Abstract
Central sensitization and purinergic receptor mechanisms have been implicated as important processes in acute and chronic pain conditions following injury or inflammation of peripheral tissues. This study has documented that application of the P2X1,2/3,3 receptor agonist αß-meATP (100 mM) to the rat tooth pulp induces central sensitization in medullary dorsal horn nociceptive neurons that is reflected in significant increases in mechanoreceptive field size and responses to noxious stimuli and decreased mechanical activation threshold. Furthermore, these responses can be blocked by pulp application of the P2X1,2/3,3 antagonist TNP-ATP and also attenuated by medullary application of TNP-ATP. These results suggest that activation of P2X1,2/3,3 receptors in orofacial tissues plays a critical role in producing central sensitization in medullary dorsal horn nociceptive neurons.
Keywords: purinergic receptors, plasticity, trigeminal nucleus, pain
1. Introduction
Central sensitization of central nociceptive neurons is reflected as an increase in mechanoreceptive field (RF) size, a decrease in mechanical activation threshold and an increase in responses to noxious RF stimuli, and has been implicated as an important process in acute and chronic pain conditions following injury or inflammation of peripheral tissues[10, 20, 21]. We have previously shown that application of the inflammatory irritant mustard oil (MO) to the rat molar tooth pulp induces central sensitization in the rat trigeminal subnucleus caudalis (also termed the medullary dorsal horn, MDH) that can be blocked by the medullary application of the P2X1,2/3,3 receptor subtype antagonist 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) [4, 5]. Furthermore, our in vitro MDH data suggest that extracellular adenosine-5′-triphosphate (ATP) molecules acting at purinergic receptor subtypes P2X1,2/3,3 are involved in the MDH mechanisms of trigeminal central sensitization [8, 11, 13]. However, it has also been shown that these P2X receptor subtypes are expressed on small-diameter trigeminal ganglion neurons including those that innervate the tooth pulp [1, 2, 19, 23] and on their central terminals in the MDH [14, 24, 25], but it is unclear if P2X receptor-dependent pulp afferent input to the medulla is sufficient to induce central sensitization in nociceptive neurons in the rat MDH. Therefore, the aim of this study was to test if central sensitization can be induced in the rat MDH by application of the P2X1,2/3,3 receptor agonist αß-meATP to the pulp and if application to the pulp or medulla of TNP-ATP blocks MDH central sensitization. Some of the data have been presented in abstract form[4].
2. Material and Methods
All procedures and surgeries were approved by the University of Toronto Animal Care Committee in accordance with the regulations of the Ontario Animal Research Act (Canada).
Animal preparation
Adult male Sprague-Dawley rats (280-400 g) were initially anesthetized by intraperitoneal α-chloralose (50 mg/kg)/urethane (1g/kg) and prepared as previously described in detail [6, 8]. Briefly, the coronal pulp of the right maxillary first molar was exposed and covered with a saline-soaked cotton pellet, and then the dorsal surface of the caudal medulla was surgically exposed. The rat then received a continuous intravenous infusion of a mixture of 70% α-chloralose/urethane solution (0.2 g/ml) and 30% pancuronium solution (1mg/ml) at a rate of 0.4 ml/h. The animal was artificially ventilated throughout the whole experiment and heart rate, expired CO2 level, and rectal temperature were continuously monitored.
Recording and stimulation procedures
Spontaneous and evoked single neuron activity was recorded in histologically verified sites in the deep laminae of MDH (lateral: 1.4-2.0 mm; posterior: 1.5-2.0 mm referred to the obex), as previously described [6, 8]. Responses to stimulation of the orofacial region were amplified and displayed on an oscilloscope and computer and the data were later analyzed off-line with Spike 2 software (Cambridge Electronic Desighn, Science Park, Milton Road, Cambridge, UK). Mechanical (brush, pressure and pinch) and noxious thermal (radiant heat 51–53°C) stimuli were applied to classify nociceptive-specific (NS) neurons in the deep laminae of MDH [6, 8]. Each NS neuron’s cutaneous orofacial RF was determined with nonserrated forceps, and its activation threshold to mechanical stimulation of its RF was assessed by force-monitoring forceps. Its responses were recorded to graded pressure applied by the forceps (25, 50, 75, 100, 125, 150 g applied in ascending order, each for 5 s at an interval of>45 s), and the number of spikes evoked by each of these graded stimuli were summed.
