Endothelial cells, which line the blood vessels throughout the body, might be considered to be THE proximal organ system for any circulating toxicant. The endothelium is crucially dependent on the proper function of the nitric oxide synthase (NOS) pathway. Nitric oxide (NO) signals numerous cellular activities in an autocrine and paracrine manner, though the most commonly studied role for endothelial-derived NO involves relaxation of vascular smooth muscle and vasodilation. Indeed, Viagra would not be so popular if it were not for impaired function of endothelial NOS (eNOS). In the present issue of Toxicological Sciences, Nurkiewicz and colleagues communicate seminal findings that inhaled particles reduce the vascular bioavailability of NO, providing a crucial clue to understanding the link between inhaled toxicants and cardiovascular disease.
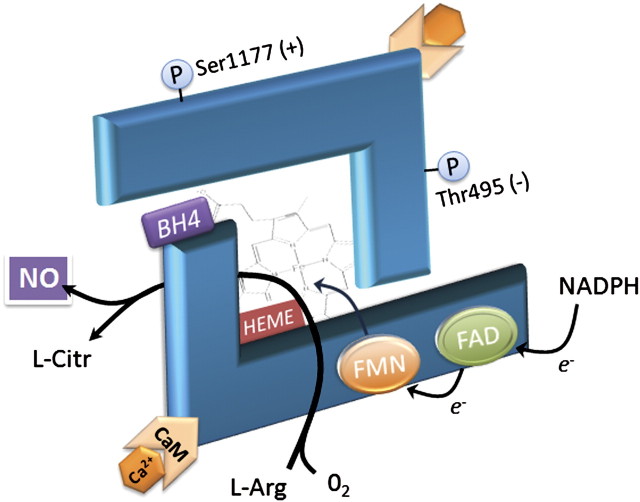
Despite its essential role in vascular physiology, eNOS appears to be a fragile enzyme system and requires several cofactors for proper activity (Fig. 1). eNOS functions as a dimer and when electrochemically uncoupled acts as a generator of superoxide. Among the necessary cofactors, tetrahydrobiopterin (BH4) has the ignoble distinction of being one of the more effective scavengers of peroxynitrite and superoxide, and its oxidized form is of little use to NOS (Kuzkaya et al., 2003). Arginine is the necessary substrate by which eNOS generates NO (and citrulline), but Arginase is commonly upregulated in systemic vascular diseases and reduces arginine availability (Bivalacqua et al., 2007). Furthermore, complex interactions with kinases may activate or deactivate NOS. Akt can activate NOS by phosphorylating the Ser1177 site, while PKC reduces function by enhancing the interaction with caveolin-1, which holds the NOS inactive (Chen et al., 2008). When electrochemically uncoupled, NOS becomes a generator of superoxide, which can scavenge more BH4 or NO to conceivably establish a chain reaction that renders the entire NOS system dysfunctional.
FIG. 1.
Endothelial NOS functions as an electrochemically coupled dimer, requiring electron donation from nicotinamide adenine dinucleotide phosphate that is transferred to a heme via flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) cofactors. BH4 is essential for NOS function, although its role is uncertain. Evidence suggests that BH4 is required for dimerization and may also increase substrate (L-arginine) affinity with the heme iron (Moens and Kass, 2006). Phosphorylation by cellular kinases can also increase (e.g., Serine 1177) or decrease (e.g., Threonine 495) NOS activity (Chen et al., 2008).
It is not surprising that eNOS is implicated in many vascular diseases and an important therapeutic route. Nitroglycerin, a NO donor, affects a dilation of coronary vessels in vasospastic or stable forms of angina. Inhaled NO is an important treatment for pulmonary hypertension in neonates. A myriad of vascular disorders, including hypertension and atherosclerosis, are associated with significant perturbations of the NOS pathway. We have long considered the potential role of NOS impairment since Robert Brook and colleagues established that vasoconstriction was enhanced in humans exposed to inhaled pollutants (Brook et al., 2002). Several subsequent studies have indirectly tied the pollutant-induced vascular dysfunction to NOS (Knuckles et al., 2008; Mills et al., 2005; Nurkiewicz et al., 2006), but the nail in the coffin has been elusive.
NOS function is extremely difficult to assess directly in its native environment. The most common activity assay measures the generation of radiolabeled citrulline. Unfortunately, this assay requires the addition of exogenous cofactors, such BH4. As intracellular cofactor depletion may be the principal reason for toxicant induced endothelial dysfunction, the citrulline assay may be confounded by this artificial supplementation. Measures of nitrate/nitrite levels in plasma are commonly reported as an index of systemic vascular NOS function, but this dramatically oversimplifies the physiology by ignoring the local control of vascular tone and the origins of NO from other cells. The use of spin-trap resonance techniques are highly specific but also highly challenging when examining specific tissues from in vivo studies and, like the citrulline assay, may be suspect due to the level of artifice. Other indirect methods, such as measuring BH4 (or expression of BH4 synthesizing enzymes) or phosphorylation of eNOS, are also commonly employed to implicate NOS impairment.
