Abstract
Genetically engineered lymphocytes hold promise for the treatment of genetic disease, viral infections and cancer. However, current methods for genetic transduction of peripheral blood lymphocytes rely on viral vectors, which are hindered by production and safety-related problems. In this study, we demonstrated an efficient novel nonviral platform for gene transfer to lymphocytes. The Sleeping Beauty transposon-mediated approach allowed for long-term stable expression of transgenes at ~50% efficiency. Utilizing transposon constructs expressing tumor antigen-specific T-cell receptor genes targeting p53 and MART-1, we demonstrated sustained expression and functional reactivity of transposon-engineered lymphocytes on encountering target antigen presented on tumor cells. We found that transposon- and retroviral-modified lymphocytes had comparable transgene expression and phenotypic function. These results demonstrate the promise of nonviral ex vivo genetic modification of autologous lymphocytes for the treatment of cancer and immunologic disease.
Keywords: Sleeping Beauty transposon, TCR gene therapy, adoptive immunotherapy, nonviral vector, T-cell gene transfer
Advances in tumor immunology have led to the development of effective immunotherapies for cancer. A number of strategies are currently employed: active vaccination, nonspecific cytokine infusion, passive antibody therapy and adoptive cell transfer.1 Significant clinical responses have been reported for a number of solid tumor histologies using adoptive cell-transfer therapies. Most notably for metastatic melanoma, adoptive immunotherapy with lymphocytes expanded ex vivo from excised tumor specimens (TIL; tumor infiltrating lymphocytes) mediated objective tumor regression in 51% of patients.2,3 There are, however, several limitations to this treatment approach for patients with solid tumors. Tumor-specific lymphocytes can only be consistently expanded from melanoma lesions, thereby severely restricting the treatable solid tumor histologies, and a significant population of patients will not have reactive lymphocytes appropriate for therapeutic transfer. Recent studies have overcome these limitations through genetic modification of normal peripheral blood lymphocytes (PBL).4
Tumor antigen recognition by lymphocytes is dependent on cell surface T-cell receptors (TCR), composed of α- and β-chains. TCR with tumor antigen specificity have been cloned for a number of tumor antigens, including MART-1, gp100, NY-ESO-1 and p53.5–8 Transfer of antigen-specific TCR genes can confer tumor antigen specificity to normal PBL. Using traditional murine-leukemia γ-retroviral transduction of MART-1 TCR genes, adoptive transfer of gene modified PBL-mediated objective tumor regression in 13% of melanoma patients.9 This trial demonstrated successful regression of meta-static melanoma after adoptive cell treatment with gene-modified lymphocytes, however, further investigation is necessary to improve response rates. Overcoming certain limitations of retroviral transduction may result in improved clinical outcomes and facilitate mainstream adoption of autologous gene-modified adoptive transfer therapies.
The development of a scalable, high-throughput, nonviral gene transfer system for clinical use may circumvent a number of limitations of the current γ-retroviral-based gene transduction systems. Biological properties of γ-retroviral-based systems allow for efficient transduction of dividing cells but are incapable of mediating transduction of quiescent cells. Murine models of adoptive immunotherapy demonstrated that minimally stimulated or quiescent cells are more effective in mediating antitumor responses.10 Another limitation of γ-retroviral vector transduction centers on transcriptional gene silencing, which compromises transgene expression through poorly defined cellular mechanisms dependent on viral sequences, may be absent in nonviral transposons.11,12 Additionally, γ-retroviruses have a pronounced preference for integration into 5′ promoter-containing regions of genes, which has resulted in insertional mutagenesis and malignant transformation in a human clinical trial.13,14 Finally, in addition to the theoretical limitation of viral vector-mediated gene transfer, there are several practical limitations including the cost and time required for the production of these clinical-grade viral reagents. Taken together, the inherent limitations of viral-based gene transfer suggest that an efficient nonviral system would be a significant advancement in the genetic modification of hematopoietic cells for the treatment of human disease including cancer.