Experimental paradigm
After baseline values of neuronal properties were assessed, phosphate-buffered saline (PBS) at pH 7.4 was continuously superfused (i.t.) over the exposed ipsilateral medulla (at a rate of 0.6 ml/h, room temperature of 24°C). At 10 and 15 min after superfusion began, two assessments of neuronal properties were carried out. Then at 30 min after PBS superfusion began, P2X agonist, P2X antagonist or vehicle control were applied (at room temperature) to the exposed pulp (which was then sealed with CAVIT (ESPE, Seefeld/Oberbayren, Germany) in 3 groups of rats (N=6/group): (I) αß-meATP (100 mM; Sigma-Aldrich, USA) was applied to the pulp (designated as the αß-meATP/pulp group), (II) vehicle (PBS) was applied to the pulp (PBS/pulp group), (III) both αß-meATP (100 mM) and TNP-ATP (0.1 mM) were co-applied to the pulp (αß-meATP/pulp & TNP-ATP/pulp group). Another (IV) group of rats was used in which αß-meATP was applied to the pulp 30 min after PBS superfusion i. t. over the exposed medulla was substituted by TNP-ATP (64 μM; freshly dissolved in PBS at 24°C; TNP-ATP i.t./αß-meATP/pulp group N=6). In all four groups, starting at three minutes after the solution was applied to the pulp, neuronal properties were assessed at 10 min intervals over the next 60 min.
Statistical analyses
Statistical analyses were based on normalized data (in percentages) of orofacial RF size, responses to graded pressure or pinch stimuli and mechanical activation threshold. Differences between baseline values and values at different time points after αß-meATP application in each of the four groups of animals (I-IV) were treated by one-way repeated measures (RM) analysis of variance (ANOVA) or ANOVA on ranks, followed by Dunnett’s test. Differences between the groups were treated by 2-way ANOVA followed by Dunnett’s test. The level of significance was set at a P value of less than 0.05. All values are presented as mean ± SEM.
3. Results
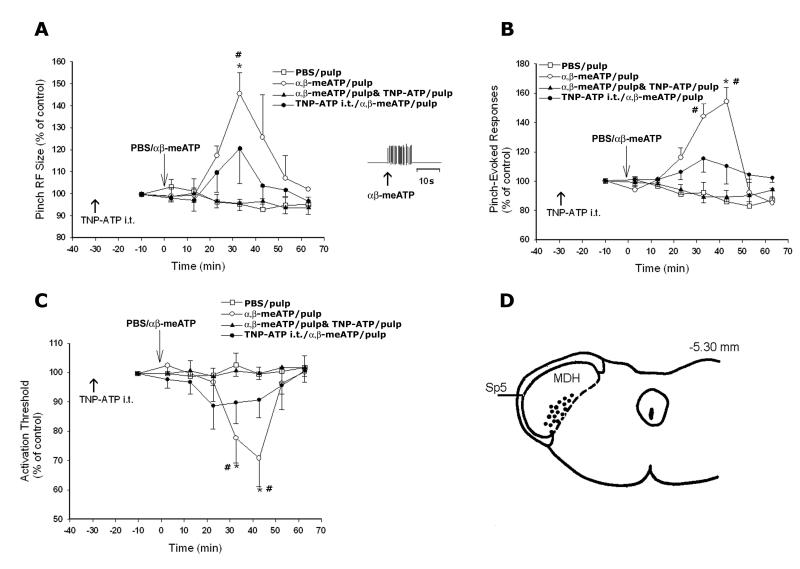
Thirty functionally identified NS neurons in the deep laminae of the MDH were studied (See Fig. 1D).
Fig. 1.
