Abstract
With modernization, the Philippines has experienced increasing rates of obesity and related cardiometabolic diseases. Studying how risk factors cluster in individuals may offer insight into cardiometabolic disease etiology. We used cluster analysis to group women who share the following cardiometabolic biomarkers: fasting triglycerides, HDL-C and LDL-C, C-reactive protein, systolic and diastolic blood pressure, homeostasis model assessment of insulin resistance, and fasting glucose. Participants included 1,768 women (36–69 y) in the Cebu Longitudinal Health and Nutrition Survey. We identified 5 distinct clusters characterized by: (1) low levels of all risk factors (except HDL-C and LDL-C) or “healthy”, (2) low HDL-C in the absence of other risk factors, (3) elevated blood pressure, (4) insulin resistance, and (5) high C-reactive protein. We identified predictors of cluster membership using multinomial logistic regression. Clusters differed by age, menopausal status, socioeconomic status, saturated fat intake, and combinations of overweight (BMI>23) and high waist circumference (>80cm). In comparison to the healthy cluster, overweight women without high waist circumference were more likely to be in the high CRP cluster (OR 4=2.26, 95% CI=1.24; 4.11), while women with high waist circumference and not overweight were more likely to be in the elevated blood pressure (OR 2.56, 95% CI=1.20; 5.46) or insulin resistant clusters (OR 4.05, 95% CI=1.39; 11.81). In addition, a diet lower in saturated fat uniquely increased the likelihood of membership to the low HDL-C cluster. Cluster analysis identified biologically meaningful groups, predicted by modifiable risk factors; this may have implications for the prevention of cardiometabolic diseases.
Keywords: cluster analysis, metabolic syndrome, cardiovascular disease, waist circumference, Asia
INTRODUCTION
Rapid nutritional and lifestyle changes in low and middle-income countries are contributing to a growing burden of overweight (OW), visceral adiposity, and associated diseases, including cardiovascular disease (CVD) and diabetes. Eighty percent of global deaths from CVD and related conditions occur in low and middle-income countries, emphasizing the need for more research to guide prevention efforts in these settings.
The Philippines exemplifies global chronic disease trends.1 Our prior work in Cebu, the second largest city in the Philippines, showed substantial age and secular trends in weight among adult women, notably a nearly 7-fold increase in overweight over a 21-year period.2 This increase is associated with adverse cardiometabolic profiles, including hypertension, elevated markers of inflammation, and adverse lipid profiles.1, 3, 4
A large literature demonstrates that cardiometabolic risk factors tend to co-occur, and may be causally interrelated.5 The definition of the “metabolic syndrome” reflects these associations. According to the guidelines established by the International Diabetes Foundation (IDF), an individual with metabolic syndrome must have central obesity plus any two of four additional factors including elevated fasting plasma glucose, high blood pressure (BP), high fasting triglycerides (TG), or low high density lipoprotein cholesterol (HDL-C).6 This metabolic syndrome concept assumes that multiple risk factors share common underlying influences, such as the link between excess body fat and multiple metabolic disturbances.
A competing interpretation of this literature argues that the risk factors included or excluded in the metabolic syndrome definition is unfounded, that the metabolic syndrome is not necessarily unified by a single etiology, and that cardiometabolic risk is dependent on the specific risk factors present.7 For example, inflammation, as indicated commonly by elevated C-reactive protein (CRP), is often not included in the classic metabolic syndrome definition, despite the fact that it predicts CVD and type II diabetes independent of metabolic syndrome status.8 Labeling an individual as having metabolic syndrome may mask the specific risk factors present, thus obscuring the etiology and most effective strategies to prevent metabolic disease.
In addition, metabolic syndrome definitions ignore the heterogeneity in the patterns of risk factor clustering, since one individual with metabolic syndrome may have central obesity, low HDL-C, and raised fasting plasma glucose, while another has central obesity, raised BP, and elevated TG. The composite metabolic syndrome definition could therefore obscure documented differences in the prevalence and patterns of cardiometabolic risk factors across ethnic, age, and sex groups.7, 9 As an example of the heterogeneity in risk factor patterning across ethnicities, low HDL-C followed by elevated BP, are the most prevalent components of the metabolic syndrome among Filipinos, whereas in the United States abdominal obesity followed by low HDL-C are the most prevalent metabolic syndrome components.10
In this paper, we seek to examine the prevalence and patterns of cardiometabolic risk factors among middle-aged Filipino women in the Cebu Longitudinal Health and Nutrition Survey (CLHNS). To avoid many of the problems noted above, we examine the patterns of cardiometabolic risk factors using cluster analysis, which identifies groups of individuals who share common cardiometabolic risk factor patterns. While some past research has used factor analysis to study patterns of cardiometabolic risk factor occurrence in Asian populations, to our knowledge no published work has investigated the clustering of cardiometabolic risk factors.11–14 We used cluster analysis rather than other techniques such as factor analysis because we aim to group individuals based on patterns/individual differences of cardiometabolic biomarkers (an alternative to using metabolic syndrome), whereas factor analysis, a variable reduction technique, would represent biomarker variables as linear combinations of a smaller set of underlying factors.
Next we examined how modifiable (dietary and lifestyle) factors predict cluster membership. The rapid transition in the CLHNS allows us to capture changes we cannot capture so readily in the US. These changes include: less physical activity and increased consumption of fat, caloric sweeteners, and meat. 15 Such diet and physical activity changes have been shown to influence cardiometabolic risk factors.16, 17 In addition, we evaluated other characteristics such as environmental cleanliness, since environmental pathogens are sources of inflammatory stimuli that result in increased production of CRP.4
Obesity and associated diseases are now the leading cause of mortality and a major public health burden in the Philippines. Cluster analysis is a valuable approach because clusters clearly reflect the prevalence and patterns of co-occurrence of risk factors in individuals. Examining how modifiable factors predict membership to clusters can provide insights into the etiology and the prevention of cardiometabolic diseases in this population.
MATERIALS AND METHODS
Survey design
The women in this study are participants in the CLHNS, which is described in detail elsewhere.18 Briefly, the CLHNS is a community-based cohort of women and their index children followed since 1983. The original participants included all pregnant women in 33 randomly selected communities of Metro Cebu, who gave birth between May 1, 1983, and April 30, 1984. A baseline interview was conducted among 3,327 women in their 6th to 7th month of pregnancy. Subsequent surveys took place immediately after birth, bimonthly for 2 years, in 1991, 1994–5, 1998–99, 2002, and 2005. Here we use data from the 2005 CLHNS, when women were 48.4 ± 6.0 y of age. All data were collected under conditions of informed consent with institutional review board approval from the University of North Carolina, Chapel Hill.
