Abstract
Paraneoplastic syndromes are an uncommon, yet well-described, phenomenon in cancer patients. The syndrome of granulocytosis caused by granulocyte colony-stimulating factor (G-CSF) production by tumors is rare and is difficult to diagnose in patients receiving treatment for metastatic disease. From January 2005 to May 2009, 626 patients were evaluated for treatment of metastatic melanoma. At initial evaluation or during the course of treatment, six patients had an elevated white blood cell count and no evidence of infection. All six had significantly elevated serum G-CSF. The level of serum G-CSF was directly correlated with the absolute neutrophil count. In-vitro assay of melanoma tumor from two patients showed elevated G-CSF in cell culture supernatant. The paraneoplastic syndrome of granulocytosis resulting from ectopic G-CSF production in patients with metastatic melanoma is rare. This diagnosis should be considered when common causes of granulocytosis have been ruled out.
Keywords: granulocyte colony-stimulating factor, melanoma, paraneoplastic syndromes
Introduction
Melanoma is the sixth most common cancer in the United States with an estimated incidence of 62 480 in 2008 with 8420 deaths [1,2]. Patients with metastatic melanoma have a median survival of 6 months and an estimated 5-year survival less than 15% [3–5]. The treatment for stage IV melanoma includes systemic chemotherapy, high-dose interleukin-2 (IL-2), and experimental therapies [6–9]. Screening laboratory studies may show an elevated white blood cell count (WBC) at diagnosis or during the treatment of melanoma. This finding may be caused by infection, bone marrow metastasis, or concomitant corticosteroid administration. In this report, we observed an elevated WBC with neutrophilia in six patients with metastatic melanoma without any of these inducing factors. After an extensive work-up for infectious disease, we obtained serum granulocyte colony-stimulating factor (G-CSF) levels in each patient. The paraneoplastic syndrome (PS) of granulocytosis was diagnosed in all six patients after the confirmation of elevated serum G-CSF.
Methods
This review includes patients referred to the Surgery Branch of the National Cancer Institute, NIH, for the treatment of metastatic melanoma from January 2005 to May 2009. All patients provided informed consent before enrollment on protocols approved by the Institutional Review Board of the National Cancer Institute. Patients with an elevated WBC before or during the treatment were evaluated initially by complete blood count with differential, comprehensive chemistry panel, blood cultures, urine analysis, and computed tomography of the chest, abdomen, and pelvis. Additional studies were performed in consultation with infectious disease specialists. Serum was sent for G-CSF level in patients who had an elevated WBC and negative work-up for infectious disease (University of Minnesota Outreach Laboratories, Minneapolis, Minnesota, USA).
Results
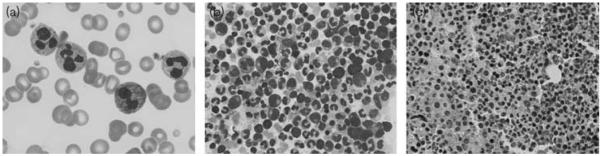
Demographic, laboratory, and treatment details of the patients are listed in Table 1. After an initial evaluation for infectious disease only one patient (E) had evidence of a probable infectious cause of leukocytosis (urinary tract infection), which was treated with antibiotic therapy. Otherwise, all patients had blood cultures, urine analysis, and computed tomographic scans which failed to identify an infectious source. Patient E, subsequent to treatment for urinary tract infection, continued to have an unexplained leukocytosis despite resolution of urinary tract infection. Patients A and B underwent bone marrow aspiration and review of the peripheral blood smear by hematology consultants. The bcr–abl gene translocation was negative by PCR in each of these patients. Peripheral blood smears of all patients in our series showed abundant mature neutrophils with occasional myelocytes or metamyelocytes, as shown in Fig. 1, consistent with the effects of excessive serum G-CSF. In addition, a transthoracic echocardiogram to rule out endocarditis in patients A, B, and C failed to show an infectious source.