Nonviral gene transfer has historically been hampered by low transduction efficiency in primary cells. Recently, transposons have been developed as a nonviral integrating system of gene delivery that is dependent on only two nonviral components for integration: (1) the donor transposon plasmid and (2) the helper transposase.15,16 The Sleeping Beauty transposon system was reconstructed from salmonid phylogenetic data and was demonstrated to mediate functional integration when introduced in mammalian cells in a number of preclinical disease models.17–19 Initial efforts with a bicistronic double-plasmid first-generation Sleeping Beauty system in lymphocytes resulted in poor gene transfer efficiency.20 Mutagenesis of the enzyme DNA-binding domain resulted in the development of hyperactive mutants with up to a 20-fold increase in native transposition efficiency.21,22 Moreover, the use of mRNA encoding transposase allows temporal control of transposase expression and eliminates the risk of recombinant elements.23 Analysis of transposon genomic integration does not reveal a marked preference for promoter regions and may indicate a safer integration profile.24,25
In this study, we developed a novel, efficient approach to introduce the two-component Sleeping Beauty transposon system into primary human PBL. We demonstrated efficient long-term transgene expression and conferral of antigen-specific antitumor reactivity compatible with the needs of genetically modified cells for adoptive transfer therapy.
Efficient long-term transposon-mediated transgene expression in peripheral blood lymphocytes
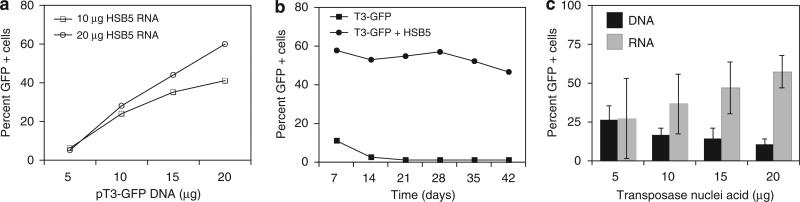
We developed a highly efficient nonviral gene transfer system for human PBL using a two-component Sleeping Beauty transposon system. Transposon plasmid DNA pT3-GFP was coelectroporated along with in vitro transcribed mRNA encoding the hyperactive Sleeping Beauty HSB5 transposase, into peripheral blood mono-nuclear cell (PBMC). Cell mortality after electroporation was approximately 70%. Cells were stimulated with OKT3 and interleukin-2 (IL2) the following day, and lymphocyte activation resulted in a 20- to 100-fold increase in cell number over 6 weeks of culture (data not shown). Titration experiments demonstrated that gene transfer efficiency increased with the amount of input RNA and DNA (Figure 1a).
Figure 1.
Optimization of transposon-mediated gene transfer into PBL. T3 transposon (pT3) was previously described21 and modified to contain the MSCV-U3 promoter, multiple cloning site and bovine growth hormone polyadenylation signal. The green fluorescent protein (GFP) reporter gene was inserted into the multiple cloning site. The Sleeping Beauty (SB) transposase was derived plasmid HSB5 as described,38 directionally cloned into T7-promoter expression plasmid pGEM4Z/GFP/64A, replacing GFP to yield pT7-HSB5-64A. In vitro transcription of RNA was previously described,39 and gene transfer of human peripheral blood mononuclear cell (PBMC) was performed using the nucleofection electroporation-procedure for human T cells as recommended by the supplier (using program U14; Amaxa Inc., Gaithersburg, MD, USA). Cells were activated next day in AIM-V medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% human serum, 50 ng ml-1 OKT3 and 6000 IU ml-1 interleukin-2 (IL2). (a) Either 10 μg or 20 μg HSB5 RNA was coelectroporated into PBMC with varying amounts of pT3-GFP transposon plasmid DNA and GFP expression measured at 10 days after gene transfer by fluorescence-activated cell sorting (FACS). (b) pT3-GFP plasmid DNA or HSB5 RNA plus pT3-GFP plasmid were coelectroporated into PBMC followed the next day by OKT3/IL2 stimulation. FACS was performed at the times indicated to measure GFP expression. (c) A fixed amount of pT3-GFP plasmid DNA (20 μg) was coelectroporated with increasing amount of HSB5 transposase plasmid DNA or in vitro transcribed HSB5 mRNA and GFP expression measured at 10 day after gene transfer by FACS. The differences in gene transfer efficiency comparing HSB5 DNA and HSB5 RNA were statistically significant (P<0.05).
Using optimal amounts of transposon plasmid and transposase RNA, PBMC were electroporated and green fluorescent protein (GFP) reporter-gene expression was monitored. Fluorescence-activated cell sorting (FACS) analysis demonstrated that stable GFP transgene expression was maintained at an efficiency of ~50% over 6 weeks (Figure 1b). In contrast, donor pT3-GFP plasmid without helper HSB5 transposase RNA resulted in GFP expression declining to background levels within 1 week after electroporation.