Graphs A, B, and C show changes in NS neuronal pinch RF size, pinch, pressure-evoked responses, and mechanical activation threshold, respectively. D shows the histologically confirmed recording sites of 18 NS neurons; the sites were plotted onto sections of the caudal medulla (−5.3 mm behind interaural line, as previously detailed [12]). The insert in A shows the response of a neuron evoked by αß-meATP (100 mM) to the tooth pulp. Tooth pulp application of PBS produced no induction of central sensitization in NS neurons in MDH compared to their baseline properties: cutaneous RF size, responses to noxious mechanical stimuli and mechanical activation threshold. However, application of αß-meATP (100 mM) to the tooth pulp induced MDH central sensitization: significant increases in RF size (A) and responses to noxious stimuli (B) and decreased mechanical activation threshold (C). Application of TNP-ATP to the tooth pulp or superfusion of TNP-ATP i.t. markedly reduced the αß-meATP-induced effects on RF size, responses to noxious stimuli, and mechanical activation threshold. *P< 0.05, 1-way ANOVA within the groups, #P< 0.05, 2-way ANOVA differences between the group of αß-meATP (100 mM) and vehicle control application to the tooth pulp.
Orofacial RF size
In the PBS/pulp group, there was no evidence of a change in RF size indicative of central sensitization in NS neurons compared to the baseline RF size (cutaneous RF size 3±0.8 %, N=6). However, application of αß-meATP (100 mM) to the pulp (αß-meATP/pulp group) induced a long-lasting and significant increase in RF size throughout the 60 min observation period, with its peak (44 ±8 %, N= 6, p<0.05, RM ANOVA) at 33 min (Fig.1). In the αß-meATP/pulp & TNP-ATP/pulp group, application of TNP-ATP (0.1 mM) to the pulp markedly reduced the αß-meATP-induced effects on RF size (N=6, p<0.05, 2-way ANOVA). TNP-ATP i.t. superfusion also reduced the αß-meATP-induced increases in RF size in the TNP-ATP i.t./ αß-meATP pulp group, resulting in a RF size that was not significantly different from that in the PBS/pulp group (2-way ANOVA, Fig. 1A).
Responses to graded pressure or pinch stimuli
In the PBS/pulp group, application of PBS to the pulp did not change responses to the graded pressure or pinch (4.4±0.7 %, N=6, RM ANOVA) compared to their baseline properties. However, responses to the graded stimuli were significantly increased after αß-meATP application to the pulp (58±6.3 %, N=6, p<0.05, RM ANOVA) in the αß-meATP/pulp group Fig. 1B). Responses to the graded stimuli were not significantly changed (N=6, RM ANOVA) when αß-meATP and TNP-ATP were applied to the pulp (αß-meATP/pulp & TNP-ATP/pulp group). TNP-ATP i.t. superfusion reduced the αß-meATP-induced increases in the responses in the TNP-ATP i.t./ αß-meATP pulp group that were not significantly different from the PBS/pulp group (2-way ANOVA, Fig. 1B).
Mechanical activation threshold
In the PBS/pulp group, application of PBS to the pulp did not change mechanical activation threshold compared to the baseline threshold (2.1±0.3 %, N=6, RM ANOVA). However, the threshold was significantly decreased (29±8.2 %, N=6, p<0.05, RM ANOVA) after application of αß-meATP (100 mM) in the αß-meATP/pulp group (Fig. 1.). Mechanical activation threshold was not significantly changed (N=6, RM ANOVA) when αß-meATP together with TNP-ATP (0.1mM) was applied to the pulp (αß-meATP/pulp & TNP-ATP/pulp group). TNP-ATP i.t. superfusion reduced the αß-meATP-induced changes in mechanical activation threshold in the TNP-ATP i.t./ αß-meATP pulp group, resulting in a threshold that was not significantly different from that in the PBS/pulp group (2-way ANOVA, Fig. 1C).
4. Discussion
This study has provided the first demonstration that activation of P2X receptors in peripheral orofacial tissues is sufficient to induce a P2X-dependent trigeminal central sensitization in single, functionally identified nociceptive neurons. It showed that since peripheral tooth pulp application of the P2X agonist (αß-meATP) produces central sensitization reflected in significant increases in pinch RF size and pinch-evoked responses and a decrease in mechanical activation threshold of MDH NS neurons that can be reversed by application to the pulp or MDH of the P2X1,2/3,3 receptor subtype antagonist TNP-ATP.
