Abstract
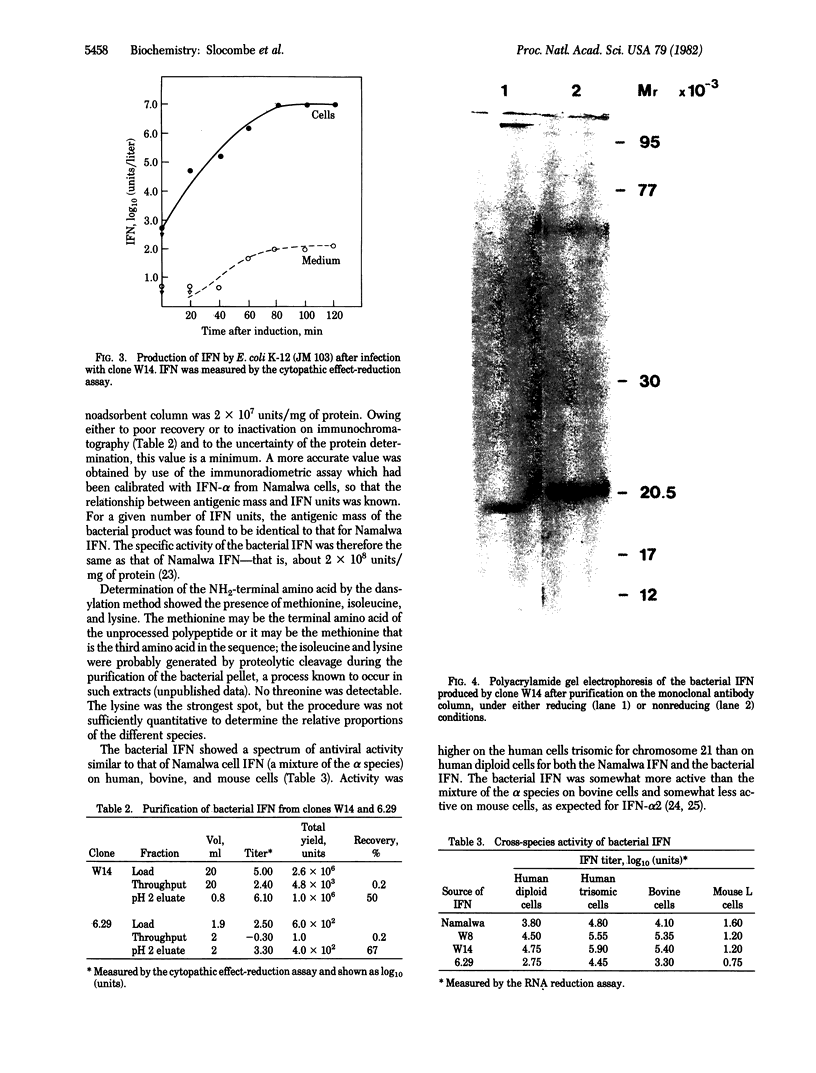
A cloned interferon alpha 2 (IFN-alpha 2) gene was partially digestd with Pvu II to give a fragment that was inserted into the HincII site of the lacZ gene of bacteriophage M13mp7. Two recombinant phages containing the IFN-alpha 2 sequences in the correct orientation for expression from the lac promoter were characterized in detail. DNA sequence analysis showed that the inserted IFN-alpha 2 gene was in phase with the initiation codon of the lacZ gene. The polypeptide product has an additional 19 amino amino acids at the amino terminus of the mature IFN-alpha 2. The first 11 amino acids originate from the amino terminus of beta-galactosidase, and the remaining 8 amino acids are part of the signal sequence of pre-IFN-alpha 2. Infection of Escherichia coli with these phage followed by induction of the lac promoter with isopropyl thiogalactoside gives high yields (up to 10(9) units/liter with an average of 1.5 X 10(8) units/liter) of the modified IFN-alpha 2. This was purified to homogeneity in a single step by immunochromatography using the monoclonal antibody NK2. The nonreduced product had an apparent molecular weight of 20,500 and was shown by immunoradiometric assay to have the same specific activity as IFN made in Namalwa cells. It exhibited the characteristic cross-species antiviral activity of IFN-alpha 2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton K. T., Burke D. C. Interferon induction by viruses and polynucleotides: a differential effect of comptothecin. J Gen Virol. 1975 Dec;29(3):297–304. doi: 10.1099/0022-1317-29-3-297. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Kurer V., Smith H. O. Sequence organization of a cloned tDNA met fragment from Xenopus laevis. Cell. 1978 Jul;14(3):713–724. doi: 10.1016/0092-8674(78)90253-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes K. H., Allen G. Specific activity of pure human interferons and a non-biological method for estimating the purity of highly purified interferon preparations. J Interferon Res. 1981;1(4):465–471. doi: 10.1089/jir.1981.1.465. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morser J., Flint J., Meager A., Graves H., Baker P. N., Colman A., Burke D. C. Characterization of interferon messenger RNA from human lymphoblastoid cells. J Gen Virol. 1979 Jul;44(1):231–234. doi: 10.1099/0022-1317-44-1-231. [DOI] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher D. S., Burke D. C. A monoclonal antibody for large-scale purification of human leukocyte interferon. Nature. 1980 Jun 12;285(5765):446–450. doi: 10.1038/285446a0. [DOI] [PubMed] [Google Scholar]
- Secher D. S. Immunoradiometric assay of human leukocyte interferon using monoclonal antibody. Nature. 1981 Apr 9;290(5806):501–503. doi: 10.1038/290501a0. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall A., Boll W., Stewart W. E., 2nd, Nagata S., Weissmann C. Target cell specificity of two species of human interferon-alpha produced in Escherichia coli and of hybrid molecules derived from them. Proc Natl Acad Sci U S A. 1981 May;78(5):2848–2852. doi: 10.1073/pnas.78.5.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980 Sep 19;209(4463):1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Guarente L., Roberts T. M., Kimelman D., Douhan J., 3rd, Ptashne M. Expression of the human fibroblast interferon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5230–5233. doi: 10.1073/pnas.77.9.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Apperson S., Stebbing N., Gray P. W., Leung D., Shepard H. M., Goeddel D. V. Antiviral activities of hybrids of two major human leukocyte interferons. Nucleic Acids Res. 1981 Nov 25;9(22):6153–6166. doi: 10.1093/nar/9.22.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelverton E., Leung D., Weck P., Gray P. W., Goeddel D. V. Bacterial synthesis of a novel human leukocyte interferon. Nucleic Acids Res. 1981 Feb 11;9(3):731–741. doi: 10.1093/nar/9.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]