Abstract
Obesity has been associated with increased cardiac sympathetic activation during wakefulness, but the effect on sleep-related sympathetic modulation is not known. The aim of this study was to investigate the effect of fat gain on cardiac autonomic control during wakefulness and sleep in humans. We performed a randomized controlled study to assess the effects of fat gain on heart rate variability (HRV). We recruited 36 healthy volunteers, who were randomized to either a standardized diet to gain approximately 4 kg over 8 weeks followed by an 8 week weight loss period (n=20), or to serve as a weight-maintainer control (n=16). An overnight polysomnogram with power spectral analysis of HRV was performed at baseline, after weight gain, and after weight loss to determine the ratio of low frequency (LF) to high frequency (HF) power, and to examine the relationship between changes in HRV and changes in insulin, leptin and adiponectin levels. Mean weight gain was 3.9 kg in the fat gain group versus 0.1 kg in the maintainer group. LF/HF increased both during wakefulness and sleep after fat gain and returned to baseline after fat loss in the fat gain group, and did not change in the control group. Insulin, leptin and adiponectin also increased after fat gain and fell after fat loss, but no clear pattern of changes were seen that correlated consistently with changes in HRV. Short-term fat gain in healthy subjects is associated with increased cardiac sympathetic activation during wakefulness and sleep but the mechanisms remain unclear.
Keywords: weight gain, heart rate variability, sympathetic nerve activity, obesity, insulin, leptin, adiponectin
There is considerable evidence that overweight or obesity increases cardiovascular morbidity and mortality.1–3 A number of mechanisms, including sympathetic activation,4 have been proposed to explain this association.5–8
Obesity has been linked with increased peripheral9–13 and cardiac14 sympathetic activation. Modest weight gain has been associated with increased muscle sympathetic nerve activity (MSNA) in non-obese men.15 Mechanisms linking obesity to alterations in neural circulatory control are not well defined, but it has been postulated that the increased circulating leptin and insulin, and decreased adiponectin16 are associated with increased cardiac sympathetic activity and vasoconstriction in obese people.17
Sleep accounts for approximately one-third of our lives and is accompanied by significant changes in autonomic and circulatory regulation. REM sleep in particular is associated with enhanced MSNA,18 striking fluctuations in heart rate, and alterations in coronary artery blood flow.19 Meanwhile, the early morning transition from sleep to wakefulness is associated with an increased risk of sudden cardiac death20 stroke21 and myocardial infarction.22 Assessment of sympathetic activation during periods of sleep and wakefulness may be clinically relevant and can be enabled by power spectral analysis of heart rate variability (HRV).23–25 Furthermore, there are no data regarding the effects of weight gain and related changes in insulin, leptin and adiponectin on sleep-related cardiac sympathetic modulation. The aim of this study, therefore, was to investigate the effects of weight gain and subsequent weight loss on cardiac autonomic control during sleep as measured by HRV in healthy subjects, and to examine the relationship between these changes and changes in insulin, leptin, and adiponectin concentration.
Methods
Subjects
This study was approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from each subject. We recruited 36 volunteers and after a weight maintenance period of 3 days, subjects were randomly assigned to be in the fat-gainer (n=20) or weight-maintainer group (n=16). Exclusion criteria included use of any tobacco products, employment in shift work, previous diagnosis of any disease including any sleep-related disorder, and use of any prescription medications other than oral contraceptives. Findings from this study relating to endothelial dysfunction have been published elsewhere.26
Weight-Gain and Weight-Loss Protocols
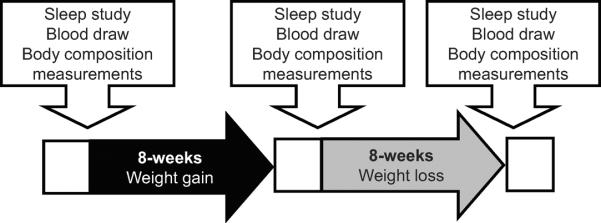
Each subject received weight-maintenance meals from our metabolic kitchen for 3 days prior to each phase. The menus were based on the standardized foods available in the metabolic kitchen at the Clinical Research Unit (CRU) of Mayo Clinic, and each subject's food preferences. Weight maintenance caloric needs were calculated per the Harris-Benedict equation,27 plus an additional 30–60% to match occupational activity needs. After the weight maintenance period of 3 days, those randomized to gain weight received a diet with 1000 kcal/day beyond their weight maintenance requirements for 8 weeks while those randomized to maintain weight continued to receive the same diet for 8 weeks. The goal was to gain approximately 3 to 4 kg of total body fat (approximately 5% increase in weight), and weight was measured at least 5 days per week. After the fat gain period, subjects underwent a supervised diet program for 8 weeks to return to their basal weight. The diet composition throughout the study was 40% carbohydrate, 40% fat, and 20% protein. Cardiopulmonary exercise testing at baseline, after weight gain and weight loss was conducted to assess levels of physical fitness. The study outline is shown in Figure 1.
