Abstract
Objective
Adverse pregnancy outcomes, including preterm birth, are markedly higher among African-Americans versus Whites. Stress-induced immune dysregulation may contribute to these effects. Epstein-Barr virus (EBV) reactivation provides a robust model for examining cellular immune competence. This study examined associations of EBV virus capsid antigen immunoglobulin G (VCA IgG) with gestational stage, race, and racial discrimination in women during pregnancy and postpartum.
Methods
Fifty-six women (38 African-American, 18 White) were included. African-Americans and Whites did not differ in age, education, income, parity, or body mass index (ps ≥.51). During the 1st, 2nd, and 3rd trimester and ~5 weeks postpartum, women completed measures of racial discrimination, perceived stress, anxiety, depressive symptoms and health behaviors. EBV VCA IgG antibody titers were measured via ELISA in serum collected at each visit.
Results
In the overall sample, EBV VCA IgG antibody titers were lower in the 3rd versus 1st trimester (p=.002). At every timepoint (1st, 2nd, 3rd trimester and postpartum), African-American women exhibited higher serum EBV VCA IgG antibody titers than Whites (ps<.001). This effect was most pronounced among African-Americans reporting greater racial discrimination [p=.03 (1st), .04 (2nd), .12 (3rd), .06 (postpartum)]. Associations of race and racial discrimination with EBV VCA IgG antibody titers were not accounted for by other measures of stress or health behaviors.
Conclusions
Compared to Whites, African-American women showed higher EBV VCA IgG antibody titers, indicative of impaired cellular immune competence, across pregnancy and postpartum. This effect was particularly pronounced among African-American women reporting greater racial discrimination, supporting a role for chronic stress in this association. In women overall, EBV antibody titers declined during late as compared to early pregnancy. This may be due to pregnancy-related changes in cell-mediated immune function, humoral immune function, and/or antibody transfer to the fetus in late gestation. As a possible marker of stress-induced immune dysregulation during pregnancy, the role of EBV reactivation in racial disparities in perinatal health warrants further attention.
Keywords: Epstein-Barr virus, EBV, race, immune function, preterm birth, psychosocial stress, racial disparities, pregnancy, perinatal health, immunity, postpartum, herpesviruses, reactivation, racial discrimination, stress
Introduction
Maternal stress during pregnancy has been associated with increased risk of adverse birth outcomes, but the mechanisms by which this occurs remain uncertain (Committee on Understanding Premature Birth and Assuring Healthy Outcomes 2007). Stress constructs associated with risk of preterm birth include perceived stress, general distress, stressful life events, anxiety, and depressive symptoms (Committee on Understanding Premature Birth and Assuring Healthy Outcomes 2007). Moreover, perceived racial discrimination has repeatedly been linked to increased risk of preterm delivery among African-American women (Rosenberg, Palmer et al. 2002; Dole, Savitz et al. 2003; Collins, David et al. 2004; Dole, Savitz et al. 2004; Mustillo, Krieger et al. 2004). Thus, the chronic stress of discriminated racial minority status has been implicated in the substantial racial disparities in preterm birth and low birth weight among African-American women versus women of other races/ethnicities in the U.S. (Giscombe and Lobel 2005).
Given the linkage of stress to adverse pregnancy outcomes, an emerging literature is focusing on potential biological mechanisms for these associations. In terms of immune parameters, inflammatory markers have been a primary focus. Factors including perceived stress, stressful life events, depressive symptoms, and trauma have been associated with higher circulating inflammatory markers including interleukin(IL)-6, tumor necrosis factor (TNF)-α, and IL-1RA in pregnant women (Coussons-Read, Okun et al. 2005; Ruiz, Stowe et al. 2007; Paul, Boutain et al. 2008; Christian, Franco et al. 2009; Blackmore, Moynihan et al. 2011; Cassidy-Bushrow, Peters et al. 2012) as well as exaggerated inflammatory responses to in vivo and in vitro immune challenges (Coussons-Read, Okun et al. 2007; Christian, Franco et al. 2010). Linking such effects to birth outcomes, in a study of 173 women followed across pregnancy, an association between prenatal stress and gestational age at birth was mediated by levels of circulating inflammatory markers (Coussons-Read, Lobel et al. 2012).
In addition to promoting inflammation, chronic stress can suppress cellular immune function. Viral reactivation provides a robust model for examining cellular immune competence. By adulthood, >90% of the U.S. population is infected with Epstein-Barr virus (EBV) (Jones and Straus 1987). Once infected, an individual carries the virus for life. The virus latently infects B-lymphocytes, which are the primary cells in which the virus is maintained. The cellular immune system plays an important role in controlling replication of the virus. However, under conditions of immunosuppression, the virus may reactivate causing a release of viral proteins and activating a humoral immune response as indexed by higher EBV VCA IgG antibody titers (Glaser and Kiecolt-Glaser 1994).
Although EBV reactivation generally causes few to no symptoms in healthy individuals, it can serve as a marker of stress-induced immune dysregulation (Glaser and Kiecolt-Glaser 2005). For example, EBV VCA IgG antibody titers are elevated among older adults experiencing the chronic stress of caregiving for a spouse with dementia versus well-matched controls (Kiecolt-Glaser, Dura et al. 1991). EBV reactivation is also responsive to transient stressors; medical students as well as military academy students exhibit higher EBV VCA IgG antibody titers during major academic exams compared to several weeks before or after exams (Glaser, Kiecolt-Glaser et al. 1985; Glaser, Friedman et al. 1999). Other research has shown that the intense stress of space flight (Payne 1999; Pierson, Stowe et al. 2005) and Antarctic expeditions (Mehta, Pierson et al. 2000) result in higher EBV antibody titers and decreased EBV-specific T-cells responses. Elevated EBV antibody titers have also been reported in the context of stress associated with rapid economic and cultural changes in communities in Siberia and Samoa (McDade, Stallings et al. 2000; Sorensen, Snodgrass et al. 2009). In addition to effects of objective stressors, impaired control of latent viruses has been associated with psychological characteristics including anxiety, tendency toward emotional repression, and loneliness (Glaser, Kiecolt-Glaser et al. 1985; Esterling, Antoni et al. 1990; Esterling, Antoni et al. 1993). Conversely, greater vigor and social support have been associated with lower EBV antibody titers in the context of major life stressors (Lutgendorf, Reimer et al. 2001; McDade 2001; Fagundes, Bennett et al. 2011).