Studies with the Sleeping Beauty transposon system have demonstrated that there was an optimal ratio of Sleeping Beauty transposase to transposon. It has been previously reported that increasing the ratio results in a decrease in transposition efficiency through mechanisms of overexpression inhibition.26 We examined transposition efficiency with a range of DNA and RNA substrates encoding the HSB5 transposase at a fixed amount of T3GFP transposon DNA (Figure 1c). Electroporation with 20 μg pT3-GFP transposon DNA and varying amounts of helper HSB5 transposase RNA or DNA was performed on using PBMC. Increasing ratios of HSB5 DNA resulted in a maximal efficiency with 5 μg HSB5 DNA plasmid and then a decrease in efficiency at higher ratios (Figure 1c). In contrast, increasing amounts of HSB5 RNA correlated to an increasing trend in stable efficiency reaching an optimal level of 50–60% with 20 μg HSB RNA (Figure 1c).
Comparison of nonviral transgene expression with virally mediated transduction in peripheral blood lymphocytes
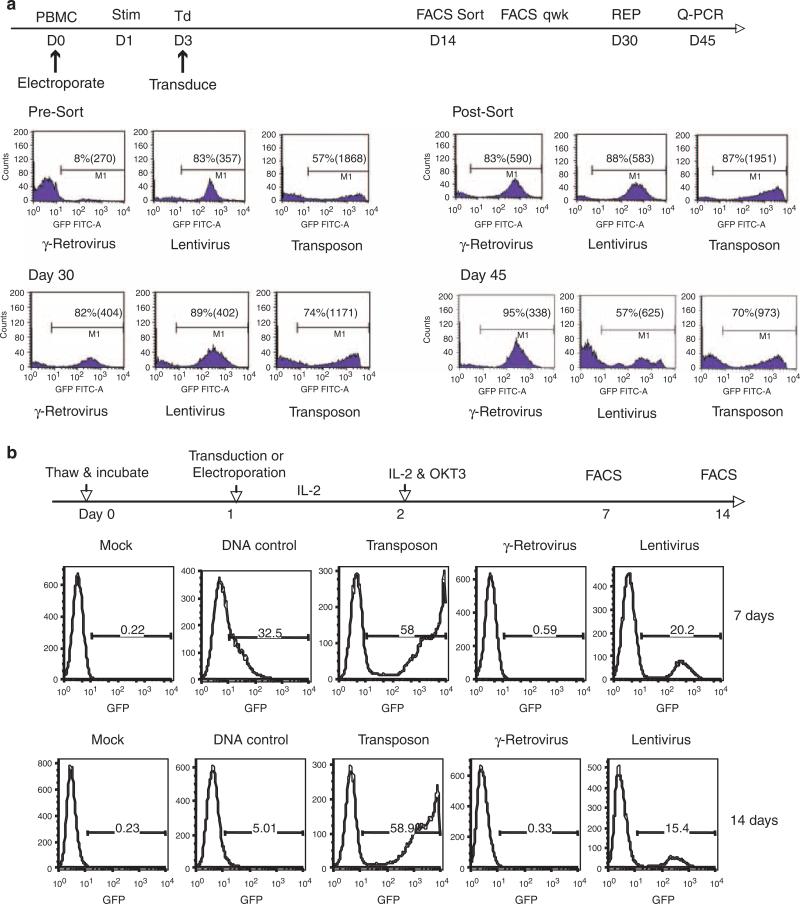
To compare transposon gene transfer to traditional viral vector-mediated gene transfer, an identical transgene expression cassette with an MSCV-U3 promoter-driving expression of a GFP reporter was inserted into the Sleeping Beauty transposon, a γ-retroviral and a lentiviral vector. Fresh PBL were isolated and virally transduced with γ-retrovirus or lentivirus, or electroporated with transposon DNA/transposase RNA, containing the identical expression cassettes (Figure 2a). To promote transduction, the γ-retroviral and lentiviral vector cultures were stimulated for 2 days in IL2 cytokine plus anti-CD3 antibody OKT3 before exposure to virus. After 2 weeks, FACS was used to isolated positive GFP-expressing cells (>80% +) which were maintained in culture and assayed for GFP reporter expression weekly thereafter. We observed that all cultures continued to express GFP, although in the transposon-engineered cultures, a slight decrease in the percent of GFP+ cells was noted (87% after sort and 74% at day 30; Figure 2a). At day 30, the cultures where subjected to a rapid expansion protocol (REP) to increase cell numbers and then assayed at day 45 for GFP expression by FACS. We observed that at every time point, mean fluorescence intensity (MFI) was significantly higher in the transposon group when compared with the γ-retrovirus and lentivirus-transduced cells (Figure 2a). To investigate this difference in MFI, relative transgene copy number was determined for each group by quantitative PCR after normalization to β-actin. Relative comparison to the γ-retrovirus group (based on initial gene transfer efficiency of 8%, we set the copy number for the γ-retrovirus to a value of 1.0 copy per cell) revealed that the lentivirus-transduced PBL population contained 5 times the copy number of the GFP transgene, whereas the transposon-engineered PBL contained 25 times the γ-retroviral vector transgene copy number. These data suggest that the differences in GFP expression per cell measured by MFI, may have resulted from higher transposon copy number per cell.