Figure 1.
Study design
Polysomnography
Patients underwent nocturnal laboratory-based attended digital polysomnography in the CTSA Sleep Facility at the Clinical Research Unit of Mayo Clinic in Rochester. Polysomnograms were recorded using a Compumedics E-Series Comprehensive Networked-Linked Amplifier (Compumedics, Abbotsford, VIC., Australia). Polysomnograms were scored by an experienced registered polysomnographic technologist in accordance with current American Academy of Sleep Medicine guidelines.28
Heart Rate Variability Spectral Analysis
HRV was measured during wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. The data obtained during wakefulness were recorded for 5 minutes at 10:00 PM before sleep onset. The data during NREM and REM sleep were visually identified from the polysomnographic recordings and whole segments from the first and second epoch of each sleep stage was selected for analysis with the results averaged. Measurements were taken only during established sleep stages during periods of stable breathing not associated with any arousals. Electrocardiographic (ECG) signals from bipolar leads were transformed to digital signals to calculate the R-R intervals at a sampling rate of 512 Hz. Power spectral analysis of HRV was performed by the MemCalc power spectral density method29 using a commercial software package (MemCalc/Win, Suwa Trust, Tokyo, Japan) that used the maximum entropy method for spectral analysis and the non-linear least squares method for fitting analysis.
Low frequency (LF) was defined as 0.04–0.15 Hz, and high frequency (HF) was defined as 0.15–0.4 Hz. The LF component was corrected to normalized units (nu) using the equation LFnu=LF/(LF+HF) and the HF component was corrected to nu as HFnu=HF/(LF+HF).
Measurements of Body Composition
Body composition was measured at baseline, after weight-gain and after recovery and included height measured by wall stadiometer, weight by an electronic scale, waist and hip circumferences by non-elastic tape, and body fat by dual-energy x-ray absorptiometry (Lunar Radiation, Madison, WI).
Blood Measurements
Fasting blood samples were obtained by venipuncture immediately after polysomnography at 6:00 am and assayed in the Immunochemical Core Laboratory of the CRU at Mayo Clinic, Rochester, MN. Plasma glucose levels were measured using the standard turbidimetric method using a Hitachi 912 (Roche Diagnostic, Basel, Switzerland), plasma insulin levels were measured using a two-site immune enzymatic assay (Beckman Instruments, Chaska, MN), plasma leptin levels were measured with commercially-available radioimmunoassay kits (Linco Research, St. Charles, MO) and plasma adiponectin levels were measured using enzyme-linked immunosorbent assay kits (Mediagnost, Reutlingen, Germany). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated with the formula as plasma insulin (μU/mL) × fasting glucose (mg/dL)/405.30 This index is considered to be a useful marker for simple assessment of insulin resistance.
Statistical Analysis
Data are summarized as number and percentage for categorical variables and means with standard error of the mean (SEM) for continuous variables. Changes in HRV between baseline and after weight gain, between weight gain and after recovery, and baseline and after recovery were pre-specified analyses evaluated by Wilcoxon Sign-Rank test. As an exploratory analysis, the correlation between circulating insulin, leptin, and adiponectin and those HRV parameters that changed significantly during the study was assessed using Spearman's correlation coefficient. Analyses were performed with JMP version 8 (SAS Institute, Cary, NC). A two-sided p-value of <0.05 was considered statistically significant, and a Bonferoni correction was used to correct for multiple comparisons involving the three measures of spectral power (LFnu, HFnu, and LF/HF, p<0.016).
