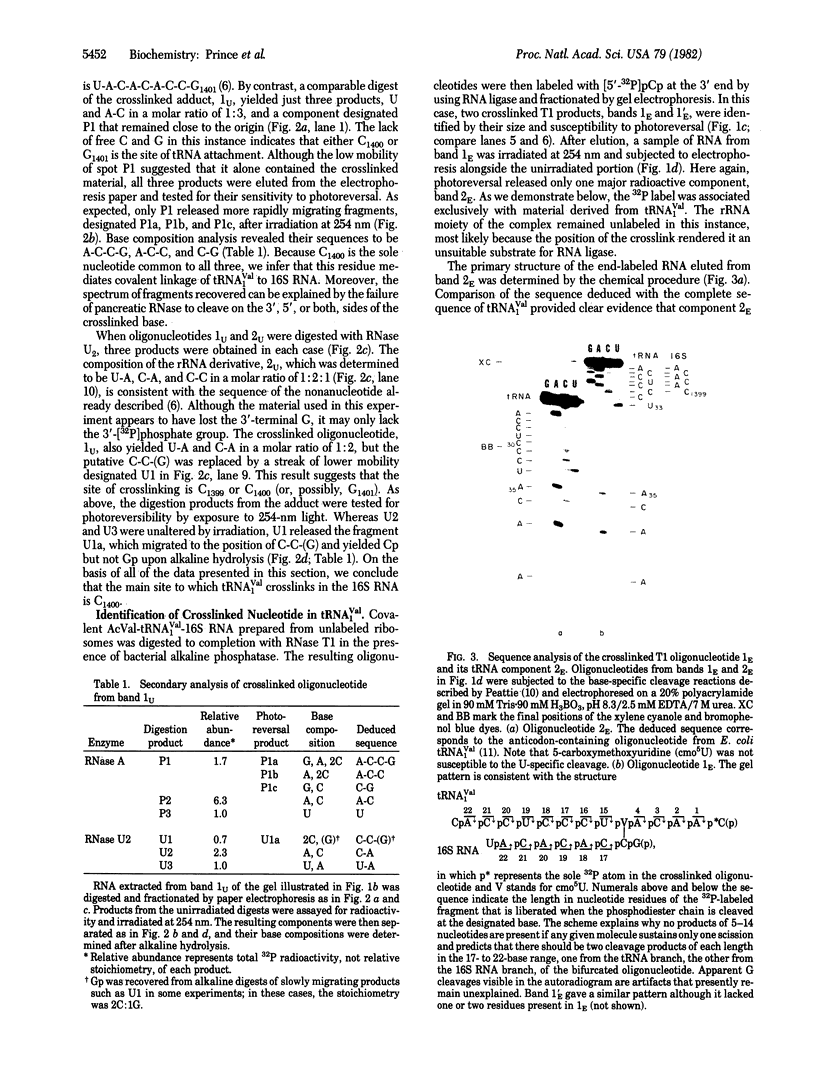
N-Acetylvalyl-tRNA1Val (AcVal-tRNA1Val) was bound to the P site of uniformly 32P-labeled 70S ribosomes from Escherichia coli and crosslinked to 16S RNA in the 30S ribosomal subunit by irradiation with light of 300-400 nm. To identify the crosslinked nucleotide in 16S RNA. AcVal-tRNA1Val-16S [32P]RNA was digested completely with RNase T1 and the band containing the covalently attached oligonucleotides from tRNA and rRNA was isolated by polyacrylamide gel electrophoresis. The crosslinked oligonucleotide, and the 32P-labeled rRNA moiety released from it by photoreversal of the crosslink at 254 nm, were then analyzed by secondary hydrolysis with pancreatic RNase A and RNase U2.The oligonucleotide derived from 16S RNA was found to be the evolutionarily conserved sequence, U-A-C-A-C-A-C-C-G1401, and the nucleotide crosslinked to tRNA1Val, C1400. The identity of the covalently attached residue in the tRNA was established by using AcVal-tRNA1Val-16S RNA prepared from unlabeled ribosomes. This complex was digested to completion with RNase T1 and the resulting RNA fragments were labeled at the 3' end with [5'-32P]pCp. The crosslinked T1 oligonucleotide isolated from the mixture yielded one major end-labeled component upon photoreversal. Chemical sequence analysis demonstrated that this product was derived from the anticodon-containing pentadecanucleotide of tRNA1Val, C-A-C-C-U-C-C-C-U-cmo5U-A-C-m6A-A-G39(cmo5U, 5-carboxymethoxyuridine). A similar study of the crosslinked oligonucleotide revealed that the residue covalently bound to 16S was cmo5U34, the 5' or wobble base of the anticodon. The adduct is believed to result from formation of a cyclobutane dimer between cmo5U34 of tRNA1Val and C1400 of the 16S RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W., Dover S. D. Optimised parameters for RNA double-helices. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1392–1399. doi: 10.1016/0006-291x(72)90867-4. [DOI] [PubMed] [Google Scholar]
- Baer R., Dubin D. T. The 3'-terminal sequence of the small subunit ribosomal RNA from hamster mitochondria. Nucleic Acids Res. 1980 Nov 11;8(21):4927–4941. doi: 10.1093/nar/8.21.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Debey P., Hui Bon Hoa G., Douzou P., Godefroy-Colburn T., Graffe M., Grunberg-Manago M. Ribosomal subunit interaction as studied by light scattering. Evidence of different classes of ribosome preparations. Biochemistry. 1975 Apr 22;14(8):1553–1559. doi: 10.1021/bi00679a001. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Maden B. E. Nucleotide sequence through the 18S-28S intergene region of a vertebrate ribosomal transcription unit. Nucleic Acids Res. 1980 Dec 20;8(24):5993–6005. doi: 10.1093/nar/8.24.