Figure 2.
Comparison of green fluorescent protein (GFP) reporter expression in γ-retroviral, lentiviral or transposon-mediated genetically modified PBL. Identical expression gene expression cassettes (MSCV-U3-GFP) were cloned into each vector system and produced as described.40,41 (a) Peripheral blood mononuclear cell (PBMC) were electroporated with transposon system on day 0 or transduced with viral vectors following 2 days of stimulation with interleukin-2 (IL2; 300 IU ml-1) and OKT3 (50 ng ml-1). On day 14, GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS) to normalize for differences in gene transfer efficiency. GFP-positive population of cells in each vector group was assayed weekly by FACS and on day 30 subject to a further expansion using the rapid expansion protocol (REP).42 Shown were the percent positive cells and mean fluorescence intensity (MFI) of each group measured at the times indicated. Transgene copy number was determined by quantitative PCR after normalization with β-actin. β-Actin and GFP primer-probes (Tamra) were from Applied Biosystems Oligonucleotide Factory (Foster City, CA, USA). Reactions performed on Applied Biosystems 7500 Fast Real-time PCR System. (b) Cryopreserved PBMC were resuspended in AIM-V medium and culture overnight. The next day, cells were subject to electroporation with the Sleeping Beauty (SB) transposon alone (DNA control) or transposon plus transposase RNA (Transposon) or transduction with γ-retroviral or lentiviral vectors. IL2 (300 IU ml-1) was added to the cultures after gene transfer and the following day T cells were stimulated with OKT3 (50 ng ml-1). The number GFP-positive cells in each vector group were determined by FACS at the time points indicated.
Using the same GFP-expressing gene transfer vectors, we compared the ability of transposons and viral vectors to engineer unstimulated PBL. It is well established that γ-retroviral vectors require target cells to undergo cell division for productive transduction, and although lentiviral vectors do not require full T-cell activation for effective gene transfer (for lentiviruses to productively transduce T cells, the T cells must receive minimal stimulation with cytokines such that they move from the G0 to G1b stage of the cell cycle27,28). Cryopreserved PBL were thawed and put into culture with media lacking cytokine and the next day subject to electroporation with the Sleeping Beauty transposon, or transduced with the γ-retrovirus or lentiviral vectors (Figure 2b). Following electroporation/transduction, IL2 was added to the cultures and on day 2 T cells were stimulated by the addition of OKT3. FACS analysis at day 7 and day 14 demonstrated τ58% GFP+ cells in transposon-electro-porated cultures, >15% GFP+ T cells in cultures transduced with the lentiviral vector, but no GFP+ cells in the γ-retroviral vector-transduced cultures. These observations confirm previous results with viral vectors and suggest that transposons may be an effective method to introduce genes into minimally stimulated T cells. T-cell stimulation may change the phenotype of T cells in ways that make them potentially less active in vivo and murine models of adoptive cell therapy have demonstrated an inverse relationship between the amount of ex vivo T-cell stimulation and in vivo antitumor efficacy.10