Table 1.
Patient demographic, treatment, and laboratory data
| Patient | Age (years) |
Sex | Metastatic sites |
Prior systemic therapy |
WBC (4–10 k/ml) |
Hgb (11–16 g/dl) |
Platelets (173–370 k/ml) |
ANC (1.5–6.1 k/ml) |
Serum G-CSF ( <39 pg/ml) |
|---|---|---|---|---|---|---|---|---|---|
| A | 49 | F | lu, ln, li, su | Temozolamide, IL-2, ACT | 54.8 | 8.7 | 228 | 52 | 273 |
| B | 24 | M | ln | IL-2 | 61.2 | 14 | 396 | 52.1 | 624 |
| C | 56 | M | lu, ad, su | SMI, IL-2 | 22.3 | 7.8 | 602 | 19.9 | 71 |
| D | 60 | M | ln, rp, su, im | IL-2 | 40.1 | 9.1 | 635 | 31.8 | 184 |
| E | 24 | F | lu, ln, su | IL-2 | 38.5 | 11.2 | 326 | 34.5 | 488 |
| F | 51 | M | ln, im, ip | IL-2 | 18.8 | 11.4 | 460 | 13.9 | 155 |
Serum G-CSF levels correspond with cell counts shown.
Normal laboratory values shown in brackets.
ACT, adoptive cell transfer; ad, adrenal glands; ANC, absolute neutrophil count; F, female; G-CSF, granulocyte colony-stimulating factor; Hgb, hemoglobin; IL-2, interleukin-2; im, intramuscular; ip, intraperitoneal; li, liver; ln, lymph nodes; lu, lungs; M, male; rp, retroperitoneum; SMI, small molecule inhibitor; su, subcutaneous tissue; WBC, white blood cell count.
Fig. 1.
Peripheral blood and bone marrow granulocytic hyperplasia in patients with paraneoplastic granulocytosis. (a) Peripheral blood smear from patient A shows markedly increased number of neutrophils. Many neutrophils have toxic granules and fewer have Döhle bodies. Bone marrow aspirate smear (b) and biopsy (c) from patient B show hypercellularity and granulocytic hyperplasia with focal left shift in myeloid maturation. Magnification (a) × 1000, (b) × 400, (c) ×200.
In all cases, a serum G-CSF level was obtained once the initial tests were negative for infection. In each patient, serum G-CSF was significantly elevated (normal < 39 pg/ml) and the degree of leukocytosis directly correlated to the level of serum G-CSF measured (Fig. 2). The time from diagnosis of metastatic melanoma to the detection of leukocytosis was 14, 2, and 9 months in patients A, B, and C, respectively. All patients in our series were treated with high-dose IL-2 before the onset of leukocytosis (range 2–12 months). In addition, one patient (C) received two experimental small molecule inhibitors before being evaluated at our institution, therefore the time from treatment to the onset of leukocytosis is difficult to ascertain. Another patient (A) received temozolamide (15 months before) and then a short course of exogenous G-CSF in conjunction with nonmyeloablative chemotherapy and adoptive cell transfer. Patient A received 10 doses of recombinant G-CSF and developed leukocytosis with neutrophilia, 4 months after completing the treatment, therefore a causal relationship does not seem plausible.
Fig. 2.

Positive correlation of serum granulocyte colony-stimulating factor (G-CSF) level to absolute neutrophil count.
In an assay, using 1 × 105 tumor cells incubated in 200 μl cell culture medium for 48 h, supernatant G-CSF levels were 360 (patient B) and 192 pg/ml (patient C). In comparison, a patient with metastatic melanoma, with a similar treatment history, and a normal WBC had tumor cell culture supernatant G-CSF level of 27 pg/ml and normal serum G-CSF level of 31 pg/ml. These latter results are suggestive of a process in which tumor cells are the putative source of elevated serum G-CSF. The case of patient F provides a clinical correlation of this proposed pathophysiologic process. At the time of evaluation, patient F’s WBC was 18.8 k/μl with serum G-CSF of 155 pg/ml. He underwent resection of a large axillary metastasis for the purpose of generating tumor infiltrating lymphocytes (TIL). There was no evidence of infection in the tumor specimen. After surgery his WBC fell to 13.1 k/μl (absolute neutrophil count 8.4 k/μl) with a corresponding serum G-CSF level of 32 pg/ml.