Results
We recruited 36 healthy volunteers, 22 men and 14 women, between the ages of 18 and 50 years (mean 29.6±1.3 years).
Glucose levels were significantly different between fat-gainers (n=20) and weight-maintainers (n=16) at baseline (98.0 vs 88.5 mg/dl, p<0.01). Baseline body fat percentage in the fat-gainers and weight-maintainers was not significantly different (31.7 vs 29.6 %, p=0.15). There were no differences in any variable measured between baseline and at the 8 weeks time point in the weight-maintainer group. In the fat-gainer group, subjects gained an average of 3.9 ± 0.2 kg in the weight gain period which was also reflected by increases in body fat, waist and hip circumference. However, blood pressure, VO2 peak, apnea-hypopnea index, total sleep time and number of arousals did not change during the study in the fat-gainer group (Table 1).
Table 1.
Subject characteristics during the fat-gain and weight-maintainance protocols
| Fat-gainers (n=20) | Weight-maintainers (n=16) | ||||
|---|---|---|---|---|---|
| Variable | Baseline | Weight gain | Recovery | Baseline | Follow up |
| Age, years | 29.7±1.4 | - | - | 29.6±2.3 | - |
| Female, % | 40 | - | - | 37.5 | - |
| Body weight, kg | 73.9±3.4 | 77.8±3.5†§ | 74.1±3.3 | 74.6±3.6 | 74.7±3.7 |
| Body fat, % | 31.7±1.9 | 34.2±2.0†§ | 32.4±2.1 | 29.6±2.2 | 29.8±2.3 |
| Body Mass Index, kg/m2 | 24.4±0.9 | 25.7±0.9†§ | 24.5±0.9 | 24.2±0.9 | 24.4±0.9 |
| Waist circumference, cm | 82.3±2.3§ | 86.8±2.4†‡ | 85.1±2.4† | 84.2±3.0 | 84.0±2.6 |
| Hip circumference, cm | 98.2±1.8 | 101.5±1.9†§ | 98.5±1.8 | 100.0±1.7 | 100.7±1.8 |
| Systolic blood pressure, mmHg | 118.3±2.9 | 119.4±3.3 | 115.4±2.8 | 116.3±3.2 | 117.2±2.8 |
| Diastolic blood pressure, mmHg | 73.7±2.5 | 74.6±2.2 | 73.0±2.3 | 72.8±2.3 | 71.7±2.6 |
| Resting heart rate, beats/minute | 67.7±2.7 | 68.3±2.6 | 67.7±2.8 | 66.9±3.7 | 64.8±3.2 |
| VO2 peak, mL/kg/min | 36.0±2.3 | 36.2±2.0 | 37.5±2.2 | 38.5±2.6 | 38.3±2.8 |
| Total Sleep Time, min | 353.0±9.5 | 367.8±8.5 | 366.8±11.8 | 350.1±9.2 | 363.7±7.1 |
| AHI, events/hour | 1.1±0.3 | 0.9±0.3 | 0.8±0.2 | 1.6±0.6 | 1.7±0.5 |
| Number of Arousals, events/hour | 19.1±2.4 | 21.9±2.8 | 17.8±1.7 | 21.2±2.3 | 21.9±2.4 |
| Glucose, mg/dL | 98.0±2.1 | 100.2±5.0 | 95.3±2.2 | 88.5±2.3∥ | 88.0±1.6 |
| Insulin, μU/mL | 5.3±0.7 | 7.1±0.9* | 6.5±1.0 | 4.3±0.4 | 4.3±0.6 |
| HOMA-IR | 1.4±0.2 | 2.0±0.3 | 1.8±0.3 | 0.9±0.1 | 0.9±0.2 |
| Leptin, ng/mL | 6.5±1.0 | 10.9±1.5†§ | 6.3±0.9 | 5.7±1.1 | 6.5±1.4 |
| Adiponectin, ng/mL | 8129±1218 | 9339±1477‡ | 7420±1296 | 8829±1273 | 8167±1141 |
Data are presented as mean±SEM.
Within group comparisons:
p<0.05,
p<0.01 when compared to baseline.
p<0.05,
p<0.01, when compared to recovery.
p<0.01 when compared to fat-gainers at baseline.