Transposon-mediated expression of tumor antigen-specific T-cell receptors in peripheral blood lymphocytes
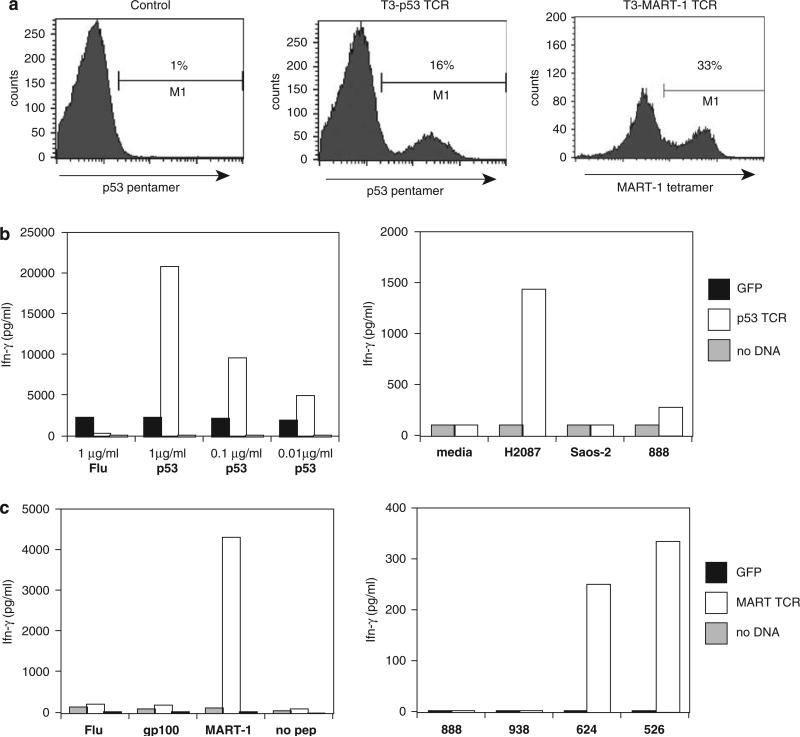
Previous studies have demonstrated that introduction of a γ-retroviral-based construct encoding α- and β-chain genes of an HLA-A2-restricted TCR specific for p53 antigen into PBL resulted in structural pentamer-specific staining, and antigen-specific reactivity, suggesting a potential method to target p53 antigen expressing epithelial tumors.5,29 A p53 TCR expression cassette was inserted in the pT3 MSCV-U3 transposon to generate pT3-p53, which was electroporated into PBMC along with mRNA encoding Sleeping Beauty HSB5 transposase. T cells were stimulated the next day with IL2 and OKT3 and expanded to test for the expression and function of the transposon introduced TCR.
Three weeks after electroporation, FACS analysis with p53 pentamer demonstrated 16% pentamer staining, compared with 1% background for control electroporation without helper transposase mRNA (Figure 3a). p53 TCR-engineered T cells were cocultured with peptide-pulsed antigen-presenting cells (APC; T2 cells pulsed with p53:264–272 peptide) and after overnight culture, effector cytokine interferon-γ levels were determined by enzyme-linked immunosorbent assay (ELISA). Results demonstrated specific interferon-γ release using APCs pulsed with p53 peptide, whereas coculture with an irrelevant flu peptide demonstrated background reactivity (Figure 3b). To test for reactivity of p53-TCR-engineered T cells with tumor cell targets, overnight coculture with tumor cell lines were conducted. Data in Figure 3b demonstrated specific reactivity dependent on both HLA-A2+ and p53 cognate antigen expressing tumor lines, as measured by interferon-γ production.
Figure 3.
Transposon-mediated transfer of antitumor antigen T-cell receptors (TCR). pT3-p53 was constructed by cloning of the anti-p53 TCR previously reported43 with the α- and β-chains being linked by a T2A ribosomal skip peptide.44 pT3-MART-1 was engineered by cloning of the σ-T2A-β-MART-1-specific TCR DMF545 containing murine constant regions46 into the pT3 transposon. (a) Peripheral blood mononuclear cell (PBMC) were coelectroporated with pT3-p53 or pT3-MART-1 and HSB5 RNA (20 μg each) and expression measured at 10 days after gene transfer by fluorescence-activated cell sorting (FACS) using MART-1 tetramer (Beckman Coulter, San Jose, CA, USA) or p53 pentamer (ProImmune, Oxford, UK). (b) Left panel: interferon-γ (IFN-γ) production assayed by enzyme-linked immunosorbent assay (ELISA; Endogen, Cambridge, MA, USA) of supernatant from overnight coculture of pT3p53/HSB5 RNA-electroporated PBMC and T2 cells pulsed with p53:264–272 peptide at labeled concentrations. Right panel: interferon-γ production assayed by ELISA of supernatant from overnight coculture of pT3-p53/HSB5 coelectroporated cells and listed tumor lines (H2087 HLA A2+/p53+, Saos2 A2+/p53 , 888 A2-/p53–). (c) Left panel: interferon-γ production assayed by ELISA of supernatant from overnight coculture of pT3-MART-1/HSB5 RNA-electroporated PBMC and melanoma tumor cell lines (888, MART-1+ /HLA-A2–, 938 MART-1+/HLA-A2–, 624 HLA-A2+/MART-1+, 528 HLA-A2+/MART-1+).
