Abstract
The role of nonchemical stressors in modulating the human health risk associated with chemical exposures is an area of increasing attention. On 9 March 2011, a workshop titled “Approaches for Incorporating Nonchemical Stressors into Cumulative Risk Assessment” took place during the 50th Anniversary Annual Society of Toxicology Meeting in Washington D.C. Objectives of the workshop included describing the current state of the science from various perspectives (i.e., regulatory, exposure, modeling, and risk assessment) and presenting expert opinions on currently available methods for incorporating nonchemical stressors into cumulative risk assessments. Herein, distinct frameworks for characterizing exposure to, joint effects of, and risk associated with chemical and nonchemical stressors are discussed.
Keywords: joint action, exposure, dose response, modeling
The risk assessment paradigm has begun to shift from assessing single chemicals using “reasonable worst case” assumptions for individuals to considering multiple chemicals and community-based models. This shift is motivated by a desire to better estimate actual public health risk from environmental exposures as well as legislative considerations, such as Executive Order 12898, Federal Actions to Address Environmental Justice In Minority and Low-Income Populations. Inherent in community-based risk assessment is examination of all stressors affecting a defined population (community), including both the complex milieu of chemicals to which the community is exposed and the specific vulnerabilities of that community. Nonchemical stressors make up an important subset of community vulnerabilities that have the potential to either directly affect the health of individuals or modulate their response to chemical exposures. Strides have been made in developing methods for conducting cumulative risk assessment for multiple chemicals, but integrating nonchemical stressors represents a new challenge. The overarching goal of developing risk assessment approaches that incorporate both chemical and nonchemical stressors is to better inform public health decisions. However, it is important to recognize that individual agencies are mandated to address certain issues and cannot address others (e.g., Environmental Protection Agency [EPA] can require cleanup of a Superfund site but cannot increase access to medical care).
Defining “nonchemical stressor” in the context of risk assessment is difficult due to the diversity of mental and physical stimuli that fall under this umbrella term. Further, distinguishing nonchemical from chemical stressors can be problematic as physiological responses to nonchemical stressors and chemical stressors may be mediated by molecular signaling along the same biochemical pathways. Nonchemical stressors are defined here as physical or psychosocial challenges and cumulative risk assessment as including both chemical and nonchemical stressors. Examples of physical stressors include noise, temperature, visual light, and atmospheric pressure (Gordon, 2003). Psychosocial stressors can arise from deficiencies in the quality of one’s environment (e.g., housing or sanitation) or resources (e.g., schools or access to medical care) and negative social factors (e.g., crime) (deFur et al., 2007). Nonchemical factors are known to impact health, although often with rough descriptions. Heat impairs judgment (Chai, 1981), stress and sleep deprivation affect immune function (McEwen, 2006; McEwen et al., 1997), vibration alters cardiovascular function (Yue and Mester, 2007), and evidence suggests that adverse childhood experiences such as maltreatment are associated with changes in the immune, endocrine, and nervous systems (Danese and McEwen, 2011). In addition, some evidence of interactions exists, including noise and solvents jointly affecting hearing (Morata, 2002), chemical exposure affecting susceptibility to disease (Eder et al., 2007), social stress altering the respiratory response to fine concentrated ambient particles (Clougherty et al., 2010), and anxiety and stress enhancing chemical toxicity (Gee and Payne-Sturges, 2004).
On 9 March 2011, a workshop titled “Approaches for Incorporating Nonchemical Stressors into Cumulative Risk Assessment” took place during the 50th Anniversary Annual Society of Toxicology Meeting in Washington D.C. Objectives of the workshop included describing the current state of the science from various perspectives (i.e., regulatory, exposure, modeling, and risk assessment) and presenting expert opinions on currently available methods for incorporating nonchemical stressors into cumulative risk assessments. Proposed frameworks are meant to build on currently available cumulative risk assessment tools or provide additional tools to risk assessors and not to imply that current practice is inherently flawed.