Although chronic stress may contribute to racial disparities in diverse health outcomes (Shonkoff, Boyce et al. 2009), studies of racial differences in EBV reactivation are limited. In a study of adults ages 25–90, greater viral reactivation was evidenced among Blacks compared to non-Hispanic Whites (Stowe, Peek et al. 2010). In addition, a study of women of childbearing age demonstrated that perceived racial discrimination was associated with greater EBV reactivation (Borders, Grobman et al. 2010). Thus, though limited, available evidence suggests that race and racial discrimination affect EBV reactivation.
It has been suggested that EBV reactivation is greater during pregnancy than non-pregnancy due to pregnancy-related suppression of the cellular immune system (Purtilo and Sakamoto 1982). However, to our knowledge, there are no available data comparing non-pregnant women to women across the course of pregnancy. These data would provide insight into typical versus atypical immune adaptation as pregnancy progresses.
Data on effects of psychosocial factors on EBV reactivation during pregnancy are also limited. One study of pregnant women in their first trimester reported greater EBV reactivation among those with a diagnosis of clinical depression versus a well-matched non-depressed comparison group (Haeri, Johnson et al. 2011). Considering the large literature in non-pregnant adults showing effects of psychosocial stress on EBV reactivation and unique immune environment in pregnancy, greater explication of stress-immune relationships in pregnancy is warranted.
As described, EBV reactivation can provide a marker of suppressed cell-mediated immunity. As such, elevated EBV VCA IgG antibody titers may serve as a non-causal marker of increased risk of adverse birth outcomes. Moreover, reactivation of latent herpesviruses has been associated with inflammation (Bennett, Glaser et al. 2012). This represents one potential causal pathway by which EBV reactivation may affect birth outcomes.
Building upon the limited existing literature, this study examined effects of gestational age, race, and subjective stress on EBV virus capsid antigen immunoglobulin G (VCA IgG) antibody titers longitudinally across pregnancy and postpartum. Considering expected down-regulation of cell-mediated immune function during pregnancy, we hypothesized that EBV VCA IgG antibody titers would increase from the 1st to 3rd trimesters and be lower postpartum than during pregnancy. Reflecting the chronic stress of racial minority status, we hypothesized that African-American women would exhibit elevated EBV VCA IgG antibody titers compared to Whites, and that subjective stress, particularly perceived racial discrimination, would exacerbate this effect. Finally, we examined the association of EBV VCA IgG antibody titers with serum proinflammatory cytokines to test the hypothesis that EBV reactivation may promote inflammatory activity.
Methods
Study Design
Sixty pregnant women were recruited from the Ohio State University Medical Center (OSUMC) Prenatal Clinic. Study visits were conducted during the 1st, 2nd, and 3rd trimesters and at 4–9 weeks postpartum. At each visit, women provided a blood sample and completed measures of psychosocial stress and health behaviors. Women were excluded from analyses if they missed more than one of the three prenatal study visits (n=3). One woman (who was White) was excluded from analyses due to EBV seronegative status at all four study timepoints, resulting in a final sample of 56 women.
Participants
All women were born and raised in the United States. Women were not eligible if they had current hypertension, diabetes, chronic conditions with implications for immune function (e.g., rheumatoid arthritis, multiple sclerosis, or human immunodeficiency virus), fetal anomaly, illicit drug use or more than two alcoholic drinks per week during pregnancy (per self-report or medical record). Women reporting acute illness (e.g., cold or flu-like symptoms) or antibiotic use within 10 days of a study visit were rescheduled. Each completed informed consent and received modest compensation. The study was approved by the OSU Biomedical Institutional Review Board.
Demographics and Birth Outcomes
Age, race/ethnicity, marital status, education, annual family income, gravidity, and parity were collected by self-report. Pre-pregnancy body mass index (BMI; kg/m2) was calculated using self-reported pre-pregnancy weight and height measured at the first visit. Gestational age at delivery was determined by medical record review.
Psychosocial Measures
Questionnaires were administered per the schedule shown in Table 1. The Experiences of Discrimination (EOD) scale is a 9-item measure assessing the occurrence and frequency of discrimination due to race/ethnicity. Specifically, participants indicated whether they have experienced discrimination over their lifetime (Yes or No) in the following settings: 1) at school, 2) getting hired or getting a job, 3) at work, 4) getting housing, 5) getting medical care, 6) getting service in a store or restaurant, 7) getting credit, bank loans or a mortgage, 8) on the street or in a public setting, 9) from the police or in the courts. For items endorsed, participants rate the frequency of this occurrence: once, 2–3 times, or 4+ times. This scale has high test-retest reliability and predictive validity for health outcomes in Black adults (Krieger 1990; Krieger and Sidney 1996; Krieger, Smith et al. 2005). Moreover, validation studies indicate that scores are not related to social desirability (Krieger, Smith et al. 2005).
Table 1.
Psychosocial Questionnaires
| Trimester | ||||
|---|---|---|---|---|
| Measure | 1st | 2nd | 3rd | Postpartum |
| Experiences of Discrimination (EOD) | X | |||
| State Anxiety Inventory (STAI) | X | X | X | X |
| Center for Epidemiologic Studies Depression Scale (CES-D) | X | X | X | X |
| Perceived Stress Scale (PSS) | X | X | X | X |
| Pittsburgh Sleep Quality Index (PSQI) | X | X | X | X |
| Revised Prenatal Distress Questionnaire (NUPDQ) | X | X | X | |
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item measure of cognitive, emotional, and somatic symptoms of depression (Radloff 1977). The CES-D is predictive of preterm birth and immune parameters in pregnant women (Orr, James et al. 2002; Christian, Franco et al. 2009; Li, Liu et al. 2009; Christian, Franco et al. 2010; Phillips, Wise et al. 2010). The 10-item Perceived Stress Scale (PSS), is a well-validated measure which assesses a construct independent of depressive symptomatology (Cohen, Kamarck et al. 1983). Scores have been associated with maternal neuroendocrine function (Wadhwa, DunkelSchetter et al. 1996; Hobel, Dunkel-Schetter et al. 1999). The 6-item short form of the State-Trait Anxiety Inventory (STAI) was used to assess state anxiety (Spielberger 1989; Marteau and Bekker 1992). The STAI shows strong criterion, discriminant, and predictive validity in perinatal populations (Meades and Ayers 2011). The Revised Prenatal Distress Questionnaire (NUPDQ) is a 17-item measure of pregnancy-specific stress including physical discomforts, financial resources to care for children, and pain during delivery (Lobel 1996).