Weight gain was associated with increased circulating concentrations of both insulin (5.3 vs 7.1 μU/mL, p<0.05) and leptin (5.2 vs 9.8 ng/mL, p<0.01) and a trend towards increased adiponectin concentration (8129 vs 9339 ng/mL, p=0.17). After weight loss, circulating levels of insulin, leptin and adiponectin fell towards baseline levels. Fasting plasma glucose concentrations did not significantly change after weight gain (98.0 vs 100.2 mg/dL, p=0.92) nor after weight loss (100.2 vs 95.3 mg/dL, p=0.59). HOMA-IR did not significantly change between any time points, and nor did VO2 peak (Table 1).
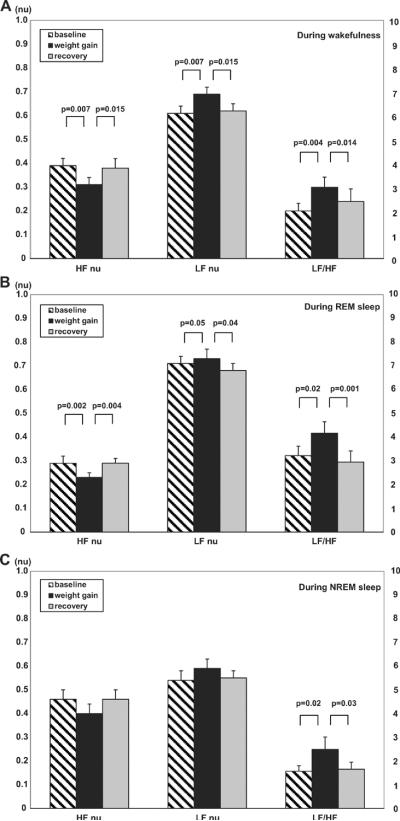
Changes in HRV during wakefulness, during REM sleep and during NREM sleep are presented in Table 2 and Figure 2. During wakefulness (Figure 2A) there was a significant decreases in HFnu along with an increase in LFnu and LF/HF ratio (0.39 vs 0.31 nu, p<0.01, 0.61 vs 0.69 nu, p<0.01 and 2.00 vs 2.99, p<0.01, respectively) after weight gain. Moreover, changes in HFnu, LFnu, and LF/HF ratio resolved with weight loss and returned towards baseline levels (0.38 vs 0.39 nu, p=0.88, 0.62 vs 0.61 nu, p=0.88, and 2.39 vs 2.00, p=0.48, respectively). During REM sleep (Figure 2B), there was a significant decrease in HFnu (0.29 vs 0.23 nu, p<0.01), and a slight increase in LFnu and LF/HF ratio (0.71 vs 0.73 nu, p=0.05 and 3.22 vs 4.16, p=0.02, respectively) after weight gain. On the other hand, HFnu was significantly increased (0.23 vs 0.29 nu, p<0.01), LFnu was slightly decreased (0.73 vs 0.68 nu, p=0.04) and LF/HF ratio was significantly decreased (4.16 vs 2.95, p<0.01) after weight loss. During NREM sleep (Figure 2C), no significant changes were observed in HFnu and LFnu, either after weight gain (0.46 vs 0.40 nu, p=0.05 and 0.54 vs 0.59 nu, p=0.13, respectively) nor after weight loss (0.40 vs 0.46 nu, p=0.07 and 0.59 vs 0.55 nu, p=0.11, respectively) although the LF/HF ratio trended up after weight gain (1.57 vs 2.48, p=0.02) and returned to approximately baseline values after recovery (2.48 vs 1.65, p=0.03). None of the HRV parameters changed between baseline and follow up in the weight-maintainer group (Table 2).
Table 2.