Discussion
Paraneoplastic syndromes (PS) result from the indirect effect of cancer on normal tissue through the production of humoral, neural, immune, or other factors [10]. Cytokines, protein hormones, their precursors, and antigen–antibody interactions are the most common mediators of PS. Diagnosis of PS requires the identification of a clinical syndrome, the detection of a neoplasm, and identification of the acting substance [10]. In 1928, Brown [11] described a case of ‘pluriglandular syndrome’ associated with oat-cell carcinoma of the lung, later known as Cushing syndrome. Four decades later, the ectopic production of adrenocorticotropic hormone by tumors was identified as the cause of this syndrome. A variety of other endocrinologic manifestations of cancer have been characterized including hematologic, neurologic, renal, cutaneous, and rheumatologic syndromes. Occasionally, the first evidence of malignancy may be the PS, such as hypercalcemia caused by parathyroid hormone-related peptide secreted by nonsmall cell lung cancer. In many instances, the successful treatment of the cancer leads to resolution of the syndrome.
Hematologic manifestations of cancer are common. Cytopenias are seen frequently in cancer patients and often attributed to chronic disease, tumor infiltration of bone marrow, or the effects of chemotherapy and radiation. Alternatively, abnormally elevated cell counts may be secondary to myeloproliferative disorders, inflammation, or excess hematopoietic growth factors. For instance, elevated erythropoietin levels observed in some renal carcinomas may cause erythrocytosis.
G-CSF is a glycoprotein that stimulates the production, maturation, function, and survival of neutrophils. G-CSF also mobilizes hematopoietic stem cells into the peripheral circulation by an unknown mechanism [12]. Administration of recombinant human G-CSF results in an increased number of circulating activated neutrophils and an increase in the number of neutrophil progenitors (promyelocytes, metamyelocytes, and myelocytes) in the bone marrow. Granulocytosis, because of excess G-CSF, is characterized by a predominance of mature neutrophils in the circulation, as opposed to chronic myelogenous leukemia, where mature neutrophils and immature progenitors (including blast forms) may be seen in comparable numbers in the peripheral blood. In chronic myelogenous leukemia, there is typically a translocation (t9;22, the Philadelphia chromosome) that results in the expression of the bcr/abl oncogenes; this can be detected by PCR, and was negative in the two initial patients (A and B) we studied for this rearrangement.
Paraneoplastic granulocytosis may be encountered in a variety of solid tumors such as small cell and nonsmall cell lung cancer, hepatocellular carcinoma, pancreatic adenocarcinoma, sarcoma, bladder carcinoma, glioblastoma, nasopharyngeal carcinoma, esophageal small cell carcinoma, and melanoma [13–21]. Melanoma has been associated with a variety of hormonal syndromes, dermatologic syndromes, and disorders involving organ systems other than the skin [22]. Reports of granulocytosis attributed to a paraneoplastic process in patients with metastatic melanoma are rare. In 1987, Lilly et al. [23] reported that a human melanoma cell line was isolated from a melanoma patient with an unexplained leukocytosis. Molecular studies showed the production of G-CSF in vitro. In a separate report by Schniewind et al. [24], a patient with rapidly progressive melanoma had leukocytosis and an elevated serum G-CSF level. The investigators suggested that G-CSF might suppress T cell-mediated immune response against human melanoma and that its production by melanoma tumors portends a poor prognosis.