TCR specific to the well-characterized melanoma tumor antigen MART-1 have been isolated and used as a γ-retroviral transgene to genetically modify PBL ex vivo in a recent clinical trial involving patients with metastatic melanoma.9 We constructed a transposon expressing an anti-MART-1 TCR and used it to electroporate PBMC along with Sleeping Beauty HSB5 transposase RNA followed by stimulation with IL2 and OKT3 for cell expansion.
At day 10 after electroporation, FACS analysis with MART-1 tetramer demonstrated 33% tetramer staining, compared with <1% background for nucleofection without helper transposase mRNA (Figure 3a). Overnight coculture with MART-1:27–35 peptide-pulsed APC demonstrated specific antigen reactivity as determined by interferon-γ ELISA (Figure 3c). Overnight coculture with tumor cell lines demonstrated specific tumor cell line recognition dependent on both HLA-A2+ and MART-1+ (determined by interferon-γ ELISA assay; Figure 3c).
Comparison of retroviral and transposon-mediated anti-MART-1 TCR genetically modified lymphocytes
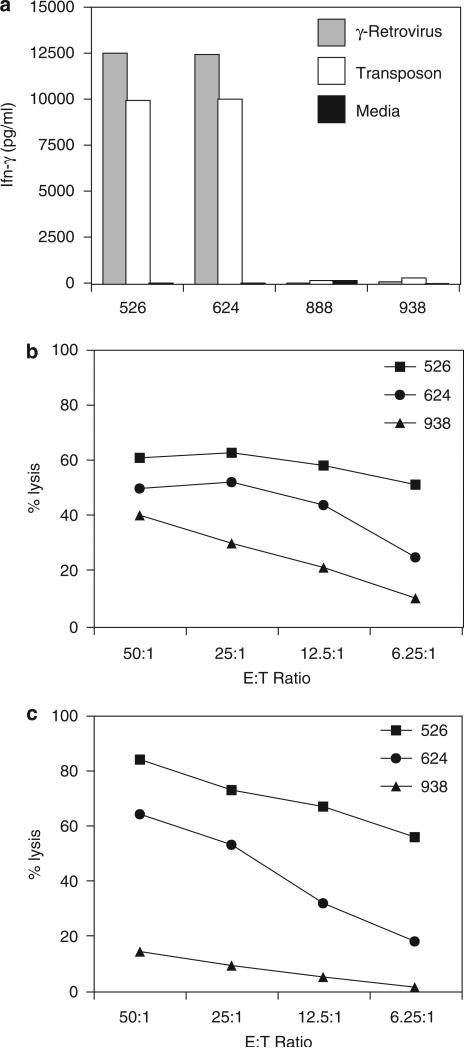
Using an identical expression cassette, PBL were genetically modified to express the anti-MART-1 TCR through either γ-retroviral transduction or transposon-mediated engineering. FACS analysis at 1 week demonstrated 28% and 36% MART-1 tetramer positivity for the γ-retrovirus and transposon vectors respectively (data not shown). The two populations of TCR-engineered T cells were then cocultured with melanoma cell lines expressing combinations of MART-1 and HLA-A2. Both groups of anti-MART-1 TCR genetically modified lymphocytes demonstrated specific reactivity to tumor lines with comparable interferon-γ production (Figure 4a). Specific cytolytic activity against A2+/MART-1+ tumor lines for lymphocytes genetically modified through γ-retroviral transduction or transposon integration was demonstrated for both vector systems by chromium-51 release assay (Figure 4b and 4c).
Figure 4.
Comparison of tumor-antigen reactivity between γ-retroviral and transposon T-cell receptor (TCR) vectors. The anti-MART-1 TCR expression cassette was engineered into both γ-retrovirus and transposon vectors. Peripheral blood mononuclear cell (PBMC) were genetically modified by the two vector systems and comparisons made in TCR expression and functional reactivity. γ-Retrovirus transduction resulted in 28%, whereas transposon resulted in 36% MART-1 tetramer staining by fluorescence-activated cell sorting (FACS) analysis (not shown). (a) Both γ-retrovirus and transposon modified lymphocytes demonstrated specific reactivity to HLA-A2+ and MART-1+ melanoma tumor cell lines by interferon-γ (IFN-γ) enzyme-linked immunosorbent assay (ELISA). MART-1 TCR transposon (b) and γ-retroviral vector (c) modified lymphocytes demonstrated increased specific cytolytic activity of HLA-A2+ and MART-1+ tumor cell lines in a chromium release assay (performed as previously described47).