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Latil-Damotte M., Jourdan R. Sequence of the 3'-terminal portion of Drosophila melanogaster 18 S rRNA and of the adjoining spacer: comparison with corresponding prokaryotic and eukaryotic sequences. FEBS Lett. 1980 Aug 11;117(1):227–231. doi: 10.1016/0014-5793(80)80951-3. [DOI] [PubMed] [Google Scholar]
- Kimura F., Harada F., Nishimura S. Primary sequence of tRNA-Val-1 from Escherichia coli B. II. Isolation of large fragments by limited digestion with RNases, and overlapping of fragments to reduce the total primary sequence. Biochemistry. 1971 Aug 17;10(17):3277–3283. doi: 10.1021/bi00793a018. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Correct codon--anticodon base pairing at the 5'-anticodon position blocks covalent cross-linking between transfer ribonucleic acid and 16S RNA at the ribosomal P site. Biochemistry. 1981 Feb 3;20(3):552–559. doi: 10.1021/bi00506a017. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Evidence for pyrimidine-pyrimidine cyclobutane dimer formation in the covalent cross-linking between transfer ribonucleic acid and 16S ribonucleic acid at the ribosomal P site. Biochemistry. 1980 Oct 14;19(21):4814–4822. doi: 10.1021/bi00562a016. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R., Kohut J., 3rd, Schwartz I., Zimmermann R. A. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Mechanism and site of reaction in transfer ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4322–4332. doi: 10.1021/bi00587a010. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of tRNALys and tRNA2Glu to 16 S RNA at the P site of Escherichia coli ribosomes. J Biol Chem. 1979 Jun 10;254(11):4745–4749. [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I., Ofengand J. Photochemical cross-linking of unmodified acetylvalyl-tRNA to 16S RNA at the ribosomal P site. Biochemistry. 1978 Jun 27;17(13):2524–2530. doi: 10.1021/bi00606a011. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Taylor B. H., Prince J. B., Ofengand J., Zimmermann R. A. Nonanucleotide sequence from 16S ribonucleic acid at the peptidyl transfer ribonucleic acid binding site of the Escherichia coli ribosome. Biochemistry. 1981 Dec 22;20(26):7581–7588. doi: 10.1021/bi00529a037. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Gates S. M., Schwartz I., Ofengand J. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Site of reaction in 16S ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4333–4339. doi: 10.1021/bi00587a011. [DOI] [PubMed] [Google Scholar]