Thus, the study by Nurkiewicz et al in the present issue of Toxicological Sciences is especially significant, as they report on the impact of titanium dioxide nanoparticles on NO bioavailability using direct NO measurements via an electrode system in an intact rat artery model. In a logical progression, the authors demonstrate that inhalation of nanoparticles lead to a reduction in endothelium-dependent vasodilation, which, by mass deposition, is far more potent than the effect of larger particulate matter (PM). The direct application of a nitric oxide donor, sodium nitroprusside, elicited dilation regardless of exposure, implying that synthesis or delivery of endogenous endothelium-derived NO is impaired.
Nitric oxide reacts rapidly with superoxide to form peroxynitrite, a highly reactive intermediate that commonly reacts with protein tyrosine moieties to form nitrotyrosine. Nitrotyrosine has been shown to be upregulated in vascular tissue following inhalation of gasoline engine emissions (Lund et al., 2007). In the present study, nanoparticles stimulate vascular oxidative stress and a significant enhancement of nitrotyrosine in both pulmonary and systemic vessels. These findings may suggest that the generation of NO from NOS was not impaired, but that the NO was rapidly scavenged by intracellular superoxide.
The bioavailability of NO was directly measured following exposure to both fine PM and nanoparticles. Perhaps not surprisingly, both exposures led to a dose-dependent reduction in measureable NO, with nanoparticles inducing a reduction that appeared twice as potent on a mass deposition basis. This entirely novel observation represents a clear explanation for the reduction in vasodilation observed from the PM exposures: NO is simply not there. Restoration of the bioavailability of NO by decreasing intracellular oxidative stress also restored vasodilation.
However, as with any biological system, the answer to the big picture regarding PM-induced cardiovascular effects remains less straightforward. For one, when the authors used an analogue of arginine to inhibit NOS, ostensibly to verify that the NO is derived from this enzyme, NO was still generated, albeit at lower levels. Moreover, at the highest levels of nanoparticle exposure, the residual NO is potentially arising from another source. Additionally, the authors did not ascertain induction of other NOS isoforms, nor has the direct impact of exposure on NOS activity or cofactor availability been assessed.
Another question that must be addressed is what happens to the scavenged NO? Assays of nitrotyrosine levels provide a clear and plausible explanation within the scope of this research model. However, NO is an interesting monoxide that, unlike its carbonaceous relative, can react and transform into nitrates, nitrites, and nitrosothiols. This latter form may have a more benign outcome. Glutathione-S-nitrosothiol formation may be a means of cellular protection from dysregulated NO production, and could potentially explain protection by GST polymorphisms or dietary supplements, such as N-acetyl cysteine. Beyond simple mechanisms of toxicity, it will be crucial in coming years to understand those circumstances which confer protection or susceptibility in human subpopulations to adequately address risk.
As pulmonary inflammation has been a component of the models used by the Nurkiewicz laboratory, there may be a broader implication for the findings of this study. Endothelial dysfunction is observed in chronic obstructive pulmonary disease (COPD) patients and is associated with a worse clinical outcome. COPD patients are at greater risk from cardiovascular death than individuals without lung disease. As the authors note, direct interaction of inhaled PM and endothelial cells is likely an academic issue, but the activation of the innate immune system via lung insults may lead to systemic inflammatory processes. Previous work from this laboratory has clearly demonstrated a role for neutrophil-derived myeloperoxidase deposition in the systemic vasculature (Nurkiewicz et al., 2006). The mechanisms underlying this link are poorly understood. Do neutrophils become “primed” in the lung and therefore have tighter interactions with endothelial surface receptors, or are cytokines released, such as transforming growth factor-β, as has been postulated in related nanoparticle research? Clearly, we have more hypotheses than actual studies, and much remains to be learned regarding interactions between inhalation exposures and vascular health.
References
- Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: Influence of in vivo gene therapy of anti-arginase. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1340–1351. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–6. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J. Biol. Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction through uncoupling of eNOS. Toxicol. Appl. Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- Lund AK, Knuckles TL, Obot Akata C, Shohet R, McDonald JD, Gigliotti A, Seagrave J, Campen MJ. Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis. Toxicol. Sci. 2007;95:485–494. doi: 10.1093/toxsci/kfl145. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci. 2009 doi: 10.1093/toxsci/kfp051. Advance Access published on March 6, 2009; doi: 10.1093/toxsci/kfp051. [DOI] [PMC free article] [PubMed] [Google Scholar]