Health Behaviors
The Pittsburgh Sleep Quality Index (PSQI), was administered at each visit (Buysse, Reynolds et al. 1989). A score > 5 is indicative of clinically disturbed sleep. Smoking, exercise and prenatal vitamin use were assessed at the first study visit. Women were classified as current, past, or never smokers. Exercise was operationalized as the frequency of engaging in vigorous physical activity long enough to build up a sweat. Prenatal vitamin use was defined as never, 1–3 days per week, 4–6 days, and 7 days.
EBV Reactivation
Serum was assayed for EBV virus capsid antigen (VCA) IgG antibody titers using Euroimmun EBV ELISA plates (Morris Plains, NJ). EBV VCA IgG antibody titers were assessed following company instructions with some modifications (Fagundes, Bennett et al. 2011). Specifically, for each ELISA plate, three controls that were included in each kit (one positive sample, one negative sample, and three calibrators) were run in duplicate. Samples were initially diluted 1:101 with a dilution buffer according to the recommended protocol provided by the company. Six serial two-fold dilutions of each sample were assayed. The last dilution factor with a positive IgG value determined the IgG antibody titer. Calculated viral titers for each sample were plotted and samples were rerun if the end point did not fall within the linear range (± 15%).
Proinflammatory Markers
Serum levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-8, and IL-1β were assayed in duplicate with ultra-sensitive multiplex kits from Meso Scale Discovery (MSD) and chemilluminescence methodology using the Immulite 1000 (Siemens Healthcare Diagnostics, Inc., 1717 Deerfield Rd., Deerfield, IL). Limits of detection were 0.61 pg/ml for IL-6, 2.4 pg/ml for TNF-α, 0.3 pg/ml for IL-8, and 0.61 pg/ml for IL-1β. All values were above the limits of detection. Assays were conducted after all data collection was complete; all assays were batched by subject and all kits were from the same lot.
Statistical Analyses
We used linear mixed models to analyze EBV VCA IgG antibody titers. The linear mixed models account for correlation in measures from the same woman over time by including a random subject effect. These models are capable of incorporating subjects with missing data points because of the restricted maximum likelihood estimation method used to fit the models. EBV titer values were log-transformed (base 10) to better satisfy normality assumptions.
We first evaluated changes in EBV VCA IgG antibody titers across pregnancy by including visit as a categorical independent variable. Differences between visits were compared by contrasts of mixed model parameters for each pair of visit timepoints, using the Tukey adjustment for multiple comparisons. Next we examined racial differences in EBV VCA IgG antibody titers by adding race (African-American or White) to the model along with the interaction between race and visit. Differences between races were tested at each visit by contrasts within the mixed model. The African-American women were then subdivided by a median split on scores on the Experiences of Discrimination (EOD) measure and EBV VCA IgG antibody titers for the resulting groups were compared at each visit within the mixed model. In secondary analyses, we used generalized linear models with a logit (dichotomous outcomes) or cumulative logit (ordered categorical outcomes) link to compare health behaviors by race at each timepoint.
Linear mixed model analyses were conducted to examine the relationship between EBV VCA IgG antibody titers and serum inflammatory markers. Correlations at each individual visit were tested as contrasts within the model. Serum inflammatory marker data were log-transformed. No outliers (datapoints ± 3 SD from the mean) were present; therefore all available data were included. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC, 2009).
Results
Sample Characteristics
Women completed study visits in the 1st trimester (Mean=11.4 weeks gestation, SD=2.2), 2nd trimester (Mean=23.4 weeks gestation, SD=2.3), 3rd trimester (Mean=31.9 weeks gestation, SD=1.8), and postpartum (Mean=5.3 weeks, SD=1.3). Complete data at all four study timepoints were available for 64% (n=36) of women. Data were missing at one study timepoint for 27% (n=15) and at two study timepoints for 9% (n=5). In this sample, 11.1% (2/18) of White women and 10.5% (4/38) of African-Americans delivered preterm (<37 weeks gestation). Two preterm deliveries were medically-indicated due to preeclampsia (1 African-American, 1 Hispanic White).
Demographics and Health Behaviors
In this sample, 64% (n=36) were African-American, 32% (n=18) were White, including two Hispanics, and 4% (n=2) reported both African-American and White race. For analytic purposes, these two women were categorized as African-American, as their experiences of racial discrimination were expected to better approximate those of African-Americans versus Whites (Campbell and Herman 2010). Whites were more likely to be married than African-Americans (p = .02). African-Americans and Whites did not differ in other demographic characteristics (Table 2) or assessed health behaviors (Table 3).
Table 2.
Demographic Characteristics
| Total (n=56) | African-American (n=38) | White (n=18) | African-American vs White | |
|---|---|---|---|---|
| Age [Mean (SD)] | 23.7 (3.76) | 23.61 (3.5) | 23.89 (4.35) | t(54) = 0.12, p = .80 |
| Marital Status [n (%)] | X2(1) = 5.21†, p = .02* | |||
| Married | 7 (12.5%) | 2 (5.3%) | 5 (27.8%) | |
| In a relationship | 35 (62.5%) | 26 (68.4%) | 9 (50.0%) | |
| Single | 14 (25.0%) | 10 (26.3%) | 4 (22.2%) | |
| Education [n (%)] | X2(1) = 0.17‡, p = .68 | |||
| Less than high school | 16 (28.6%) | 11 (28.9%) | 5 (27.8%) | |
| High school graduate | 16 (28.6%) | 10 (26.3%) | 6 (33.3%) | |
| Some college | 18 (32.1%) | 14 (36.8%) | 4 (22.2%) | |
| College degree (2 or 4 yr) | 6 (10.7%) | 3 (7.9%) | 3 (16.7%) | |
| Income [n (%)] | X2(1) = 0.29^, p = .60 | |||
| <$15,000 | 37 (66.1%) | 26 (68.4%) | 11 (61.1%) | |
| $15,000–29,999 | 13 (23.2%) | 11 (28.9%) | 2 (11.1%) | |
| ≥$30,000 | 6 (10.7%) | 1 (2.6%) | 5 (27.8%) | |
| Primigravid [n(%)] | 4 (7.1%) | 2 (5.3%) | 2 (11.1%) | p=.587# |
| Nulliparous [n(%)] | 6 (10.7%) | 4 (10.5%) | 2 (11.1%) | p= 1.0# |
| BMI [Mean (SD)] | 29.51 (8.21) | 30.01 (8.13) | 28.45 (8.51) | t(54)= −0.66, p = .51 |
p < .05
married versus unmarried
high school versus greater education
above versus below $15,000
Fisher’s exact test
Table 3.