Several studies have demonstrated the involvement of the peripheral P2X1,2/3,3 receptors in the induction and maintenance of nociceptive responses in the trigeminal system [1, 18, 22]. Furthermore, the presence of P2X receptors in the pulp and on the trigeminal ganglion neurons supplying the pulp has been shown histologically and physiologically [1, 2, 9]. It was also demonstrated that αß-meATP is a selective agonist of P2X1,2/3,3 receptors, while TNP-ATP is a selective antagonist of P2X1,2/3,3 receptors [3]. In this study we demonstrated the involvement of pulpal P2X1,2/3,3 receptors in the induction of MDH central sensitization by showing that the αß-meATP effects can be blocked by TNP-ATP application to the pulp.
The present study also demonstrated that medullary application of the selective P2X1,2/3,3 receptor antagonist TNP-ATP can also block the αß-meATP-evoked MDH central sensitization. We have previously demonstrated that pulpal application of the inflammatory irritant MO produces central sensitization that was blocked by medullary superfusion of TNP-ATP [6, 8]. Also Nakagawa et al. [16] reported that activation of P2X1,2/3,3 receptors in spinal cord produces long-lasting allodynia reflecting central sensitization, the initiation of which can be blocked by a selective P2X2/3,3 antagonist.These findings are consistent with the results of the present study where MDH central sensitization evoked by the pulp application of a P2X agonist was blocked by i.t. superfusion of TNP-ATP. It has been shown previously in a spinal cord slice preparation that glutamate release in the spinal dorsal horn can be reduced by the wide spectrum P2X antagonist pyridoxal-phosphate-6-azophenyl-2, 4-disulphonic acid tetra-sodium (PPADS) [17]. We have also demonstrated that both PPADS and TNP-ATP applied to the medulla blocks MO-induced MDH central sensitization [8], and that the MO-induced release of glutamate in the MDH can also be blocked by the medullary application of TNP-ATP or apyrase, an enzyme that catalyzes the hydrolysis of ATP [15]. In addition, we have previously demonstrated the involvement of P2X and N-methyl D-aspartate (NMDA) receptors in the nociceptive transmission in deep laminae of MDH [6, 13], including modulation of presynaptic P2X receptors that have been documented on the central terminal of trigeminal afferents [14, 24, 25]. These previous findings, together with our current data, support the idea of ATP receptor involvement in evoking and regulating central sensitization in the MDH. Since trigeminal central sensitization is a process that involves glial cell activation as well as P2X receptor mechanisms [5, 7, 8, 11, 16], we cannot exclude the possibility that P2X receptors on glial cells may contribute to the mechanisms of αß-meATP-induced central sensitization in the MDH.
In conclusion, these novel findings indicate that activation of P2X1,2/3,3 receptors in tooth pulp tissues can produce central sensitization in functionally identified nociceptive MDH neurons and that peripheral (pulp) and central (MDH) endogenous ATP is an important mediator contributing to the development of central sensitization in MDH nociceptive neurons. The findings suggest that these purinergic-dependent mechanisms may be important in the initiation of central sensitization in dental inflammatory pain states. Further understanding of the mechanisms of central sensitization in the MDH may lead to the development of novel therapeutic approaches for pathological pain states in the orofacial area.
Highlights.
ATP contributes to the development of central sensitization (CS) in medullary nociceptive neurons
application of purinergic agonist to the rat tooth pulp induces CS in medullary nociceptive neurons
central sensitization can be blocked by pulp application of the purinergic antagonist TNP-ATP
central sensitization can be attenuated by medullary application of TNP-ATP
Acknowledgments
The authors acknowledge Ms S. Carter for her technical assistance. This study was supported by NIH grant DE-04786.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding disclosure: This study was supported by NIH grant DE-04786.