Adoptive immunotherapy with gene-modified lymphocytes against cancer has demonstrated clinical efficacy, however, safety and production issues involving viral vectors limit its use. Recent studies using a nonviral transposon-based system indicate the possibility of overcoming issues regarding viral vectors but were hampered by low efficiency gene transduction. Our study demonstrates that a nonviral transposon-based system can mediate high-efficiency gene transfer to PBL and transposon-based transfer of an antitumor antigen-specific TCR can confer a specific antitumor phenotype comparable to γ-retroviral transduction. Recently, two groups demonstrated similar results using transposon-based chimeric antigen receptors (CAR) targeting the CD19 antigen, which is upregulated in certain leukemias.30,31 TCR-based therapies may have an advantage over CAR-based therapies in that they use natural T-cell signaling mechanisms. Clinical reports on the utility of these two approaches to tumor antigen recognition have demonstrated cell persistence and antitumor efficacy for TCR therapy9 but not yet for CAR-based therapies.32–34
An inherent risk in viral production is the potential generation of replication-competent retroviruses through recombination events. By utilizing an HSB5 transposase RNA substrate, we eliminate the risk of DNA recombination with the transposon and also provide temporal control of the transient transposase expression, thereby minimizing the risk of transposon remobilization. Additionally, our studies demonstrated that overexpression inhibition, which is a transposon phenomenon limiting high efficiency transposition to optimally titrated ratios of donor/helper, is not a significant factor when using an RNA transposase substrate. Transposition efficiency was not inhibited at higher ranges of HSB5 transposase RNA tested, and moreover, MFI appeared positively correlated to the amount of HSB5 transposase RNA (data not shown). It may be possible that the RNA-mediated transient expression of transposase allows for inherent titration to optimal ratios.
Functional studies of transposon-mediated gene-modified lymphocytes were performed with transfer of antitumor antigen-specific TCR targeting p53 and MART-1 tumor antigen epitopes. Clinical tumor regression was recently demonstrated using adoptive transfer of gene-modified lymphocytes targeting the melanoma tumor antigen MART-1. In this study, we demonstrate that transposon-mediated gene transfer of TCR targeting p53 and MART-1 can confer specific cognate antigen-dependent antitumor reactivity. Gene-modified reactive lymphocytes sustained expression of the TCR and were functionally reactive in interferon-γ release and tumor cell lysis assays. Although the clinical application of electroporation has been demonstrated with both dendritic cells35 and T lymphocytes following marker gene selection,36 robust methods for the electroporation (without marker gene selection) of large numbers of T cells required for adoptive immunotherapy are still under development.
For gene transfer vectors, sustained levels of gene expression may be dependent on diverse mechanisms, including silencing, metabolic activity and integration site preference. Comparisons of GFP expression after transduction mediated by γ-retrovirus, lentivirus or transposons revealed consistently higher transposon-mediated expression assessed by MFI. To investigate the role of transgene copy number in reporter gene expression, we performed quantitative PCR on genomic DNA from purified GFP-positive PBL transduced with γ-retrovirus, lentivirus or transposon and demonstrated higher relative copy numbers for transposon-engineered cultures. These data suggest that increased integrated transgene copy number may be involved in the observed differential transgene expression observed for transposons. The increased transposon copy number per cell (compared to γ-retroviral-engineered cells) may lessen any potential safety advantages transposons may have in relation to integration site preference.25 Efforts are also underway to develop sequence-targeted transgene insertion with DNA-binding domain-chimeric transposase enzymes.37,38
These novel findings indicate that the transposon-mediated nonviral TCR gene modification of PBL is efficient and transgene levels are sustained in vitro. When compared to viral vectors, the more scalable nature and simpler production methods of this plasmid/RNA-based platform suggest that current efforts in expanding to clinical scale may have considerable utility in translating adoptive cell transfer of genetically modified hematopoietic cells for treatment of cancer and immunologic diseases.