Health Behaviors
| Total (n=56) | African-American (n=38) | White (n=18) | African-American vs White | |
|---|---|---|---|---|
| Smoking Status | X2(1) = .24†, p = .71 | |||
| Current | 10 (17.9%) | 6 (15.8%) | 4 (22.2%) | |
| Past | 16 (28.6%) | 12 (31.6%) | 4 (22.2%) | |
| Never | 30 (53.6%) | 20 (52.6%) | 10 (55.6%) | |
| Impaired Sleep Quality‡ | generalized linear model | |||
| 1st trimester | .57 | .57 | .58 | p = .98 |
| 2nd trimester | .61 | .58 | .67 | p = .53 |
| 3rd trimester | .66 | .63 | .72 | p = .50 |
| Postpartum | .71 | .71 | .72 | p = .93 |
| Prenatal Vitamin Use | X2(1) = 2.95^, p = .08 | |||
| Never | 23 (41.1%) | 18 (47.4%) | 5 (27.8%) | |
| Some days (1–3/week) | 5 (8.9%) | 4 (10.5%) | 1 (5.6%) | |
| Most days (4–6/week) | 7 (12.5%) | 5 (13.2%) | 2 (10.5%) | |
| Every day (7 days/week) | 21 (37.5%) | 11 (28.9%) | 10 (55.6%) | |
| Exercise | X2(1) = 2.29@, p = .13 | |||
| Less than once per month | 18 (32.1%) | 14 (36.8%) | 4 (22.2%) | |
| Once per month | 8 (14.3%) | 4 (10.5%) | 4 (22.2%) | |
| 2–3 times per month | 7 (12.5%) | 7 (18.4%) | 0 (0%) | |
| Once per week | 13 (23.2%) | 8 (21.1%) | 5 (27.8%) | |
| More than once per week | 10 (17.9%) | 5 (13.2%) | 5 (27.8%) |
Current smoker versus current non-smoker
Pittsburgh Sleep Quality Index (PSQI) Score > 5, model estimated proportion
never/some days versus most days/every day
Greater or equal to once per week versus less exercise
Reported Racial Discrimination
Among the African-American women, 21 reported no experience of racial discrimination in the major life domains assessed; 17 reported experiencing discrimination in one or more life domains. Women were divided into two groups: those who reported racial discrimination (high discrimination) and those reporting no occurrence (low discrimination). Among those in the high discrimination category, four reported discrimination in one life domain, eight reported discrimination in two life domains, and five reported discrimination in three or more life domains. The domains in which discrimination was reported, in order of frequency of endorsement were: getting service in a store or restaurant (n=9), on the street or in a public setting (n=9), at school (n=5), at work (n=5), getting hired or getting a job (n=4), getting housing (n=4), getting credit, bank loans, or a mortgage (n=2), from the police or in courts (n=2), and getting medical care (n=1).
Stage of Gestation and EBV Reactivation
We utilized linear mixed models to assess changes in EBV VCA IgG antibody titers longitudinally across pregnancy and postpartum, permitting use of all available data. Contrary to prediction, EBV VCA IgG antibody titers were significantly lower in the 3rd trimester as compared to the 1st trimester [log10 model-adjusted mean titer (95% CI): 2.94 (2.83, 3.05) vs. 3.01 (2.90, 3.12), adjusted p=.002]. Postpartum EBV VCA IgG antibody titers [2.98 (2.87, 3.10)] approached the peak level observed in the 1st trimester, but were not significantly different than any of the pregnancy timepoints (adjusted p=.10 for 3rd trimester vs. postpartum).
Racial Difference in EBV Reactivation
Shown in Figure 1, linear mixed models demonstrated that EBV VCA IgG antibody titers were significantly greater among African-American versus White women during each trimester of pregnancy as well as postpartum [log10 model-adjusted mean titers (95% CIs), African-American vs. White: 1st trimester 3.13 (3.02, 3.26) vs. 2.73 (2.56, 2.90); 2nd trimester 3.13 (3.01, 3.25) vs. 2.62 (2.45, 2.80); 3rd trimester 3.09 (2.97, 3.20) vs. 2.64 (2.47, 2.81); postpartum 3.14 (3.02, 3.26) vs. 2.66 (2.48, 2.83), ps< .001].
Figure 1. Epstein-Barr Virus (EBV) VCA IgG antibody titers during pregnancy and postpartum.
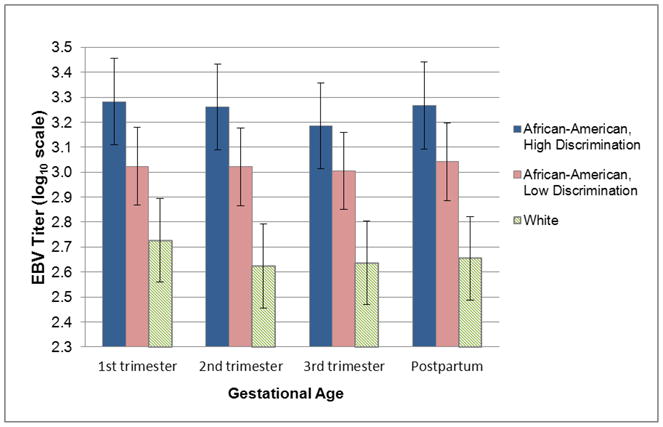
(Means and 95% confidence intervals.) In the sample overall, EBV VCA IgG antibody titers were lower during the 3rd trimester as compared to the 1st trimester (p = .002). Compared to White women (n=18) African-American women (n=38) exhibited significantly higher EBV VCA IgG antibody titers at each stage of gestation and at postpartum (ps < 0.001). This effect was most pronounced among African-American women endorsing high racial discrimination (n=17) versus those endorsing low racial discrimination (n=21) [p = .03 (1st), .04 (2nd), .12 (3rd), .06 (postpartum)].