HRV data during the fat-gain and weight-maintainance protocols
| Fat-gainers (n=20) | Weight-maintainers (n=16) | ||||
|---|---|---|---|---|---|
| Variable | Baseline | Weight gain | Recovery | Baseline | Follow up |
| HFnu during wakefulness | 0.39±0.03 | 0.31±0.03*† | 0.38±0.04 | 0.39±0.04 | 0.38±0.04 |
| LFnu during wakefulness | 0.61±0.03 | 0.69±0.03*† | 0.62±0.03 | 0.61±0.04 | 0.62±0.04 |
| LF/HF during wakefulness | 2.00±0.32 | 2.99±0.43*† | 2.39±0.53 | 2.13±0.43 | 2.21±0.46 |
| HFnu during REM sleep | 0.29±0.03 | 0.23±0.02*† | 0.29±0.02 | 0.25±0.04 | 0.24±0.04 |
| LFnu during REM sleep | 0.71±0.03 | 0.73±0.04 | 0.68±0.03 | 0.75±0.04 | 0.75±0.04 |
| LF/HF during REM sleep | 3.22±0.39 | 4.16±0.48† | 2.95±0.46 | 5.40±1.29 | 5.73±1.50 |
| HFnu during NREM sleep | 0.46±0.04 | 0.40±0.04 | 0.46±0.04 | 0.46±0.06 | 0.47±0.05 |
| LFnu during NREM sleep | 0.54±0.59 | 0.59±0.04 | 0.55±0.03 | 0.52±0.06 | 0.52±0.04 |
| LF/HF during NREM sleep | 1.57±0.23 | 2.48±0.53 | 1.65±0.30 | 2.05±0.57 | 1.57±0.27 |
Data are presented as mean±SEM.
Within group comparisons:
p<0.016 when compared to baseline.
p<0.016 when compared to recovery.
There was no significant difference at baseline between fat-gainers and weight-maintainers.
Figure 2.
Changes in HRV during wakefulness (Figure 2A), during REM sleep (Figure 2B) and during NREM sleep (Figure 2C). Data are presented as mean±SEM. HF, high frequency, LF, low frequency, nu, normalized units.
Changes of heart rate during wakefulness, during REM sleep and during NREM sleep are presented in Table 3. During wakefulness, during REM sleep and during NREM sleep, heart rate was significantly increased after weight gain (60.3 vs 64.5 beats/minute, p=0.03, 58.8 vs 62.1 beats/minute, p=0.02 and 56.9 vs 61.0 beats/minute, p<0.01, respectively) and decreased after weight loss (64.5 vs 57.6 beats/minute, p<0.01, 62.1 vs 54.5 beats/minute, p<0.01 and 61.0 vs 54.7 beats/minute, p<0.01, respectively). Heart rate in recovery decreased slightly from baseline during wakefulness and during NREM sleep (57.6 vs 60.3 beats/minute, p=0.05, 54.7 vs 56.9 beats/minute, p=0.06, respectively) and significantly decreased during NREM sleep (54.5 vs 58.8 beats/minute, p<0.01). Heart rate did not change between baseline and follow up in the weight-maintainer group (Table 3).
Table 3.
HR data during the fat-gain and weight-maintainance protocols
| Fat-gainers (n=20) | Weight-maintainers (n=16) | ||||
|---|---|---|---|---|---|
| Heart Rate, beats/minute | Baseline | Weight gain | Recovery | Baseline | Follow up |
| During wakefulness | 60.3±2.3 | 64.5±1.8*‡ | 57.6±1.7 | 60.3±3.0 | 60.2±2.8 |
| During REM sleep | 58.8±1.9‡ | 62.1±1.8*‡ | 54.5±1.9† | 58.3±3.0 | 58.3±2.7 |
| During NREM sleep | 56.9±2.1 | 61.0±1.8†‡ | 54.7±2.1 | 57.5±3.3 | 58.2±3.0 |
Data are presented as mean±SEM.
Within group comparisons:
p<0.05,
p<0.01 when compared to baseline.
p<0.01 when compared to recovery.
There was no significant difference at baseline between fat-gainers and weight-maintainers
Changes in HRV measurements after weight gain were not associated with changes in insulin, leptin or adiponectin levels during wakefulness nor during NREM sleep. During REM sleep, the only significant correlation was between LFnu and leptin level (r=0.59, p=0.02, Table 4).
Table 4.