Myeloid-derived suppressor cells (MDSC) represent a population of immature cells in cancer patients that mediate immune suppression through multiple proposed mechanisms. For example, MDSC levels are inversely correlated to T-cell receptor zeta chain expression and reduced interferon-γ and IL-2 production in T cells of cancer patients [25,26]. It is conceivable that tumorderived cytokines, such as G-CSF, may induce the expansion of MDSC and thus facilitate the unimpeded growth of tumor. In the six patients presented here, the phenotype suggestive of MDSC in peripheral blood mononuclear cells is not known. Further investigation in these patients could yield a population of MDSC that is associated with the unrestrained growth of immunosuppressive melanoma tumors.
We report on six patients with stage IV melanoma who developed granulocytosis with neutrophilia (Table 1). All patients in this series shared the diagnosis of metastatic melanoma and had a persistently elevated WBC. After an infectious etiology was not identified, a serum G-CSF level was obtained and was abnormally elevated in all patients. The source of this hormone was believed to be the metastatic melanoma. The degree of leukocytosis was directly correlated with the level of measured G-CSF (Fig. 2), suggesting that the paraneoplastic effect was because of increased G-CSF production rather than acquired sensitivity to G-CSF by myeloid progenitors. All patients in this series were pretreated with IL-2 and one patient received a short course of exogenous G-CSF after nonmyeloablative chemotherapy. There was no temporal relation to IL-2 administration and onset of leukocytosis with neutrophilia. In fact, a well-studied phenomenon of high-dose IL-2 therapy is peripheral lymphocytosis observed in patients receiving treatment for metastatic melanoma and renal carcinoma. In addition, the half-life of recombinant human G-CSF is approximately 3.5 h. Therefore, it is unlikely that the exogenous administration of G-CSF in one patient had any impact on the development of leukocytosis 4 months later.
Most cancers arise following a sequence of specific genetic mutations [27]. It is possible that the accumulation of genetic mutations over time leads to abnormal and unregulated production of G-CSF in metastatic melanoma. A de-differentiated melanoma tumor may activate previously silenced genes, such as G-CSF, and acquire an otherwise atypical phenotype. Our observation of three patients over a period of several months is that the onset of paraneoplastic granulocytosis did not coincide with the appearance of metastatic melanoma, but instead developed at a later point in the course of disease. Without a more detailed molecular analysis of tumor cell lines from patients such as ours, the true cause of G-CSF-secreting melanoma tumors remains uncertain. It is also unclear whether these tumors respond differently to standard or experimental therapy, and whether the paraneoplastic process is indicative of an aggressive tumor phenotype.
Hematologic paraneoplastic syndromes can be particularly difficult to diagnose, especially in the case of neutrophilia because of ectopic G-CSF production. Patients with metastatic melanoma may be malnourished, functionally immunosuppressed, and prone to infections. Therefore, the most common cause of an elevated WBC in this patient population is infection. In some patients, however, an infectious source cannot be identified. Six out of 626 patients were found to have leukocytosis with neutrophilia, a negative infectious work-up, and elevated serum G-CSF. The incidence of paraneoplastic syndrome because of ectopic G-CSF production in patients with metastatic melanoma was 0.95% in this series. Therefore, in patients with an unexplained rise in WBC, ectopic production of G-CSF should be considered.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society . Cancer facts and figures 2008. American Cancer Society; Atlanta, GA: 2008. [Google Scholar]