Psychosocial Stress and EBV Reactivation
EBV VCA IgG antibody titers did not differ based on depressive symptoms, perceived stress, state anxiety, or pregnancy-specific stress during pregnancy or postpartum in the full sample (ps>.11) or among African-Americans when assessed separately (ps>.17). African-American women were classified as high versus low based on median split in terms of the number of situations in which they reported discrimination on the EOD scale. In a mixed linear model, those reporting high versus low discrimination showed significantly higher EBV VCA IgG antibody titers during the first (p=0.03) and second trimesters of pregnancy (p=.04; Figure 1). A similar, though non-significant, pattern was observed in the 3rd trimester (p=.12) and at postpartum (p=.06). When compared to White women, both groups of African-American women had elevated EBV VCA IgG antibody titers at all three trimesters and at postpartum [high discrimination: ps < .001; low discrimination: p=.01 (1st), .001 (2nd), .002 (3rd), .001 (postpartum); Figure 1].
EBV VCA IgG antibody Titers and Serum Inflammatory Markers
To determine the extent to which EBV reactivation may promote inflammatory activity, we examined EBV VCA IgG antibody titer status in relation to serum IL-6, IL-8, TNF-α, and IL-1β. Results of linear mixed model analyses indicated that none of the serum inflammatory cytokines were significantly associated with EBV VCA IgG antibody titer status at any of the visits (ps > 0.18).
Race, Stress, and Health Behaviors
As described above, African-American women did not differ significantly from Whites on any health behaviors assessed (Table 3). Among African-American women, those reporting high versus low discrimination also did not differ in health behaviors: current smoking (X2(1)=.08, p = .78), vigorous exercise (once per week versus less; X2(1)=.02, p = .90), prenatal vitamin use (X2(1)=1.45, p = .22), or rates of clinically disturbed sleep during any trimester of pregnancy (PSQI score > 5; ps > .17).
Discussion
This study provides novel data on longitudinal stability in EBV VCA IgG antibody titers across the course of pregnancy. Based on the limited prior literature (e.g., Purtilo and Sakamoto 1982), we hypothesized that EBV antibody titers would be greater with increasing gestational age due to suppression of cell-mediated immune function. Contrary to prediction, EBV VCA IgG antibody titers were significantly lower in the 3rd trimester of pregnancy as compared to the 1st trimester. Overall, the stability of EBV VCA IgG antibody titers across pregnancy was remarkable. Across the course of the study, including postpartum follow-up, no woman exhibited a change of more than one dilution in EBV VCA IgG antibody titers.
As an opportunistic latent virus, factors inducing immunosuppression allow EBV to reactivate. Reactivation can be associated with the release of viral antigens and complete infectious virus particles, resulting in the initiation of an antibody response. Thus, EBV VCA IgG antibody titers can provide an indirect measure of cell-mediated immune function. Reactivation may also result in the expression of viral proteins which by themselves may induce immune dysregulation (Glaser, Litsky et al. 2006). In non-pregnant adults, increases in EBV VCA IgG antibody titers are commonly interpreted as an indication of impaired cell-mediated immunity (e.g., Glaser and Kiecolt-Glaser 1994; Stowe, Pierson et al. 2001) and decreases in antibody titers as indicative of improved cell-mediated immunity (e.g., Esterling, Antoni et al. 1992).
In the context of pregnancy, the observed decrease in antibody titers in later pregnancy may be due to one or more factors. Prior research has demonstrated the overall serum concentrations of IgG, as well as IgA and IgM, decrease during normal pregnancy (Amino, Tanizawa et al. 1978; Malek, Sager et al. 1996). It has been suggested that this may result from suppression of humoral immunity (Amino, Tanizawa et al. 1978; Malek, Sager et al. 1996). In addition, IgG is actively transported across the placenta, with the largest transfer occurring in the third trimester (Simister 2003; Kane and Acquah 2009). Although blood volume increases by 40–50% during pregnancy (Monga 2009), prior data suggest that hemodilution only modestly contributes to the overall decrease in IgG observed in pregnancy (Amino, Tanizawa et al. 1978). Moreover, in the current dataset, decreased EBV VCA IgG antibody titers were observed only in the 3rd trimester. If this effect were due to hemodilution, decreases in the 2nd trimester would be expected as well, as blood volume increases considerably through approximately week 28 after which levels remain relatively stable.
Therefore, while scientifically accurate, the finding of decreased EBV VCA IgG antibody titers in late pregnancy is unexpected and not easily explained considering the complex and multifactorial physiological changes that occur during pregnancy. Given the unique context of pregnancy, this study would have been strengthened by the inclusion of direct measures of viral replication. Although EBV antibody titers provide a useful indirect measure of reactivation, it is not possible to determine whether the observed decrease in antibody levels in late pregnancy was due to a general inhibition of antibody production or a change in the expression of latent virus. A more direct method of determining viral reactivation/replication is via measurement of viral DNA levels. This should be considered in future studies.
In terms of racial differences, as hypothesized, African-Americans exhibited substantially higher EBV VCA IgG antibody titers than Whites during each trimester of pregnancy and postpartum. Supporting a role for chronic stress in this relationship, this effect was significantly stronger among African-American women reporting greater lifetime occurrence of racial discrimination.
Most prior studies of EBV reactivation in non-pregnant adults have not included a sufficient number of African-Americans to examine racial differences. As reviewed, one study reported greater viral reactivation among Black adults ages 25–90 as compared to non-Hispanic Whites (Stowe, Peek et al. 2010). Also, among non-pregnant women, perceived racial discrimination was associated with greater EBV reactivation (Borders, Grobman et al. 2010). A study of depressed versus non-depressed pregnant women found no difference in EBV reactivation by race (Haeri, Johnson et al. 2011). However, only 9.5% of women were African-American and only 14% were very low income (i.e., Medicaid recipients). The current sample was 68% African-American and 66% reported an annual family income <$15,000. Thus, the power to detect effect of race and racial discrimination was likely enhanced in the current study by the high representation of African-Americans as well as greater vulnerability to stressors among economically disadvantaged women.