Creating the knowledge base (the necessary data and databases) to quantify the joint impact of exposure to chemical and nonchemical stressors is complicated by a lack of both consistent exposure metrics and quantitative exposure-response relationships for adverse health outcomes. Further, methods are not available for assessing the additional risk (if any) that may be imposed on segments of the population due to the cumulative impacts of chemical and nonchemical stressors. Herein, distinct frameworks for characterizing exposure to, joint effects of, and risk associated with chemical and nonchemical stressors from the workshop are presented and discussed.
CHARACTERIZING CUMULATIVE EXPOSURES TO CHEMICAL AND NONCHEMICAL STRESSORS
Monitoring and Modeling Cumulative Exposures
Methodologies for assessing cumulative exposures to multiple stressors from multiple sources fall into two broad classes, monitoring and modeling. Monitoring approaches seek to collect data on the status of stressors in individuals at a specific point in time. The techniques include collection of data on the levels of chemicals in food, air, water, and on the surfaces in residences and the collection of demographic and behavioral information (activity patterns and employment, etc.). Exposure to nonchemical stressors includes collection of data on parameters such as weather conditions in the community, income, education, status of home life, emotional health, use of recreational drugs, and misuse of alcohol. Recent advances in technology have greatly improved personal monitoring, allowing for collection of longitudinal data (information on how stressors and stress levels encountered change over time) and how the individual physically moves across the community (Borrell, 2011).
The second class of methodologies is simulation modeling. The goal of such models is to capture the behavior of factors identified as critical for predicting health outcomes in simulations of the individuals in a community (Georgopoulos et al., 2008; Price and Chaisson, 2005; Price et al., 2001). Models and monitoring provide complementary means for characterizing exposure, which is a critical step in risk assessment. For example, simulation models can be used to extrapolate from snapshot monitoring data to a more realistic picture of exposure over time. Furthermore, models can guide monitoring efforts by identifying the aspects of a community that are most important to measure or portions of the community most at risk.
Modeling Framework for Cumulative Exposure
Simulation models provide an opportunity for integrating nonchemical stressors into characterizations of cumulative exposures to chemicals. For example, the person-oriented modeling (POM) approach (Price and Chaisson, 2005) offers a useful framework for assessing nonchemical and chemical stressors. The POM is an approach for designing computer simulation software that centers on building internally consistent models of a person and attempts to capture patterns of exposure and areas of uncertainty for the person. This process is repeated for multiple individuals to produce descriptions of exposed populations.
The goal of simulation models is to define the processes that govern the values of the health-related parameters for an individual over time (intraindividual variation) and define the variation in values across individuals in a community (interindividual variation). Under such models, a person at a given point in time is defined as a vector of exposure-related “parameters.” The parameter values specify the individual’s personal characteristics (including capacity to tolerate stressors), the individual’s specific environment at that point in time, and the status of the chemical and nonchemical stressors affecting the individual. These data are used in biologically based response models to predict the occurrence of adverse effects in the individual over time. Values of certain parameters change over time resulting in different vectors over time. This results in a two-dimensional table of the parameter values at specific points in time (once a minute, once an hour, and once a day, etc.). The status of a population in a community over time can be viewed as a series of these tables that form a three-dimensional table.
The status of the community over time can be defined in a similar manner. Spatial and temporal variation of conditions in the physical and social status of the community can be modeled using the same multidimensional framework. This approach was used in the LifeLine (2006) aggregate pesticide exposure software.
Defining the Population to Model
Populations can be defined in three ways: in terms of the specific sources of exposure included in the assessment (a source-centric approach), the availability of data (individuals in the NHEXAS or CSFII surveys), or the use of independent demographics (live in a specific metropolitan area). Problems encountered using a source-centric approach include difficulties in reconciling differences between the population defined by a specific source and the total population in the community and difficulties in the modeling of communities with multiple sources of stressors. Defining populations in terms of the availability of data greatly limits the communities that can be investigated. The alternative approach, advocated here, is to define the population in terms of specific demographics where persons in the population are included regardless of actual exposure and the model determines if they are exposed or not exposed (predicted dose of “zero” or “no impact” for a nonchemical stressor). The result is a prediction of the distribution of doses in a population that includes some fraction of the population having zero doses (Price and Chaisson, 2005).