Anthropometry
Body weight, height, and waist circumference (WC) were measured using standard anthropometric techniques.19 Body mass index (BMI) was calculated as the ratio of weight (kg) to height (m2). We used WHO cutpoints for Asians to define OW as a BMI ≥ 23 kg/m2 20. We defined high WC or central obesity, specific to women, as WC ≥ 80 cm.6
Cardiometabolic disease biomarkers
Fasting cardiometabolic biomarkers included TG, HDL-C, LDL cholesterol (LDL-C), total cholesterol, glucose, insulin, and C-reactive protein (CRP). Blood samples were collected in participants’ homes in the morning after an overnight fast. Venous blood was collected in EDTA anti-coagulant vacutainer tubes. After mixing to inhibit clotting, glucose was measured immediately using the glucose dehydrogenase method (One Touch Ultra Blood Glucose Monitoring System, Lifescan Johnson and Johnson). Blood samples were stored on ice for no more than 2 hours and were then centrifuged to separate plasma prior to freezing at −70C. After separation, samples were frozen and remained frozen at −80 °C until ready for analysis. Total lipid concentrations were measured at the Emory Lipid Research Laboratory using enzymatic methods with reagents from Beckman Diagnostics on the Beckman Diagnostics CX5 chemistry analyzer (Fullerton, CA). HDL-C was determined using the homogenous assay direct HDL-C (Genzyme Corporation, Exton, PA). LDL-C was determined using the Friedewald formula, except if triglycerides exceeded 400 mg/dl then LDL-C was directly determined using a homogenous assay (Genzyme, Exton, PA). The Emory Lipid Research Laboratory is a participant in the CDC/NHLBI Lipid Standardization Program to ensure accuracy and precision of the determinations.
Plasma insulin was measured using automated Bayer® ADVIA Centaur chemiluminescent methods.22 CRP concentrations were determined using a high sensitivity immunoturbidimetric method (Synchron LX20, lower detection limit: 0.1 mg/L).
Other cardiometabolic biomarkers included homeostatic model assessment insulin resistance (HOMA-IR), and systolic and diastolic BP. We calculated HOMA-IR as 22.5/(insulin X glucose). Systolic and diastolic BP were measured in triplicate after a 10 minute seated rest using a mercury sphygmomanometer. The mean of the three measurements was used.
Risk factor cutpoints
We used cutpoints for these biomarkers based on recommendations from the IDF, the American Heart Association cutpoints, and other previously recognized and accepted cutpoints (Table 1). The HDL-C cutpoint was specific to women. CRP levels greater than 10 mg/L may indicate an acute inflammatory process such as an infectious disease; therefore we excluded women with such values.23 Before using cutpoints to identify participants with impaired fasting glucose, we applied a glucose correction factor to all fasting glucose levels. Glucometers overestimate glucose concentrations in whole venous blood as compared with standard laboratory methods.24, 25 Therefore we subtracted 0.97 mmol/l from fasting glucose values to obtain the best equivalent to venous plasma as analysed by a laboratory autoanalyser.24 The corrected fasting glucose values are reported in the analyses and tables.
TABLE 1.
Criteria for defining high cardiometabolic risk†
| Risk factors | Cutpoint |
|---|---|
| TG | ≥ 150 mg/dL 6 |
| HDL-C | < 50 mg/dL 6 |
| Systolic BP | ≥ 130 mm Hg6 |
| Diastolic BP | ≥ 85 mm Hg 6 |
| Glucose | ≥ 5.6 mmol/L 6 |
| Cholesterol | ≥ 200 mg/dL 49 |
| CRP | > 3.0 mg/dL 23 |
| LDL-C | ≥ 130 mg/dL 49 |
| HOMA-IR | ≥ 4.65 mg/dL x μg/mL 23 |
| Insulin | ≥ 109 pmol 50 |
| Hcy | ≥16 μmol/L 51 |
Cutpoints represent biomarker levels at which there are an increased risk of CVD. All plasma measures obtained after an overnight fast (see Methods).
Sociodemographic and lifestyle characteristics
We included the following sociodemographic and lifestyle characteristics: age, menopausal status, level of energy expenditure at work, environmental hygiene, socioeconomic status (SES), cigarette smoking, and alcohol drinking.
Age was categorized as ≤44 y, 45–49 y, 50–54 y, and ≥55 y to account for the nonlinear relationship between age and several biomarkers.
Level of energy expenditure at work served as a proxy for physical activity because a large percentage of women reported working, most moderate-vigorous physical activity is performed at work, and leisure time activity is uniformly sedentary in this population.26 Each occupation was categorized according to the level of physical demand, and energy expenditure values were assigned for specific occupations common among Filipino women based on field studies conducted by Tuazon et al. supplemented with data from the compendium of physical activity.27, 28 We created a categorical variable that represents the activity level of the woman’s occupation. This variable took on values from 0 to 4, where the value 0 indicated a woman not working for pay, while values 1 through 4 indicated physical activity ranging from sedentary (1.44 METS, including jobs with minimal demand, done while sitting) to more demanding (>4.1 METS, including jobs such as laundress).2
We measured environmental cleanliness using a hygiene score constructed from data on the interviewer’s rating of cooking area and immediate area around the house, as well as toilet type and water source. The score ranges from 0 to 9 with larger values indicating more environmental cleanliness.4
An SES factor score was based on a principal components analysis of household ownership of key assets such as television, vehicles, and furniture.
Smoking and alcohol use were categorized as none vs. any, since amounts were low among users.
Dietary data
Dietary data were derived from two 24-hour dietary recalls; we used the mean intakes of two days in our analysis. A nutritionist reviewed all dietary recalls immediately after collection. When implausible values were found, interviewers revisited respondents to verify reports. Energy and nutrient intakes were calculated using the Philippines Food Composition Tables produced by the Food and Nutrition Research Institute of the Philippines.29, 30 In our analysis, we used the nutrient residual method for energy adjustment to control for confounding and to remove extraneous variation due to total energy intake.31 We computed residuals of saturated fat intake by regressing saturated fat intake of individuals on their total energy intake. The residuals from the regression represent the differences between each individual’s actual saturated fat intake and the intake predicted by their total energy intake; these residuals are uncorrelated with total energy.
Final sample
Complete anthropometric, CVD biomarker, environmental, sociodemographic, and diet data were available for 1780 women. We excluded 2 pregnant (2 individuals) and non-fasting women (at the time of the blood draw) (10 individuals). None of the remaining women had CRP levels greater than 10 mg/L. This yielded a final analytic sample of 1768 women.