Correlation coefficients (rho) between changes in HRV measurements and changes in metabolic markers
| Changes in Insulin | Changes in Leptin | Changes in Adiponectin | ||||
|---|---|---|---|---|---|---|
| From baseline to weight gain | rho | p | rho | p | rho | p |
| Change HFnu during wakefulness | −0.17 | 0.67 | −0.26 | 0.34 | −0.45 | 0.11 |
| Change LFnu during wakefulness | 0.17 | 0.67 | 0.26 | 0.34 | 0.45 | 0.11 |
| Change LF/HF during wakefulness | −0.25 | 0.51 | 0.10 | 0.72 | 0.30 | 0.29 |
| Change HFnu during REM sleep | −0.31 | 0.46 | −0.48 | 0.08 | −0.28 | 0.35 |
| Change LFnu during REM sleep | 0.31 | 0.46 | 0.59 | 0.02 | 0.34 | 0.25 |
| Change LF/HF during REM sleep | 0.24 | 0.57 | 0.31 | 0.29 | 0.01 | 0.99 |
| Change HFnu during NREM sleep | -* | -* | -* | -* | -* | -* |
| Change LFnu during NREM sleep | -* | -* | -* | -* | -* | -* |
| -Change LF/HF during NREM sleep | −0.04 | 0.89 | −0.04 | 0.89 | 0.03 | 0.91 |
| Changes in Insulin | Changes in Leptin | Changes in Adiponectin | ||||
|---|---|---|---|---|---|---|
| From weight gain to recovery | rho | p | rho | p | rho | p |
| Change HFnu during wakefulness | 0.27 | 0.49 | −0.44 | 0.10 | −0.59 | 0.03 |
| Change LFnu during wakefulness | −0.27 | 0.49 | 0.43 | 0.11 | 0.58 | 0.03 |
| Change LF/HF during wakefulness | −0.09 | 0.81 | 0.34 | 0.22 | 0.73 | <0.01 |
| Change HFnu during REM sleep | 0.23 | 0.56 | 0.18 | 0.53 | −0.20 | 0.51 |
| Change LFnu during REM sleep | −0.23 | 0.56 | −0.19 | 0.51 | 0.28 | 0.35 |
| Change LF/HF during REM sleep | −0.19 | 0.62 | 0.05 | 0.87 | 0.47 | 0.11 |
| Change HFnu during NREM sleep | -* | -* | -* | -* | -* | -* |
| Change LFnu during NREM sleep | -* | -* | -* | -* | -* | -* |
| Change LF/HF during NREM sleep | 0.50 | 0.20 | −0.07 | 0.80 | 0.45 | 0.10 |
Not presented as this HRV variable did not change with weight-gain or recovery
Changes in HRV measurements from weight gain to recovery were not associated with changes in insulin or leptin during wakefulness, during REM sleep or during NREM sleep. Changes in adiponectin concentration correlated with changes in HFnu (r =−0.59, p=0.03), LFnu (r=0.58, p=0.03) and LF/HF (r=0.73, p<0.01) only during wakefulness but not during REM sleep or NREM sleep (Table 4).
Discussion
The novel finding of this study is that modest short-term weight gain is associated with changes in cardiac sympathovagal balance favoring sympathetic drive not only during wakefulness but also during sleep, and this increased sympathetic activation resolves with weight loss. In the same way, modest short-term weight gain is associated with parasympathetic attenuation, during wakefulness and REM sleep which resolves with weight loss. To the best of our knowledge, this is the first report of the effect of short term weight gain followed by weight loss on cardiac autonomic control during wakefulness and sleep in healthy humans.
The increase in LFnu and decrease in HFnu suggest an increase in cardiac sympathetic activation together with a reduction in parasympathetic (vagal) activation.23, 24, 31, 32 While previous studies have suggested that weight gain and obesity are associated with increased sympathetic nerve activity,33–35 that weight loss is associated with a reduction in sympathetic nerve activity in obese subjects,36 and that fat gain influences both the sympathetic and parasympathetic nervous systems in humans,37 the cross-sectional or observational nature of these prior studies limited the ability to assess causality. The prospective, randomized, longitudinal nature of our study, on the other hand, allows us to conclude that the increase in sympathetic activity is likely due to fat gain. Moreover, our study shows that increased cardiac sympathetic activity associated with weight gain and the decreased cardiac sympathetic activity associated with weight loss are evident not only during wakefulness, but also during sleep.