The current dataset did not provide an adequate sample size to examine EBV antibody titers in relation to adverse birth outcomes, such as preterm birth; only 6 preterm births occurred in this sample. Data relating EBV reactivation to adverse pregnancy outcomes is limited mostly to case reports of primary infection (Brown and Stenchever 1978; Goldberg, Fulginiti et al. 1981; Fleisher and Bolognese 1983; Fleisher and Bologonese 1984; Schuster, Janssen et al. 1993). While primary infection occurs in only 0.05–1.5% of pregnancies (Le, Chang et al. 1983; Eskild, Bruu et al. 2005), >90% of women carry EBV in a latent state and are therefore susceptible to reactivation. At least one study found no association between EBV reactivation and length of gestation or congenital abnormalities (Avgil, Diav-Citrin et al. 2008). Two studies have linked EBV reactivation to adverse pregnancy outcomes including stillbirth, birth defects, shorter gestation, and lower birth weight (Icart, Didier et al. 1981; Eskild, Bruu et al. 2005). Also, some data link maternal EBV reactivation to risk of leukemia and testicular cancer in offspring (Lehtinen, Koskela et al. 2003; Tedeschi, Bloigu et al. 2007; Holl, Surcel et al. 2008). Future studies with considerably larger sample sizes are needed to examine the current racial differences in EBV antibody titers in relation to birth outcomes.
Available data do not indicate whether EBV may play a causal role in adverse perinatal health outcomes or if it serves as a general marker of a pathological process (i.e., impaired cellular immune competence). Work from our laboratory has shown that at least one EBV encoded early protein, dUTPase, can induce the synthesis of several proinflammatory cytokines including IL-8, IL-6 and IL-1β (Glaser, Litsky et al. 2006). The target cells are dendritic cells and macrophages (Ariza, Glaser et al. 2009). As reviewed, a growing literature supports a role for inflammation in the relationship between stress and preterm birth (Coussons-Read, Okun et al. 2005; Coussons-Read, Okun et al. 2007; Ruiz, Stowe et al. 2007; Paul, Boutain et al. 2008; Christian, Franco et al. 2009; Christian, Franco et al. 2010; Blackmore, Moynihan et al. 2011; Cassidy-Bushrow, Peters et al. 2012; Coussons-Read, Lobel et al. 2012). There were no significant associations between EBV VCA IgG antibody titers and serum proinflammatory cytokines in the current sample, suggesting that EBV reactivation did not significantly promote inflammatory activity. However, further work in larger samples is needed to understand these interactions and implications for pregnancy.
We examined health behaviors as a potential mediator of the relationship between stress and EBV VCA IgG antibody titers. Women did not differ in body mass index, smoking, exercise, sleep, or prenatal vitamin use based on either race or perceived racial discrimination. Thus, health behaviors did not explain the observed differences in EBV VCA IgG antibody titers, supporting the role for direct physiological pathways in the relationship between stress and immune dysregulation. A limitation of these data is that they were obtained by self-report. All self-reported pre-pregnancy weights were plausible given the woman’s weight as measured by scale at the first study visit. However, women are more likely to under-report rather than over-report their pre-pregnancy weight (Kovalchik 2009). Similarly, self-reported smoking among pregnant women likely underestimates true smoking behavior (Shipton, Tappin et al. 2009).
Contrary to prior studies, aside from racial discrimination, other stress parameters (e.g., depressive symptoms, perceived stress) were not associated with EBV VCA IgG antibody titers in this sample. This may reflect statistical power; these effects may be smaller than those exerted by race and racial discrimination, thereby requiring a larger sample size to permit detection. In addition, greater range and representation of women with lower stress may provide greater power to detect effects of other stress parameters.
Our goal in utilizing EBV VCA IgG antibody titers as a marker in the current investigation was to use this as an indicator of cellular immune competence. That is, we hypothesize that impaired cell-mediated immune function, rather than EBV reactivation per se, may contribute to adverse perinatal health outcomes. However, it is possible that EBV itself may play a causal role. Given this possibility, effects of stress and pregnancy status on other latent viruses warrant attention as well, including herpes simplex virus (HSV) I and II, varicella-zoster virus (VZV), and cytomegalovirus (CMV). For example, CMV and HSV-1 are carried latently by approximately 60% of adults in the U.S. by age 40 (Staras, Dollard et al. 2006; Xu, Sternberg et al. 2006) and both may be reactivated in conditions of stress (Glaser, Kiecolt-Glaser et al. 1985; Schaeffer, Mckinnon et al. 1985; Mehta, Stowe et al. 2000). Moreover, inflammation associated with viral reactivation may only be detectable in the context of reactivation of multiple herpesviruses (Bennett, Glaser et al. 2012). Thus, assessment of seropositive status and reactivation of multiple herpesviruses would be informative in future studies.
The literature linking stress and immune parameters in human pregnancy is growing (Christian 2012). Our data indicate that EBV VCA IgG antibody titers are significantly lower in later pregnancy as compared to early pregnancy. Compared to Whites, African-American women showed greater EBV VCA IgG antibody titers across pregnancy and postpartum. This difference was the most considerable among African-American women reporting greater racial discrimination, supporting a role for chronic stress in this association. As a possible indicator of stress-induced immune dysregulation during pregnancy, EBV provides a novel viral model which may elucidate biological pathways underlying racial disparities in perinatal health. Continued research is needed to determine the reliability of these findings in larger cohorts and the extent to which EBV reactivation may causally contribute to adverse pregnancy outcomes or instead serve as a marker of general immune dysregulation. In sum, this study provides novel data regarding the association of stage of pregnancy, race, and psychosocial stress with EBV VCA IgG antibody titers. These findings add to a growing literature showing that stress is associated with altered immune function during pregnancy.
Highlight.
Compared to Whites, African-Americans exhibit higher Epstein-Barr virus antibody titers in pregnancy and postpartum, particularly those reporting greater racial discrimination.