Statistical Analysis
We performed cluster analysis to identify groups of women with similar cardiometabolic risk factor patterns using SAS PROC FASTCLUS (SAS version 9.2, SAS Institute, Cary, NC). This procedure implements the K-means clustering algorithm (least squares method). K-means clustering uses the Euclidean distance, computed from input variables, to assign cluster membership by minimizing the distance among subjects in a cluster while maximizing the distance between clusters. The procedure first selects cluster seeds, a set of points calculated as a first guess of the cluster means. Next it calculates the Euclidean distance from each subject to each cluster seed; each subject is assigned to the nearest seed to form temporary clusters. The means of each of the temporary clusters are calculated and replace the seed values. Distance calculation and member assignment progress in an iterative fashion until no further changes occur.32, 33
Final cluster solutions are sensitive to initial seed values. To remedy this problem and to use a more objective approach to picking a cluster solution we created an algorithm modified from a previous clustering algorithm.34 This algorithm performed 1,000 iterations of each cluster procedure using randomly generated initial cluster seeds. For each of the 1,000 cluster solutions it calculated the ratio of between-cluster variance to within-cluster variance or R2/(1 − R2), where R2, pooled across all variables, represented the ability to predict each input variable from the cluster.33 We wanted to maximize the ratio of between-cluster variance to within-cluster variance and therefore wanted to find the largest R2. The algorithm identified the iteration/cluster solution with the largest R2.34
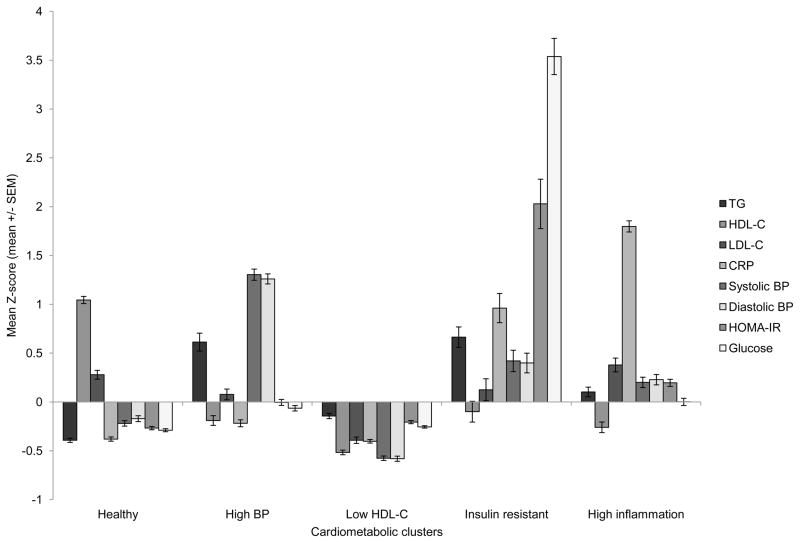
The variables entered into the cluster analysis were sample-specific Z-scores of eight cardiometabolic risk factors (Figure 1): diastolic BP, systolic BP, CRP, fasting glucose, HDL-C, HOMA-IR, LDL-C, and TG. We chose these cardiometabolic risk factors because they concisely represent hypertension, inflammation, insulin resistance, and lipid abnormalities. The cardiometabolic risk factor variables were standardized because they are measured in different units and cannot be assumed to have equal variance.
FIGURE 1.
Mean Z-scores of fasting CVD biomarkers by cardiometabolic cluster
Mean Z-scores by cardiometabolic cluster for the eight fasting CVD biomarkers used as input variables in the cluster analysis.
Using the algorithm we created, we found a 5-cluster solution with R2 = 0.39. We also conducted a series of cluster analyses with 3 to 6 clusters specified, but we chose to use a 5-cluster solution because these results yielded distinct cardiometabolic risk factor patterns and each cluster had sufficient numbers (approximately >5% of the sample).34 We identified the five clusters in the solution based on their dominant key features: “healthy”, “high BP”, “low HDL-C”, “insulin resistant”, and “high CRP”. We named the clusters according to their predominant pattern of mean Z-scores of cardiometabolic risk factors (namely, what risk factor(s) had the highest mean relative to other clusters).
We used multinomial logistic regression in Stata version 11.0 (Stata Corporation, College Station, TX, 2006) to determine how women’s age, menopausal status, combinations of WC and OW status, physical activity at work, average daily energy and saturated fat intake, smoking, alcohol drinking, hygiene score, and SES factor score related to cluster membership. We included variables, which distinguished combinations of OW and high WC, namely OW without high WC, high WC without OW, and both OW and high WC; these combinations were all compared to individuals with neither OW nor high WC. Age and physical activity at work were categorical variables; women 44 years and younger and level 1 physical activity (lowest physical activity at work) were respectively used as reference groups. Throughout our analysis we used α < 0.05 as the criterion for significance.
RESULTS
Cluster Analysis
CVD biomarker patterns
Mean Z-scores of the eight CVD biomarkers varied markedly by cluster (Figure 1), as did the prevalence of risk factors defined by IDF and other cutpoints to represent “high risk” (Table 2).
TABLE 2.