There is evidence linking changes in cardiac autonomic drive to arousals from sleep and obstructive events,38 and while this seems unlikely to explain our results as we observed neither an increase in the number of arousals nor apnea-hypopnea index during the study, it is possible that more subtle changes in respiratory mechanics occurred.
As leptin increases with weight gain,39, 40 it has been speculated that the effect of increased body fat on sympathetic drive is mediated by this adipokine.41 We confirmed that leptin increased after short-term experimental weight gain, and found that changes in leptin correlated with changes in LF, but not LF/HF, during REM sleep. Similarly, it has been reported that hyper-insulinemia increases sympathetic activity.42 However, our data do not show a relationship between changes in circulating insulin and changes in HRV. Adiponectin is a protein secreted from adipose tissue that activates the AMP-activated protein kinase in the peripheral tissues. Adiponectin increases insulin sensitivity and decreases insulin concentration,43 and therefore may indirectly influence sympathetic activity. We noted a correlation between changes in adiponectin concentration and changes in HRV between weight gain and weight loss only during wakefulness.
The major strengths of our study include its longitudinal experimental design and inclusion of normal healthy subjects without medical conditions or medications that might have confounded our results. Furthermore, our rigorous laboratory-based polysomnography and HRV analyses strengthen our conclusions. Changes in physical conditioning are also unlikely to explain our findings since exercise tolerance was unchanged at the different stages of the protocol. However some limitations should be considered. The magnitude, rate, and duration of fat gain in this study likely do not reflect the long-term severe and chronic weight gain and so our results may not be readily extrapolated to obese people in the general population. Moreover, we were not able to determine whether the observed changes were related to changes in the diet or to weight gain. Finally, we examined just three potential mechanisms that may link weight gain with cardiac sympathetic activity, namely, circulating concentrations of insulin, leptin and adiponectin. It is unclear if the lack of clear association between changes in these hormones and cardiac sympathetic activity was due to our sample size, the short-term nature of our study of a chronic process, or reflective of a more complex relationship between insulin, insulin resistance,44 leptin, leptin resistance,45 adiposity,46 and neurohormonal changes that occur with fat gain.
The increase in sympathetic balance during sleep associated with weight gain may have important clinical implications. The conversion from sleep to wakefulness is associated with an increased risk of sudden cardiac death,20 stroke,21 and myocardial infarction.22 Hence, fluctuation of autonomic nervous activity during both wakefulness and sleep are likely of clinical importance. During REM sleep there is normally intense vascular sympathetic activation associated with wide fluctuations in cardiac autonomic drive.18 These changes are associated with a reduction in coronary artery blood flow in the setting of coronary stenosis19 and with variant angina in some patients.47 Increased cardiac sympathetic balance during sleep may exacerbate this phenomenon and perhaps contribute to increased cardiovascular morbidity and mortality associated with obesity. The reduction of cardiac sympathetic drive after weight loss suggests that the changes associated with short-term weight gain are reversible. Whether reduction of body fat after years of chronic severe obesity results in a similar effect is unknown and would have significant implications for global public health.
Perspectives
Our findings suggest that modest, short-term weight gain is associated with increased cardiac sympathetic activity not only during wakefulness but also during sleep, which is reversible by weight loss in healthy individuals. These data may be relevant to our understanding of mechanisms underlying the association between weight gain and cardiovascular morbidity and mortality.
Acknowledgements
We greatly thank Mrs. Debra L. Pfeifer and Mrs. Ann B. Peterson for administrative assistance and Mr. Toru Suzuki and Mr. Wataru Hayashi of GMS, Co. for guidance with HRV measurements.
Sources of Funding Taro Adachi was supported by the Japanese Heart Foundation and the Japanese Society of Electrocardiology. Fatima H. Sert-Kuniyoshi was supported by AHA grant 09-20069G. Andrew D. Calvin is supported by the Mayo Clinic Clinician-Investigator Training Program. Dr. Somers is supported by NIH Grants HL73211, HL65176, R21 HL96071-01 and 1 UL1 RR024150.