Acknowledgments
Role of the Funding Sources
This study was supported by NICHD (R21HD061644, LMC and R21HD067670, LMC) and NIAID (AI084898, RG). The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources, Grant UL1RR025755 and is now at the National Center for Advancing Translational Sciences, Grant 8UL1TR000090-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We appreciate the contributions of Clinical Research Assistants Colleen Sagrilla, Kelly Marceau, and Rebecca Long to data collection. We thank Marshall Williams for his thoughtful comments on the manuscript. We also thank the staff and patients of the Ohio State University Wexner Medical Center Prenatal Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amino N, Tanizawa O, et al. Changes of Serum Immunoglobulins Igg, Iga, Igm, and Ige during Pregnancy. Obstetrics and Gynecology. 1978;52(4):415–420. [PubMed] [Google Scholar]
- Ariza ME, Glaser R, et al. The EBV-Encoded dUTPase Activates NF-kappa B through the TLR2 and MyD88-Dependent Signaling Pathway. Journal of Immunology. 2009;182(2):851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- Avgil M, Diav-Citrin O, et al. Epstein-Barr virus infection in pregnancy--a prospective controlled study. Reprod Toxicol. 2008;25(4):468–471. doi: 10.1016/j.reprotox.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Bennett JM, Glaser R, et al. Inflammation and reactivation of latent herpesviruses in older adults. Brain, Behavior, and Immunity. 2012;26(5):739–746. doi: 10.1016/j.bbi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, et al. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic Medicine. 2011;73(8):656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders AE, Grobman WA, et al. The relationship between self-report and biomarkers of stress in low-income reproductive-age women. American Journal of Obstetrics and Gynecology. 2010;203(6):577, e571–578. doi: 10.1016/j.ajog.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZA, Stenchever MA. Infectious mononucleosis and congenital anomalies. Am J Obstet Gynecol. 1978;131(1):108–109. doi: 10.1016/0002-9378(78)90484-2. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Campbell ME, Herman MR. Politics and policies: attitudes toward multiracial Americans. Ethnic and Racial Studies. 2010;33(9):1511–1536. [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, et al. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. Journal of Reproductive Immunology. 2012;94(2):202–209. doi: 10.1016/j.jri.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Christenfeld NJ, Sloan RP, et al. Risk factors, confounding, and the illusion of statistical control. Psychosom Med. 2004;66(6):868–875. doi: 10.1097/01.psy.0000140008.70959.41. [DOI] [PubMed] [Google Scholar]
- Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience and Biobehavioral Reviews. 2012;36 doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, et al. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity. 2009;23(6):750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, et al. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behavior, and Immunity. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, et al. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Collins JW, David RJ, et al. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. American Journal of Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm birth: causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. [Google Scholar]
- Coussons-Read ME, Lobel M, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behavior and Immunity. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, et al. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, et al. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine. 2005;67(4):625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, et al. Maternal stress and preterm birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, et al. Psychosocial factors and preterm birth among African American and White women in central North Carolina. American Journal of Public Health. 2004;94(8):1358–1365. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskild A, Bruu AL, et al. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. British Journal of Obstetrics and Gynaecology. 2005;112(12):1620–1624. doi: 10.1111/j.1471-0528.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- Esterling BA, Antoni M, et al. Emotional repression, stress disclosure responses, and Epstein-Barr viral capsid antigen titers. Psychosomatic Medicine. 1990;52:397–410. doi: 10.1097/00006842-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Esterling BA, Antoni MH, et al. Defensiveness, trait anxiety, and Epstein-Barr viral capsid antigen antibody titers in healthy college students. Health Psychology. 1993;12(2):132–139. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- Esterling BA, Antoni MH, et al. Psychosocial modulation of antibody to Epstein-Barr viral capsid antigen and human herpesvirus type-6 in HIV-1-infected and at-risk gay men. Psychosomatic Medicine. 1992;54(3):354–371. doi: 10.1097/00006842-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychology. 2011 doi: 10.1037/a0025599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher G, Bolognese R. Persistent Epstein-Barr virus infection and pregnancy. J Infect Dis. 1983;147(6):982–986. doi: 10.1093/infdis/147.6.982. [DOI] [PubMed] [Google Scholar]
- Fleisher G, Bologonese R. Infectious mononucleosis during gestation: report of three women and their infants studied prospectively. Pediatr Infect Dis. 1984;3(4):308–311. doi: 10.1097/00006454-198407000-00006. [DOI] [PubMed] [Google Scholar]
- Giscombe CL, Lobel M. Explaining Disproportionately High Rates of Adverse Birth Outcomes Among African Americans: The Impact of Stress, Racism, and Related Factors in Pregnancy. Psychological Bulletin. 2005;131(5):662–683. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Friedman SB, et al. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain, Behavior, and Immunity. 1999;13(3):240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation and its implications for reactivation of latent herpesviruses. In: Glaser R, Jones J, editors. Herpesvirus infections. New York: Marcel Dekker; 1994. pp. 245–270. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, et al. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, et al. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, et al. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, et al. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346(1):205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Goldberg GN, V, Fulginiti A, et al. In utero Epstein-Barr virus (infectious mononucleosis) infection. JAMA. 1981;246(14):1579–1581. [PubMed] [Google Scholar]
- Haeri S, Johnson N, et al. Maternal depression and epstein-barr virus reactivation in early pregnancy. Obstetrics and Gynecology. 2011;117(4):862–866. doi: 10.1097/AOG.0b013e31820f3a30. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, et al. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Holl K, Surcel HM, et al. Maternal Epstein-Barr virus and cytomegalovirus infections and risk of testicular cancer in the offspring: a nested case-control study. Acta Pathologica et Microbiologica Scandinavica. 2008;116(9):816–822. doi: 10.1111/j.1600-0463.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- Icart J, Didier J, et al. Prospective study of Epstein Barr virus (EBV) infection during pregnancy. Biomedicine. 1981;34(3):160–163. [PubMed] [Google Scholar]
- Jones JF, Straus SE. Chronic Epstein-Barr Virus infection. Annual Review of Medicine. 1987;38:195–209. doi: 10.1146/annurev.me.38.020187.001211. [DOI] [PubMed] [Google Scholar]
- Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. American Journal of Gastroenterology. 2009;104(1):228–233. doi: 10.1038/ajg.2008.71. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, et al. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53(4):345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kovalchik S. Validity of adult lifetime self-reported body weight. Public Health Nutrition. 2009;12(8):1072–1077. doi: 10.1017/S1368980008003728. [DOI] [PubMed] [Google Scholar]
- Krieger N. Racial and Gender Discrimination - Risk-Factors for High Blood-Pressure. Social Science and Medicine. 1990;30(12):1273–1281. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- Krieger N, Sidney S. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. American Journal of Public Health. 1996;86(10):1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, et al. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science and Medicine. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Le CT, Chang RS, et al. Epstein-Barr virus infections during pregnancy. A prospective study and review of the literature. Am J Dis Child. 1983;137(5):466–468. doi: 10.1001/archpedi.1983.02140310048014. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Koskela P, et al. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. American Journal of Epidemiology. 2003;158(3):207–213. doi: 10.1093/aje/kwg137. [DOI] [PubMed] [Google Scholar]
- Li D, Liu L, et al. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146–153. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- Lobel M. The revised prenatal distress questionnaire (NUPDQ) Stony Brook; New York: State University of New York; 1996. [Google Scholar]
- Lutgendorf SK, Reimer TT, et al. Effects of housing relocation on immunocompetence and psychosocial functioning in older adults. J Gerontol A Biol Sci Med Sci. 2001;56(2):M97–105. doi: 10.1093/gerona/56.2.m97. [DOI] [PubMed] [Google Scholar]
- Malek A, Sager R, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. American Journal of Reproductive Immunology. 1996;36(5):248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) British Journal of Clinical Psychology. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- McDade TW. Lifestyle incongruity, social integration, and immune function in Samoan adolescents. Social Science and Medicine. 2001;53(10):1351–1362. doi: 10.1016/s0277-9536(00)00414-7. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, et al. Culture change and stress in Western Samoan youth: Methodological issues in the cross-cultural study of stress and immune function. American Journal of Human Biology. 2000;12(6):792–802. doi: 10.1002/1520-6300(200011/12)12:6<792::AID-AJHB7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Meades R, Ayers S. Anxiety measures validated in perinatal populations: A systematic review. Journal of Affective Disorders. 2011;133(1–2):1–15. doi: 10.1016/j.jad.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Pierson DL, et al. Epstein-Barr virus reactivation associated with diminished cell-mediated immunity in antarctic expeditioners. J Med Virol. 2000;61(2):235–240. doi: 10.1002/(sici)1096-9071(200006)61:2<235::aid-jmv10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Stowe RP, et al. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182(6):1761–1764. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- Monga M. Maternal Cardiovascular, Respiratory, and Renal Adapation to Pregnancy. In: Creasy RRRK, Iams JD, editors. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 6. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- Mustillo S, Krieger N, et al. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA Study. American Journal of Public Health. 2004;94(12):2125–2131. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. Final Natality Data. Retrieved May 3, 2012, from www.marchofdimes.com/peristats.
- Orr ST, James SA, et al. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- Paul K, Boutain D, et al. The relationship between racial identity, income, stress and C-reactive protein among parous women: Implications for preterm birth disparity research. Journal of the National Medical Association. 2008;100(5):540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- Payne DA, Mehta SK, Tyring SK, Stowe RP, Pierson DL. Incidence of Epstein-Barr virus in astronaut saliva during spaceflight. Aviation space and environmental medicine. 1999;70(12):1211–1213. [PubMed] [Google Scholar]
- Phillips GS, Wise LA, et al. Prepregnancy depressive symptoms and preterm birth in the Black Women’s Health Study. Ann Epidemiol. 2010;20(1):8–15. doi: 10.1016/j.annepidem.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson DL, Stowe RP, et al. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun. 2005;19(3):235–242. doi: 10.1016/j.bbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Purtilo DT, Sakamoto K. Reactivation of Epstein-Barr Virus in Pregnant-Women - Social-Factors, and Immune Competence as Determinants of Lymphoproliferative Diseases - a Hypothesis. Medical Hypotheses. 1982;8(4):401–408. doi: 10.1016/0306-9877(82)90033-0. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rosenberg L, Palmer JR, et al. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. 2002;13(6):646–652. doi: 10.1097/00001648-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Stowe RP, et al. The relationships among acculturation, body mass index, depression, and interleukin 1-receptor antagonist in Hispanic pregnant women. Ethn Dis. 2007;17(2):338–343. [PubMed] [Google Scholar]
- Schaeffer MA, Mckinnon W, et al. Immune Status as a Function of Chronic Stress at Three-Mile-Island. Psychosomatic Medicine. 1985;47(1):85–85. [Google Scholar]
- Schuster V, Janssen W, et al. Congenital Epstein-Barr virus infection. Monatsschr Kinderheilkd. 1993;141(5):401–404. [PubMed] [Google Scholar]
- Shipton D, Tappin DM, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. British Medical Journal. 2009;339 doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, et al. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Sorensen MV, Snodgrass JJ, et al. Lifestyle incongruity, stress and immune function in indigenous Siberians: the health impacts of rapid social and economic change. American Journal of Physical Anthropology. 2009;138(1):62–69. doi: 10.1002/ajpa.20899. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anxiety inventory: a comprehensive bibliography. Palo Alto, CA (577 College Ave., Palo Alto 94306): Consulting Psychologists Press; 1989. [Google Scholar]
- Staras SA, Dollard SC, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clinical Infectious Diseases. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, et al. Herpesvirus reactivation and socioeconomic position: a community-based study. Journal of Epidemiology and Community Health. 2010;64(8):666–671. doi: 10.1136/jech.2008.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Pierson DL, et al. Elevated stress hormone levels relate to Epstein-Barr virus reactivation in astronauts. Psychosom Med. 2001;63(6):891–895. doi: 10.1097/00006842-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Tedeschi R, Bloigu A, et al. Activation of maternal Epstein-Barr virus infection and risk of acute leukemia in the offspring. American Journal of Epidemiology. 2007;165(2):134–137. doi: 10.1093/aje/kwj332. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, DunkelSchetter C, et al. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58(5):432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Xu FJ, Sternberg MR, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama-Journal of the American Medical Association. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]