Cardiometabolic risk factors by cluster†
| CVD risk factors | Healthy (n= 476) | High BP (n= 313) | Low HDL-C (n= 654) | Insulin resistant (n= 84) | High CRP (n= 241) |
|---|---|---|---|---|---|
| Variables in cluster analysis | |||||
| TG, mg/dL | 97.4 ± 1.9 | 183.5 ± 7.9 | 118.3 ± 2.3 | 187.5 ± 8.9 | 139.6 ± 4.2 |
| HDL-C, mg/dL | 51.5 ± 0.4 | 38.9 ± 0.5 | 35.5 ± 0.2 | 39.8 ± 1.1 | 38.1 ± 0.6 |
| LDL-C, mg/dL | 128.6 ± 1.5 | 122.1 ± 1.9 | 106.1 ± 1.1 | 123.6 ± 3.8 | 132.1 ± 2.4 |
| Systolic blood pressure, mmHg | 115.2 ± 0.6 | 145.9 ± 1.2 | 107.9 ± 0.5 | 128.0 ± 2.2 | 123.6 ± 3.8 |
| Diastolic blood pressure, mmHg | 77.5 ± 0.4 | 95.4 ± 0.6 | 72.3 ± 0.3 | 84.7 ± 1.3 | 82.5 ± 0.7 |
| Fasting glucose, mmol/L | 4.0 ± 0.0 | 5.4 ± 0.1 | 5.0 ± 0.0 | 12.5 ± 0.4 | 5.5 ± 0.1 |
| CRP, mg/dL | 0.9 ± 0.0 | 1.2 ± 0.1 | 0.8 ± 0.0 | 3.7 ± 0.3 | 5.5 ± 0.1 |
| HOMA-IR, mg/dL x μg/mL | 2.1 ± 0.1 | 3.1 ± 0.1 | 2.3 ± 0.1 | 10.9 ± 1.0 | 3.9 ± 0.1 |
| Prevalence of risk indicators, % | |||||
| Elevated TG | 10.3 ± 1.4 | 49.2 ± 2.8 | 23.4 ± 1.7 | 69.0 ± 5.1 | 35.3 ± 3.1 |
| Low HDL-C | 50.5 ± 2.3 | 89.1 ± 1.8 | 99.2 ± 0.3 | 84.5 ± 4.0 | 90.5 ± 1.9 |
| Elevated LDL-C | 44.8 ± 2.3 | 38.3 ± 2.8 | 20.0 ± 1.6 | 38.1 ± 5.3 | 50.2 ± 3.2 |
| Hypertension | 25.9 ± 2.0 | 95.8 ± 1.1 | 7.8 ± 1.1 | 51.2 ± 5.5 | 49.8 ± 3.2 |
| Elevated fasting glucose | 12.6 ± 1.5 | 31.0 ± 2.6 | 13.6 ± 1.3 | 100.0 ± 0.0 | 34.4 ± 3.1 |
| Elevated CRP | 4.2 ± 0.9 | 9.3 ± 1.7 | 4.5 ± 0.8 | 45.2 ± 5.5 | 94.6 ± 1.5 |
| Elevated HOMA-IR | 5.7 ± 1.1 | 18.6 ± 2.2 | 8.0 ± 1.1 | 76.2 ± 4.7 | 29.0 ± 2.9 |
| Other indicators | |||||
| Total cholesterol, mg/dL | 199.7 ± 1.6 | 196.4 ± 2.2 | 165.4 ± 1.3 | 202.9 ± 4.3 | 199.1 ± 2.5 |
| Elevated total cholesterol, % | 45.3 ± 2.3 | 47.3 ± 2.8 | 14.6 ± 1.4 | 50.0 ± 5.5 | 45.6 ± 3.2 |
| Fasting insulin, pmol/L | 46.5 ± 1.3 | 64.4 ± 2.3 | 51.3 ± 1.3 | 133.2 ± 15.9 | 79.3 ± 2.7 |
| Elevated fasting insulin, % | 4.6 ± 1.0 | 13.8 ± 2.0 | 6.0 ± 0.9 | 33.3 ± 5.2 | 22.4 ± 2.7 |
Results are means ± SE for continuous variables and percent ± SE categorical variables
Women in the healthy cluster (n = 476, 27%) had low mean values of all risk factors (except HDL-C and LDL-C) relative to the other clusters. Women in the high BP cluster (n = 313, 18%) had elevated systolic and diastolic BP, and most women this group were hypertensive (96%). This group also had a high prevalence of elevated TG (49%) and fasting glucose (31%). The low HDL-C cluster (n = 654, 37%) was the largest of the five clusters. Nearly all of these women (99%) had low HDL-C, in addition they had the lowest prevalence of high LDL-C (20%), hypertension (8%), and elevated total cholesterol (15%). The insulin resistant cluster (n = 84, 5%) was the smallest of the five clusters. All women in this cluster had elevated fasting glucose as well as the highest prevalence of elevated TG (69%), fasting insulin (33%), and HOMA-IR (76%). In addition, a high proportion of these insulin resistant women had elevated CRP levels (45%) and hypertension (51%). The high CRP cluster (n = 241, 14%) was characterized by a high prevalence of elevated CRP (95%), a marker of chronic low-grade inflammation. This cluster also had a high prevalence of the following: elevated LDL-C (50%), low HDL-C (91%), elevated fasting glucose (34%), elevated fasting insulin (22%), and elevated HOMA-IR (29%).
Sociodemographic and lifestyle factors (Table 3)
TABLE 3.
Sociodemographic, body composition, and dietary characteristics by cluster†
| Cluster characteristics | Healthy (n= 476) | High BP (n= 313) | Low HDLC (n= 654) | Insulin Resistant (n= 84) | High CRP (n= 241) |
|---|---|---|---|---|---|
| Socioeconomic characteristics | |||||
| Age | 47.8 ± 0.3 | 50.6 ± 0.3 | 47.4 ± 0.2 | 50.0 ± 0.7 | 48.7 ± 0.4 |
| Age group | |||||
| ≤44 years, % | 36.1 ± 2.2 | 18.2 ± 2.2 | 37.8 ± 1.9 | 27.4 ± 4.9 | 30.7 ± 3.0 |
| 45–49 years, % | 32.6 ± 2.2 | 30.7 ± 2.6 | 32.9 ± 1.8 | 25.0 ± 4.8 | 30.7 ± 3.0 |
| 50–54 years, % | 17.4 ± 1.7 | 26.8 ± 2.5 | 17.9 ± 1.5 | 27.4 ± 4.9 | 22.8 ± 2.7 |
| ≥55 years, % | 13.9 ± 1.6 | 24.3 ± 2.4 | 11.5 ± 1.2 | 20.2 ± 4.4 | 15.8 ± 2.4 |
| Postmenopausal status, % | 36.8 ± 2.2 | 49.8 ± 2.8 | 31.8 ± 1.8 | 45.2 ± 5.5 | 43.2 ± 3.2 |
| Level of energy expenditure at work | |||||
| Not working, % | 19.7 ± 1.8 | 24.0 ± 2.4 | 19.4 ± 1.5 | 21.4 ± 4.5 | 19.5 ± 2.6 |
| level 1, % | 7.1 ± 1.2 | 3.8 ± 1.1 | 5.5 ± 0.9 | 9.5 ± 3.2 | 6.2 ± 1.6 |