Footnotes
Disclosures Dr. Somers has served as a Consultant for ResMed, Cardiac Concepts, Apnex Medical, and Sova Pharmaceuticals and has been a principal investigator or co-investigator on research grants funded by the Respironics Foundation and the Sorin Corporation. FHSK became an employee of Philips Respironics, Inc. after the collection of the data presented in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 2.Molenaar EA, Hwang SJ, Vasan RS, Grobbee DE, Meigs JB, D'Agostino RB, Sr., Levy D, Fox CS. Burden and rates of treatment and control of cardiovascular disease risk factors in obesity: the Framingham Heart Study. Diabetes Care. 2008;31:1367–1372. doi: 10.2337/dc07-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert YE, Linas S. The role of obesity in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2009;5:101–111. doi: 10.1038/ncpneph1022. [DOI] [PubMed] [Google Scholar]
- 6.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, Thomas RJ, Singh P, Hoffmann M, Okcay A, Korinek J, Wolk R, Somers VK. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5:418–425. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Milani RV, Ventura HO. Untangling the heavy cardiovascular burden of obesity. Nat Clin Pract Cardiovasc Med. 2008;5:428–429. doi: 10.1038/ncpcardio1257. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin JS, Juhan-Vague I, Hawe E, Humphries SE, di Minno G, Margaglione M, Tremoli E, Kooistra T, Morange PE, Lundman P, Mohamed-Ali V, Hamsten A. Low-grade inflammation may play a role in the etiology of the metabolic syndrome in patients with coronary heart disease: the HIFMECH study. Metabolism. 2004;53:852–857. doi: 10.1016/j.metabol.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 10.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–635. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 11.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 12.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 14.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 15.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 16.Maser RE, Lenhard MJ. An overview of the effect of weight loss on cardiovascular autonomic function. Curr Diabetes Rev. 2007;3:204–211. doi: 10.2174/157339907781368931. [DOI] [PubMed] [Google Scholar]
- 17.Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13:215–223. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 19.Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function during stenosis. Physiol Behav. 1989;45:1017–1020. doi: 10.1016/0031-9384(89)90231-x. [DOI] [PubMed] [Google Scholar]
- 20.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 21.Wroe SJ, Sandercock P, Bamford J, Dennis M, Slattery J, Warlow C. Diurnal variation in incidence of stroke: Oxfordshire community stroke project. BMJ. 1992;304:155–157. doi: 10.1136/bmj.304.6820.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 23.Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, Iellamo F. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33:71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Montano N, Cogliati C, Dias da Silva VJ, Gnecchi-Ruscone T, Malliani A. Sympathetic rhythms and cardiovascular oscillations. Auton Neurosci. 2001;90:29–34. doi: 10.1016/S1566-0702(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 25.Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, Singh P, Pusalavidyasagar S, Huyber C, Votruba S, Lopez-Jimenez F, Jensen MD, Somers VK. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 56:662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JA, Benedict FG. A biometric study of basal metabolism in man. 1919;(No. 279) doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iber CA-I, S, Chesson A, Quan SF, The American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. 2007. [Google Scholar]
- 29.Sawada Y, Ohtomo N, Tanaka Y, Tanaka G, Yamakoshi K, Terachi S, Shimamoto K, Nakagawa M, Satoh S, Kuroda S, Iimura O. New technique for time series analysis combining the maximum entropy method and non-linear least squares method: its value in heart rate variability analysis. Med Biol Eng Comput. 1997;35:318–322. doi: 10.1007/BF02534083. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 32.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 33.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 34.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 35.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- 37.Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988;318:1077–1083. doi: 10.1056/NEJM198804283181701. [DOI] [PubMed] [Google Scholar]
- 38.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 40.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 41.Paolisso G, Manzella D, Montano N, Gambardella A, Varricchio M. Plasma leptin concentrations and cardiac autonomic nervous system in healthy subjects with different body weights. J Clin Endocrinol Metab. 2000;85:1810–1814. doi: 10.1210/jcem.85.5.6511. [DOI] [PubMed] [Google Scholar]
- 42.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 44.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 45.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39(2 Pt 2):486–490. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 46.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 47.King MJ, Zir LM, Kaltman AJ, Fox AC. Variant angina associated with angiographically demonstrated coronary artery spasm and REM sleep. Am J Med Sci. 1973;265:419–422. doi: 10.1097/00000441-197305000-00009. [DOI] [PubMed] [Google Scholar]