Acknowledgements
We thank Dr Steven R Yant and Dr Mark A Kay of the Departments of Pediatrics and Genetics, Stanford University School of Medicine, Stanford, CA, for their generous gift of the Sleeping Beauty transposon plasmids and hyperactive transposase, as well as for their helpful suggestions on this project. We thank FACS lab and TIL lab in Surgery Branch for providing technical support and maintenance of tumor cells from patients. This work is supported by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [erratum appears in J Immunol. 2006 Oct 15; 177(8):5746]
- 6.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes.[see comment]. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells.[see comment]. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannell D, Ellis J. Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol. 2001;11:205–217. doi: 10.1002/rmv.316. [DOI] [PubMed] [Google Scholar]
- 12.Swindle CS, Klug CA. Mechanisms that regulate silencing of gene expression from retroviral vectors. J Hematother Stem Cell Res. 2002;11:449–456. doi: 10.1089/15258160260090915. [DOI] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency.[see comment]. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration [see comment]. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 15.Hackett PB, Ekker SC, Largaespada DA, McIvor RS. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv Genet. 2005;54:189–232. doi: 10.1016/S0065-2660(05)54009-4. [DOI] [PubMed] [Google Scholar]
- 16.Ivics Z, Izsvak Z. Transposons for gene therapy! Curr Gene Ther. 2006;6:593–607. doi: 10.2174/156652306778520647. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Mah C, Fletcher BS. Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon. Mol Ther. 2006;13:1006–1015. doi: 10.1016/j.ymthe.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz-Urda S, Lin Q, Yant SR, Keene D, Kay MA, Khavari PA. Sustainable correction of junctional epidermolysis bullosa via transposon-mediated nonviral gene transfer. Gene Therapy. 2003;10:1099–1104. doi: 10.1038/sj.gt.3301978. [DOI] [PubMed] [Google Scholar]
- 19.Yant SR, Meuse L, Chiu W, Ivics Z, Izsvak Z, Kay MA. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet. 2000;25:35–41. doi: 10.1038/75568. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Wilber AC, Bao L, Tuong D, Tolar J, Orchard PJ, et al. Stable gene transfer and expression in human primary T cells by the Sleeping Beauty transposon system. Blood. 2006;107:483–491. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yant SR, Park J, Huang Y, Mikkelsen JG, Kay MA. Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells. Mol Cell Biol. 2004;24:9239–9247. doi: 10.1128/MCB.24.20.9239-9247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zayed H, Izsvak Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004;9:292–304. doi: 10.1016/j.ymthe.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Wilber A, Frandsen JL, Geurts JL, Largaespada DA, Hackett PB, McIvor RS. RNA as a source of transposase for Sleeping Beauty-mediated gene insertion and expression in somatic cells and tissues. Mol Ther. 2006;13:625–630. doi: 10.1016/j.ymthe.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Geurts AM, Yae K, Srinivasan AR, Fahrenkrug SC, Largaespada DA, et al. Target-site preferences of Sleeping Beauty transposons. J Mol Biol. 2005;346:161–173. doi: 10.1016/j.jmb.2004.09.086. [DOI] [PubMed] [Google Scholar]
- 25.Yant SR, Wu X, Huang Y, Garrison B, Burgess SM, Kay MA. High-resolution genome-wide mapping of transposon integration in mammals. Mol Cell Biol. 2005;25:2085–2094. doi: 10.1128/MCB.25.6.2085-2094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, et al. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003;8:108–117. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 27.Cavalieri S, Cazzaniga S, Geuna M, Magnani Z, Bordignon C, Naldini L, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Cui Y, Huang X, Yu Z, Thomas AM, Ye Z, et al. Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum Gene Ther. 2003;14:1089–1105. doi: 10.1089/104303403322124800. [DOI] [PubMed] [Google Scholar]
- 29.Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Guo H, Kang J, Choi S, Zhou TC, Tammana S, et al. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol Ther. 2008;16:580–589. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 34.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 36.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivics Z, Katzer A, Stuwe EE, Fiedler D, Knespel S, Izsvak Z. Targeted Sleeping Beauty transposition in human cells. Mol Ther. 2007;15:1137–1144. doi: 10.1038/sj.mt.6300169. [DOI] [PubMed] [Google Scholar]
- 38.Yant SR, Huang Y, Akache B, Kay MA. Site-directed transposon integration in human cells. Nucleic Acids Res. 2007;35:e50. doi: 10.1093/nar/gkm089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lizee G, Gonzales MI, Topalian SL. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther. 2004;15:393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- 42.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 43.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-Cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]