| level 2, % | 50.6 ± 2.3 | 48.2 ± 2.8 | 44.2 ± 1.9 | 45.2 ± 5.5 | 53.9 ± 3.2 |
| level 3, % | 16.2 ± 1.7 | 17.9 ± 2.2 | 20.3 ± 1.6 | 19.0 ± 4.3 | 15.4 ± 2.3 |
| level 4, % | 22.5 ± 1.9 | 24.0 ± 2.4 | 30.9 ± 1.8 | 23.8 ± 4.7 | 20.3 ± 2.6 |
| Hygiene score | 6.2 ± 0.1 | 6.0 ± 0.1 | 5.8 ± 0.1 | 6.1 ± 0.2 | 6.1 ± 0.1 |
| SES factor score | 0.2 ± 0.1 | 0.0 ± 0.1 | −0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Cigarette smoking, % | 12.8 ± 1.5 | 16.0 ± 2.1 | 17.4 ± 1.5 | 15.5 ± 4.0 | 19.9 ± 2.6 |
| Alcohol drinking, % | 46.2 ± 2.3 | 39.6 ± 2.8 | 38.4 ± 1.9 | 32.1 ± 5.1 | 38.6 ± 3.1 |
| Body composition and dietary characteristics | |||||
| Waist circumference, cm | 78.5 ± 0.5 | 84.2 ± 0.5 | 78.0 ± 0.4 | 86.9 ± 1.1 | 88.1 ± 0.7 |
| High waist circumference, % | 40.1 ± 2.2 | 69.3 ± 2.6 | 42.0 ± 1.9 | 72.6 ± 4.9 | 74.7 ± 2.8 |
| BMI, kg/m2 | 23.6 ± 0.2 | 25.3 ± 0.2 | 23.2 ± 0.2 | 25.7 ± 0.5 | 27.0 ± 0.3 |
| Overweight status, % | 52.3 ± 2.3 | 72.5 ± 2.5 | 49.5 ± 2.0 | 72.6 ± 4.9 | 82.2 ± 2.5 |
| Energy, kJ | 4,925.0 ± 91.6 | 4,890.3 ± 135.6 | 4,387.2 ± 74.3 | 5,011.0 ± 236.9 | 5,022.8 ± 130.0 |
| Saturated fat, g | 8.8 ± 0.4 | 8.4 ± 0.6 | 6.2 ± 0.3 | 8.6 ± 1.0 | 8.9 ± 0.6 |
| Metabolic syndrome‡, % | 27.3 ± 2.0 | 69.0 ± 2.6 | 36.4 ± 1.9 | 67.9 ± 5.1 | 69.3 ± 3.0 |
Results are means ± SE for continuous variables and percent ± SE categorical variables
Metabolic syndrome based on IDF criteria
The low HDL-C cluster had the youngest mean age (47.4 ± 0.2y) while the high BP cluster (50.6 ± 0.3y) had the highest mean age. Similarly the low HDL-C cluster had the lowest proportion of postmenopausal women (32%) while the high BP cluster had the largest proportion of postmenopausal women (50%). Women across all clusters showed similar levels of physical activity at work. About half of all women across all clusters fell into the sedentary category of physical activity at work. All clusters had similar hygiene scores, but the low HDL-C cluster had the lowest SES factor score. Smoking prevalence was greatest in the high CRP cluster (20%). The healthy cluster had the highest proportion of women consuming alcohol (46%) while the insulin resistant cluster had the lowest (32%).
Anthropometrics and dietary patterns
Large differences were observed in anthropometrics and diet across clusters (Table 3). Women in the high CRP cluster had the highest mean WC and BMI as well as the highest average daily energy and saturated fat intake. Women in the healthy and low HDL-C clusters had the lowest WC and BMI. The average daily intake of energy and saturated fat were lowest in the low HDL-C cluster.
Metabolic syndrome
For comparative purposes, we used the IDF criteria to estimate the prevalence of metabolic syndrome across clusters. Nearly 46% of the women met the criteria for metabolic syndrome, and of these 73% were in one of the “non-healthy” clusters. Within the clusters, the prevalence of metabolic syndrome varied from 27% among “healthy” women, to 69% among the high BP, insulin resistant and high CRP clusters (Table 3). Of the women in the “healthy” cluster with metabolic syndrome, the most prevalent risk factor was reduced HDL-C (75%).
Multivariable Analysis
The following results used the healthy cluster as the reference group (Table 4). Using the coefficients from the multinomial logistic model, we estimated the effects of combinations of OW and high WC on cluster membership: OW alone, high WC alone, and OW and high WC. Here the reference group was those without both risk factors. We found that OW alone predicted membership to the high CRP cluster (OR 2.26, 95%CI=1.24:4.11). High WC alone predicted membership to the high BP (OR 2.56, 95%CI=1.20:5.46) and insulin resistant clusters (OR 4.05, 95%CI=1.39:11.81). Lastly, having both risk factors predicted the membership to the high BP (OR 4.67, 95%CI=3.23:6.75), insulin resistant (OR 4.59, 95%CI=2.48:8.49), and high CRP clusters (OR 6.85, 95%CI=4.44:10.56); these higher magnitude odds ratios (compared to each risk factor alone) suggest a synergistic effect of high WC and OW. Diet, behavioral, and SES effects were most prominent as predictors of the low HDL-C cluster. The likelihood of being in this cluster was increased by abstinence from alcohol, a lower SES factor score, premenopausal status, and lower saturated fat intake. Cigarette smoking uniquely predicted membership in the high CRP cluster.
TABLE 4.