- 4.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 5.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 8.Ahmann DL, Creagan ET, Hahn RG, Edmonson JH, Bisel HF, Schaid DJ. Complete responses and long-term survivals after systemic chemotherapy for patients with advanced malignant melanoma. Cancer. 1989;63:224–227. doi: 10.1002/1097-0142(19890115)63:2<224::aid-cncr2820630203>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, McClay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 2002;29:413–426. doi: 10.1053/sonc.2002.35237. [DOI] [PubMed] [Google Scholar]
- 10.Boyiadzis M, Lieberman FS, Geskin LJ, Foon KA. Paraneoplastic syndromes. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: principles and practice of oncology. 8th ed Lippincott, Williams & Wilkins; New York: 2008. pp. 2343–2362. [Google Scholar]
- 11.Brown WH. A case of pluriglandular syndrome: diabetes of bearded women. Lancet. 1928;2:1022–1023. [Google Scholar]
- 12.Kurkjian CD, Ozer H. Leukopenia anemia and thrombocytopenia. In: DeVita VTLT, Rosenberg SA, editors. Cancer: principles and practice of oncology. 8th ed Lippincott, Williams & Wilkins; New York: 2008. pp. 2617–2633. [Google Scholar]
- 13.Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92:2399–2405. doi: 10.1002/1097-0142(20011101)92:9<2399::aid-cncr1588>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Furihata M, Sonobe H, Iwata J, Ido E, Ohtsuki Y, Asahi Y, et al. Lung squamous cell carcinoma producing both parathyroid hormone-related peptide and granulocyte colony stimulating factor. Pathol Int. 1996;46:376–379. doi: 10.1111/j.1440-1827.1996.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 15.Araki K, Kishihara F, Takahashi K, Matsumata T, Shimura T, Suehiro T, et al. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27:716–721. doi: 10.1111/j.1478-3231.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi KM, Raman AK, Tan D, Fakih MG. Leukemoid reaction in pancreatic cancer: a case report and review of the literature. Jop. 2006;7:631–634. [PubMed] [Google Scholar]
- 17.Jardin F, Vasse M, Debled M, Dominique S, Courville P, Callonnec F, et al. Intense paraneoplastic neutrophilic leukemoid reaction related to a G-CSF-secreting lung sarcoma. Am J Hematol. 2005;80:243–245. doi: 10.1002/ajh.20454. [DOI] [PubMed] [Google Scholar]
- 18.Turalic H, Deamant FD, Reese JH. Paraneoplastic production of granulocyte colony-stimulating factor in a bladder carcinoma. Scand J Urol Nephrol. 2006;40:429–432. doi: 10.1080/00365590600679350. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa K, Kitamura T, Oka T, Matsushita H. Bladder tumor producing granulocyte colony-stimulating factor and parathyroid hormone related protein. J Urol. 2002;167:2130. [PubMed] [Google Scholar]
- 20.Melhem MF, Meisler AI, Saito R, Finley GG, Hockman HR, Koski RA. Cytokines in inflammatory malignant fibrous histiocytoma presenting with leukemoid reaction. Blood. 1993;82:2038–2044. [PubMed] [Google Scholar]
- 21.Hintzen RQ, Voormolen J, Sonneveld P, van Duinen SG. Glioblastoma causing granulocytosis by secretion of granulocyte-colony-stimulating factor. Neurology. 2000;54:259–261. doi: 10.1212/wnl.54.1.259. [DOI] [PubMed] [Google Scholar]
- 22.Wagner RF, Jr, Nathanson L. Paraneoplastic syndromes, tumor markers, and other unusual features of malignant melanoma. J Am Acad Dermatol. 1986;14(2 Pt 1):249–256. doi: 10.1016/s0190-9622(86)70029-7. [DOI] [PubMed] [Google Scholar]
- 23.Lilly MB, Devlin PE, Devlin JJ, Rado TA. Production of granulocyte colony-stimulating factor by a human melanoma cell line. Exp Hematol. 1987;15:966–971. [PubMed] [Google Scholar]
- 24.Schniewind B, Christgen M, Hauschild A, Kurdow R, Kalthoff H, Klomp HJ. Paraneoplastic leukemoid reaction and rapid progression in a patient with malignant melanoma: establishment of KT293, a novel G-CSF-secreting melanoma cell line. Cancer Biol Ther. 2005;4:23–27. doi: 10.4161/cbt.4.1.1447. [DOI] [PubMed] [Google Scholar]
- 25.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 26.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 27.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]