References
- [1].Adachi K, Shimizu K, Hu JW, Suzuki I, Sakagami H, Koshikawa N, Sessle BJ, Shinoda M, Miyamoto M, Honda K, Iwata K. Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain. 2010;6:59. doi: 10.1186/1744-8069-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alavi AM, Dubyak GR, Burnstock G. Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res. 2001;80:476–483. doi: 10.1177/00220345010800021501. [DOI] [PubMed] [Google Scholar]
- [3].Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- [4].Cherkas PS, Dostrovsky JO, Sessle BJ. Peripheral P2X mechanisms are involved in the production of central sensitization in rat dorsal horn nociceptive neurons (266.7/AA8) Society for Neuroscience; Chicago, USA: 2009. [Google Scholar]
- [5].Chiang CY, Dostrovsky JO, Iwata K, Sessle BJ. Role of glia in orofacial pain. Neuroscientist. 17:303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- [6].Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998;80:2621–2631. doi: 10.1152/jn.1998.80.5.2621. [DOI] [PubMed] [Google Scholar]
- [7].Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chiang CY, Zhang S, Xie YF, Hu JW, Dostrovsky JO, Salter MW, Sessle BJ. Endogenous ATP involvement in mustard-oil-induced central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 2005;94:1751–1760. doi: 10.1152/jn.00223.2005. [DOI] [PubMed] [Google Scholar]
- [9].Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- [10].Dubner R. Plasticity in Central Nociceptive Pathways. In: Merskey H, Loeser JD, Dubner R, editors. The paths of pain 1975–2005. IASP Press; Seattle: 2005. pp. 101–115. [Google Scholar]
- [11].Hu B, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. P2X receptors in trigeminal subnucleus caudalis modulate central sensitization in trigeminal subnucleus oralis. J Neurophysiol. 2002;88:1614–1624. doi: 10.1152/jn.2002.88.4.1614. [DOI] [PubMed] [Google Scholar]
- [12].Hu JW, Dostrovsky JO, Sessle BJ. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J Neurophysiol. 1981;45:173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- [13].Jennings EA, Christie MJ, Sessle BJ. ATP potentiates neurotransmission in the rat trigeminal subnucleus caudalis. Neuroreport. 2006;17:1507–1510. doi: 10.1097/01.wnr.0000234740.97076.95. [DOI] [PubMed] [Google Scholar]
- [14].Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- [15].Kumar N, Cherkas PS, Chiang CY, Dostrovsky JO, Sessle BJ, Coderre TJ. Involvement of ATP in noxious stimulus-induced release of glutamate in the trigeminal subnucleus caudalis (medullary dorsal horn): A microdialysis study in anesthetized rats (764.9/CC49) Society for Neuroscience; Chicago, USA: 2009. [Google Scholar]
- [16].Nakagawa T, Wakamatsu K, Zhang N, Maeda S, Minami M, Satoh M, Kaneko S. Intrathecal administration of ATP produces long-lasting allodynia in rats: differential mechanisms in the phase of the induction and maintenance. Neuroscience. 2007;147:445–455. doi: 10.1016/j.neuroscience.2007.03.045. [DOI] [PubMed] [Google Scholar]
- [17].Nakatsuka T, Tsuzuki K, Ling JX, Sonobe H, Gu JG. Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol. 2003;89:3243–3252. doi: 10.1152/jn.01172.2002. [DOI] [PubMed] [Google Scholar]
- [18].Oliveira MC, Parada CA, Veiga MC, Rodrigues LR, Barros SP, Tambeli CH. Evidence for the involvement of endogenous ATP and P2X receptors in TMJ pain. Eur J Pain. 2005;9:87–93. doi: 10.1016/j.ejpain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- [19].Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–250. [PubMed] [Google Scholar]
- [20].Salter MW. Cellular neuroplasticity mechanisms mediating pain persistence. J Orofac Pain. 2004;18:318–324. [PubMed] [Google Scholar]
- [21].Sessle B. Orofacial pain. In: Merskey H, Loeser JD, Dubner R, editors. The paths of pain 1975–2005. IASP Press; Seattle: 2005. pp. 131–150. 2005. [Google Scholar]
- [22].Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain. 2005;116:42–51. doi: 10.1016/j.pain.2005.03.042. [DOI] [PubMed] [Google Scholar]
- [23].Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience. 2007;144:208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- [25].Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X(1)-P2X(6) receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups. J Comp Neurol. 2000;427:485–507. [PubMed] [Google Scholar]