Predictors of cluster membership†
| Cluster | High BP | Low HDL-C | Insulin resistant | High CRP | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Age group | ||||||||
| >45 y | (Reference) | |||||||
| 45–49 y | 2.01 (1.33, 3.04) | 0.001 | 1.12 (0.83, 1.52) | 0.453 | 1.02 (0.53, 1.97) | 0.956 | 1.06 (0.70, 1.61) | 0.776 |
| 50–54 y | 3.57 (2.10, 6.07) | 0.000 | 1.22 (0.79, 1.88) | 0.372 | 2.18 (0.99, 4.79) | 0.053 | 1.42 (0.82, 2.49) | 0.214 |
| ≥55 y | 4.60 (2.50, 8.46) | 0.000 | 0.97 (0.58, 1.64) | 0.912 | 2.45 (0.95, 6.29) | 0.063 | 1.42 (0.73, 2.75) | 0.298 |
| Postmenopausal status | 0.85 (0.55, 1.31) | 0.455 | 0.63 (0.44, 0.91) | 0.014 | 0.86 (0.44, 1.71) | 0.677 | 1.19 (0.75, 1.88) | 0.475 |
| Not OW‡ and not high WC§ | (Reference) | |||||||
| OW only | 1.52 (0.88, 2.62) | 0.136 | 0.71 (0.48, 1.04) | 0.080 | 1.20 (0.44, 3.22) | 0.723 | 2.26 (1.24, 4.11) | 0.007 |
| High WC only | 2.56 (1.20, 5.46) | 0.015 | 0.88 (0.45, 1.74) | 0.719 | 4.05 (1.39, 11.81) | 0.011 | 2.11 (0.77, 5.77) | 0.146 |
| OW and high WC | 4.67 (3.23, 6.75) | 0.000 | 1.24 (0.93, 1.64) | 0.131 | 4.59 (2.48, 8.49) | 0.000 | 6.85 (4.44, 10.56) | 0.000 |
| Level of energy expenditure at work | ||||||||
| Not working | 2.17 (1.02, 4.61) | 0.044 | 1.09 (0.63, 1.91) | 0.754 | 0.80 (0.31, 2.06) | 0.638 | 1.10 (0.53, 2.29) | 0.793 |
| Level 1 | (Reference) | |||||||
| Level 2 | 1.51 (0.74, 3.09) | 0.256 | 1.00 (0.60, 1.68) | 0.998 | 0.60 (0.25, 1.44) | 0.252 | 1.04 (0.53, 2.03) | 0.918 |
| Level 3 or 4 | 1.84 (0.86, 3.95) | 0.115 | 1.28 (0.74, 2.23) | 0.377 | 0.86 (0.33, 2.26) | 0.764 | 1.05 (0.50, 2.21) | 0.891 |
| Energy¶ | 1.04 (0.76, 1.43) | 0.811 | 0.70 (0.53, 0.93) | 0.014 | 1.04 (0.64, 1.71) | 0.868 | 0.98 (0.70, 1.38) | 0.928 |
| Saturated fat residual | 1.00 (0.97, 1.02) | 0.802 | 0.97 (0.95, 0.99) | 0.014 | 0.99 (0.95, 1.03) | 0.551 | 1.00 (0.97, 1.02) | 0.861 |
| Cigarette smoking | 1.37 (0.88, 2.13) | 0.163 | 1.28 (0.89, 1.83) | 0.181 | 1.68 (0.84, 3.35) | 0.144 | 2.19 (1.39, 3.45) | 0.001 |
| Alcohol drinking | 0.79 (0.58, 1.07) | 0.126 | 0.68 (0.53, 0.88) | 0.003 | 0.57 (0.34, 0.95) | 0.030 | 0.71 (0.50, 0.99) | 0.042 |
| Hygiene score | 0.94 (0.83, 1.07) | 0.353 | 1.08 (0.98, 1.21) | 0.132 | 0.89 (0.72, 1.09) | 0.248 | 0.89 (0.78, 1.03) | 0.107 |
| SES factor score | 0.90 (0.78, 1.05) | 0.195 | 0.75 (0.66, 0.85) | 0.000 | 1.09 (0.86, 1.37) | 0.478 | 1.04 (0.89, 1.23) | 0.590 |
The “healthy cluster” is the referent outcome
Overweight (BMI ≥ 23 kg/m2)
High waist circumference
Energy intake was scaled when imputed in the multinomial logistic regression to ease interpretation; units were kJ/1000
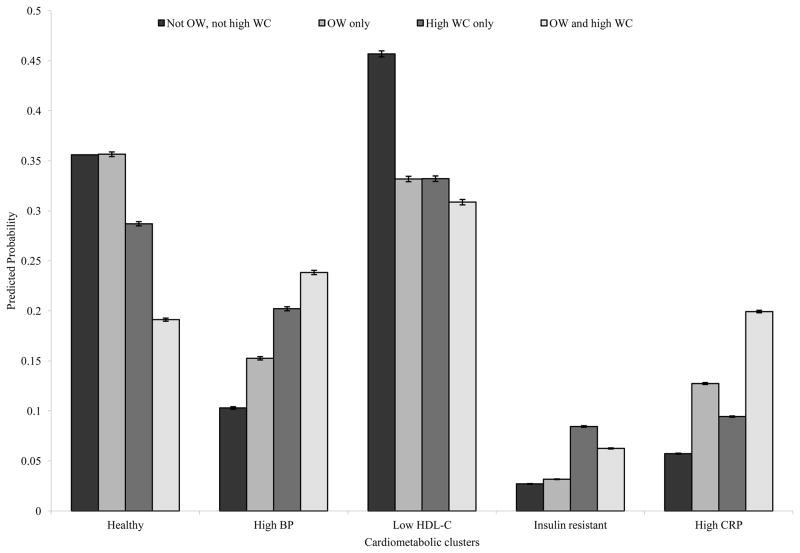
To aid in the interpretation of the results, we calculated the predicted probabilities of cluster membership after assigning different combinations of WC and OW status, holding all other covariates constant (Figure 2). The highest predicted probability of membership in each cluster occurred with the following assignments: For the healthy cluster, not OW and not high WC followed by OW alone; for the high BP cluster, OW and high WC, followed by high WC alone; for the low HDL-C cluster, not OW and not high WC; for the insulin resistant cluster, high WC alone, followed by high WC and OW; and for the high CRP cluster, OW and high WC, followed by OW only and high WC only.
FIGURE 2.
Predicted probabilities of cluster membership with different combinations of high waist circumference and overweight status
Predicted probabilities of being in one of the cardiometabolic clusters given four different populations: a population where no one is overweight (OW) nor with high waist circumference (WC), a population where everyone is OW in the absence of high WC, a population where everyone has high WC in the absence of OW, and a population where everyone is both OW and with high WC. Probabilities were calculated after running the multinomial logistic regression model.
DISCUSSION
Cluster analysis of eight cardiometabolic risk factors revealed five biologically consistent clusters in this population of middle-aged Filipino women. High WC significantly predicted membership in all of the cardiometabolic clusters relative to the healthy cluster, and the combination of high WC with OW status was associated with a large increase in risk, relative to either condition alone. The synergistic effect of having both risk factors was particularly strong in predicting membership in the high CRP cluster.
The finding that WC was a strong predictor of cluster membership was anticipated, and underscores the adverse health effects of excess visceral fat deposition to women in Cebu, assuming WC is an indicator of visceral fat. 35, 36 WC is among the best-established predictors of cardiometabolic risk and past work in the CLHNS and studies in other Asian populations support this notion.1, 4, 13, 38 Research has also demonstrated that increased WC predicts cardiometabolic abnormalities in both normal weight and overweight/obese individuals, highlighting the potential for visceral fat to influence development of cardiometabolic risk factors independent of overall BMI status.39
The inclusion of inflammation in the cluster analysis, a risk factor not commonly included in definitions of the metabolic syndrome, allowed us to identify a distinct group characterized primarily by high CRP. Interestingly, OW status in the absence of high WC uniquely predicted membership in this group, suggesting that some aspect of adiposity, independent of visceral adiposity (proxied by WC), might influence inflammation to a greater extent than other cardiometabolic disease markers. Work by Rexrode and colleagues conducted in a similar-age population of women found that CRP levels were strongly correlated with BMI throughout the full range of relative weight.40 The combination of high WC and OW status was particularly risky for this high CRP cluster (OR 6.85, 95%CI=4.44:10.56). Our prior work in Cebu identified WC as the strongest anthropometric predictor of elevated CRP, although this analysis did not distinguish between different profiles of high WC and OW.4 As mentioned above, VAT is an important a source of pro-inflammatory cytokines. In our study population, VAT might be a particularly important source of inflammation, since previous research demonstrates that Filipino women have a higher proportion of VAT compared with European or African-American women with the same WC.41
The low HDL-C cluster included the largest number of women. Other studies have shown similar results. Using the Philippines National Nutrition and Health Survey (NNHeS) data, Morales et al. demonstrated that among women (≥ 20 y) low HDL-C was the most prevalent component of metabolic syndrome (81%).10 Our recent work in the same CLHNS women showed that the prevalence of the “isolated” low HDL-C phenotype, defined as HDL-C<35 mg/dL with normal TG (<200 mg/dL), was 28.8%, which is much higher than the 2.10% prevalence in similar-aged American women from NHANES.3
The etiology of low HDL-C, while poorly understood, most likely includes some combination of nutritional, developmental, and genetic factors.3 For example from a developmental perspective, poor maternal energy was inversely associated with HDL-C concentrations in male offspring in the CLHNS population.42 Thirty-three percent of the offspring of the women studied here had HDL-C less than 35 mg/dL when they were adolescents, suggesting early development of adverse lipid profiles in this population.43
In relation to dietary intake, we found that low intake of saturated fat uniquely predicted membership in the low HDL-C cluster. Most dietary recommendations suggest limiting saturated fat intake, since it elevates total and LDL cholesterol. However, recent studies have shown that lauric acid has a more favorable effect on the total cholesterol to HDL cholesterol ratio than any other fatty acid, either saturated or unsaturated, primarily by increasing HDL-C levels.44 The most common cooking oil in Cebu is coconut oil, which is rich in lauric acid.43 Our results suggest that decreased saturated fat intake, perhaps from coconut oil, increase the likelihood of membership into the low HDL-C cluster. This is supported by recent findings by Feranil et al. that dietary coconut oil intake was positively associated with HDL-C levels in pre-menopausal CLHNS women.45
Epidemiological studies show an inverse relationship between HDL-C levels and incidence of CVD.46 There is increasing evidence that low HDL-C, in isolation from other lipids, is an independent factor for CVD risk.47 Since cardiovascular diseases are the leading cause of death in the Philippines, the widespread prevalence of low HDL-C in this population requires further attention.43 It is notable that a recent genome wide association study that included CLHNS data identified several loci with powerful influence on HDL-C levels;48 this might contribute to the common occurrence of the isolated low HDL-C phenotype in this population.
Cluster analysis was a useful tool for our study for identifying groups of women sharing similar cardiometabolic risk factor patterns. A limitation of cluster analysis is that not all individuals within a certain cluster necessarily share all characteristics, for example in our “healthy” cluster we found the average Z-scores for cardiometabolic risk biomarkers were relatively low (except HDL-C), however we cannot attribute these characteristics to each individual in the cluster. A significant strength of using cluster analysis is that we were able to avoid using the metabolic syndrome definition, which ignores the heterogeneity in the patterns of CM risk factor clustering. For example, 46% of the population is categorized as having metabolic syndrome based upon IDF criteria, while in contrast our cluster analysis approach found that 73% of women clustered into “non-healthy” cardiometabolic risk factor groups. Most of the women not captured by the IDF definition were in the low HDL-C cluster. In addition, we did not include WC as a criterion for the clustering algorithm, unlike the IDF, which requires elevated WC in the definition. This allowed us to distinguish for which clusters of women high WC was a risk factor.
Another limitation to our study included not taking into account medication use when classifying individuals according to risk factor cutpoints, which could have resulted in misclassification. However overall medication use in the study sample was low: 2 individuals took statins, 1.75% took diabetes medication, and 4% took anti-hypertensive medications. However if we had excluded these individuals our sample would be biased, therefore we chose to keep these individuals in our analysis.
Lastly, attrition was largely due to out-migration. Compared with those lost to follow-up, women who participated in the 2005 survey were less educated and came disproportionately from rural, poorer households. Given that permanent migrants from the Metro Cebu area were not followed, the remaining sample is therefore selective of households with more residential stability and lower SES.
Overall by using cluster analysis to evaluate how anthropometric measures influence cardiometabolic biomarkers, we made fewer assumptions regarding the underlying etiology and allowed relationships to emerge from the data themselves. In conclusion, the identification of modifiable risk factors for cardiometabolic risk patterns can help create targeted prevention strategies for cardiometabolic related diseases in this population.
ABBREVIATIONS
- BMI
body mass index
- BP
blood pressure
- CLHNS
Cebu Longitudinal Health and Nutrition Survey
- CRP
C-reactive protein
- CVD
cardiovascular disease
- HDL-C
high density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- IR
insulin resistance
- LDL-C
low density lipoprotein cholesterol
- OW
overweight
- TG
triglyceride
- WC
waist circumference
Footnotes
CONFLICTS OF INTEREST
N. Zubair, C.W. Kuzawa, T.W. McDade, and L.S. Adair have no conflicts of interest
FUNDING DISCLOSURE
Financial support comes from UNC and also the National Institutes of Health: (R01-HL085144-03, R01-HD054501, and R01-TW05596).
CONTRIBUTION:
N. Zubair and L.S. Adair had full access to all study data and take full responsibility for the integrity of the data and accuracy of the analysis. L.S. Adair is the Principal Investigator for which the study was based; N. Zubair and L.S. Adair designed research; C.W. Kuzawa, and T.W. McDade led the laboratory data analysis; N. Zubair and L.S. Adair performed the statistical analysis; N. Zubair wrote the initial draft of the manuscript; N. Zubair, L. S. Adair, C.W. Kuzawa, and T.W. McDade reviewed and revised the drafts. All authors read and approved the final manuscript.
Contributor Information
Niha Zubair, Email: zubair@email.unc.edu.
Chris W. Kuzawa, Email: kuzawa@northwestern.edu.
Thomas W. McDade, Email: t-mcdade@northwestern.edu.
Linda S. Adair, Email: linda_adair@unc.edu.
REFERNCES
- 1.Rutherford JN, McDade TW, Lee NR, Adair LS, Kuzawa C. Change in waist circumference over 11 years and current waist circumference independently predict elevated CRP in Filipino women. Am J Hum Biol. 2010;22:310–5. doi: 10.1002/ajhb.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adair LS, Gultiano S, Suchindran C. 20-year trends in Filipino women weight reflect substantial secular and age effects. J Nutr. 2011;141:667–73. doi: 10.3945/jn.110.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford JN, McDade TW, Feranil AB, Adair LS, Kuzawa CW. High prevalence of low HDL-c in the Philippines compared to the US: population differences in associations with diet and BMI. Asia Pac J Clin Nutr. 2010;19:57–67. [PMC free article] [PubMed] [Google Scholar]
- 4.McDade TW, Rutherford JN, Adair L, Kuzawa C. Adiposity and pathogen exposure predict C-reactive protein in Filipino women. J Nutr. 2008;138:2442–7. doi: 10.3945/jn.108.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–31. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 7.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Wilson PWF, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 9.Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care. 2010;33:2457–61. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales DD, Punzalan FE, Paz-Pacheco E, Sy RG, Duante CA. Metabolic syndrome in the Philippine general population: prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Dis Res. 2008;5:36–43. doi: 10.3132/dvdr.2008.007. [DOI] [PubMed] [Google Scholar]
- 11.Das M, Pal S, Ghosh A. Factor analysis of risk variables associated with metabolic syndrome in adult Asian Indians. J Cardiovasc Dis Res. 2010;1:86–91. doi: 10.4103/0975-3583.64442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JY, Hong YS, Sung YA, Barrett-Connor E. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004;27:2027–32. doi: 10.2337/diacare.27.8.2027. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Yamuna A, Murugesan N, Narayan KM. Insulin resistance and clustering of cardiometabolic risk factors in urban teenagers in southern India. Diabetes Care. 2007;30:1828–33. doi: 10.2337/dc06-2097. [DOI] [PubMed] [Google Scholar]
- 14.Wu CZ, Lin JD, Li JC, Hsiao FC, Hsieh CH, Kuo SW, et al. Factor analysis of metabolic syndrome using direct measurement of insulin resistance in Chinese with different degrees of glucose tolerance. Indian J Med Res. 2008;127:336–43. [PubMed] [Google Scholar]
- 15.Barker DJ. The fetal and infant origins of adult disease. Br Med J. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glew RH, Williams M, Conn CA, Cadena SM, Crossey M, Okolo SN, et al. Cardiovascular disease risk factors and diet of Fulani pastoralists of northern Nigeria. Am J Clin Nutr. 2001;74:730–6. doi: 10.1093/ajcn/74.6.730. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Lichtenstein AH, Roberts SB, Ma G, Gao S, Tucker KL, McCrory MA. Relative influence of diet and physical activity on cardiovascular risk factors in urban Chinese adults. Int J obes. 2003;27:920–32. doi: 10.1038/sj.ijo.0802308. [DOI] [PubMed] [Google Scholar]
- 18.Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort profile: the Cebu Longitudinal Health and Nutrition Survey. Int J Epidemiol. 2011;40:619–25. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman TJ, Roache AF, Martorell R. Anthropometric Standardization Reference Manual. Med Sci Sports Exerc. 1992;24:952. [Google Scholar]
- 20.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53:922–32. doi: 10.1373/clinchem.2006.077784. [DOI] [PubMed] [Google Scholar]
- 22.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 23.Kumar CPTG, Sng CPTBL, Kumar COLS. Correlation of capillary and venous blood glucometry with laboratory determination. Prehospital Emergency Care. 2004;8:378–83. doi: 10.1016/j.prehos.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clinica Chimica Acta. 2008;396:10–3. doi: 10.1016/j.cca.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Jennings A. Identifying the determinants of coexisting over and undernutrition in Cebu, Philippines. Chapel Hill: The University of North Carolina at Chapel Hill; 2008. [Google Scholar]
- 26.Tuazon MA, van Raaij JM, Hautvast JG, Barba CV. Energy requirements of pregnancy in the Philippines. Lancet. 1987;2:1129–31. doi: 10.1016/s0140-6736(87)91555-8. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 28.FNRI. Food Composition Tables Recommended for Use in the Philippines. Manila, Philippines: Food and Nutrition Research Institute; 1997. [Google Scholar]
- 29.FNRI. Philippine Nutrition Facts & Figures. Manila, Philippines: Food and Nutrition Research Institute; 2001. [Google Scholar]
- 30.Willett W. Nutritional Epidemiology. 2. Oxford: OUP; 1998. [Google Scholar]
- 31.Aldenderfer M, Blashfield R. Cluster Analysis. Newbury Park, CA: SAGE Publications, Inc; 1984. [Google Scholar]
- 32.Boone-Heinonen J, Gordon-Larsen P, Adair L. Obesogenic clusters: multidimensional adolescent obesity-related behaviors in the U.S. Ann Behav Med. 2008;36:217–30. doi: 10.1007/s12160-008-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–8. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 34.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–8. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 35.Satoh H, Fujii S, Furumoto T, Kishi R, Tsutsui H. Waist circumference can predict the occurrence of multiple metabolic risk factors in middle-aged Japanese subjects. Ind Health. 2010;48:447–51. doi: 10.2486/indhealth.ms1121. [DOI] [PubMed] [Google Scholar]
- 36.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 37.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13:674–82. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 38.Araneta MRG, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obesity. 2005;13:1458–65. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 39.Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. Am J Clinical Nutr. 2003;77:960–6. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- 40.Kuzawa CW, Adair LS, Avila JL, Cadungog JH, Le NA. Atherogenic lipid profiles in Filipino adolescents with low body mass index and low dietary fat intake. Am J Hum Biol. 2003;15:688–96. doi: 10.1002/ajhb.10200. [DOI] [PubMed] [Google Scholar]
- 41.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 42.Feranil AB, Duazo PL, Kuzawa CW, Adair LS. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac J Clin Nutr. 2011;20:190–5. [PMC free article] [PubMed] [Google Scholar]
- 43.Tall AR. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990;86:379–84. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruckert E, Hansel B. HDL-c is a powerful lipid predictor of cardiovascular diseases. Int J Clin Pract. 2007;61:1905–13. doi: 10.1111/j.1742-1241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 45.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Cholesterol Education Program Expert Panel on Detection E. Treatment of High Blood Cholesterol in A Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 47.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 48.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999;99:178–82. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]