Once a population has been defined, distributions for exposure-related parameters are identified and developed in order to estimate: the probability of exposure to a stressor; the intensity of exposure; and the individual’s capacity to tolerate other stressors based on their general health status. Parameters that predict the potential for exposure may include age, gender, income, housing, and geographical location. Parameters that affect the intensity of the exposure and the resulting dose would include for example weight, breathing rate, and behaviors such as the frequency and duration of hand-to-mouth events. Parameters that influence the health risks would include general health, age, gravidity, gender, etc.
Demographic-based approaches also can reduce the confusion in cumulative risk terminology. The concepts of chemicals in the environment, exposure to chemicals, and chemical doses are well defined. However, cumulative assessments are often less clear for nonchemical stressors. According to some definitions, a stressor is necessarily an external factor, whereas a vulnerability is a characteristic of an individual that reflects sensitivity to a stressor. Existing cumulative assessments, however, have been troubled by a lack of consistency in terminology. Depending on the study, a parameter (such as drug use) may be considered to be an external stressor, a vulnerability, or simply a personal characteristic. Under the proposed framework, there is no attempt to define how a parameter is viewed. Instead, a person at a given point in time is defined as a vector of exposure-related “parameters.”
Modeling Correlation and Autocorrelation
A major concern in simulation modeling of the exposure parameters is the correlation among values for different factors across individuals in a population and across individuals over time. Failure to capture correlations in the model can affect the estimates of the distributions of dose and risk. There are two approaches to defining correlations. The first, the record-based approach, relies on data taken from a single person; thus, correlations between the values of a person’s exposure factors are captured empirically. This approach is limited to instances where monitoring data are available.
The alternative approach is to construct the person’s exposure-related characteristics based on multiple sources of data. This approach requires methods that avoid assigning inappropriate values to a person. In the POM framework, the approach is to constrain the range of likely values for a person using a hierarchical system for assigning values to each of the person’s characteristics (Price and Chaisson, 2005). The goal of this hierarchy is to construct models that initially set values for those characteristics that can be assigned with confidence and use those values to constrain the range of possible values for the remaining characteristics. The approach begins by classifying the person’s exposure parameters into different categories depending on how they vary with time. The categories are fixed and variable. Variable characteristics are further subdivided into four groups: long-term trends, episodic, cyclic, and ephemeral (see Table 1).
TABLE 1.
Categories, Descriptions, and Examples of Categories of Exposure Parameters
| Categories | Subcategories | Description | Examples |
| Fixed | Constant over lifetime | Gender, race, ethnicity, birth date, and body type | |
| Variable | Long-term trend | Vary in predictable pattern | Physiological characteristics (e.g., height) and some exposure sources |
| Episodic | Nonperiodic state changes | Associated with major life changes (e.g., residence, occupation, exposure in institutional setting) | |
| Cyclic | Vary with periodicity (e.g., seasonal or related to the day of the week) | Activity patterns, diet, and product use | |
| Ephemeral | Vary from day to day or moment to moment without periodicity | Random activity (one-time use of pesticide) or constrained random activity i.e., partially random and partially cyclical or episodic |
Rules for Assigning Values to Characteristics
Once inputs are categorized, the following rules are used to evaluate the temporal changes in input values in a simulation of a person's life (Lifeline, 2006; Price et al. 2001). The goal of the rules is to assure that correlation and autocorrelation in values will be captured in the model.
A person’s fixed characteristics are always assigned first. This allows the fixed characteristics to be used in the consistent selection of subsequent variables.
A person’s time-varying inputs are assigned for each time step of the person’s life. Assignment of the values on any time step is contingent on the values assigned to prior time steps.
Each day of a person’s life is defined in terms of season and whether it is a weekend or weekday. This allows the model to take advantage of the relative consistency between work (or school) days and weekends. In addition, many sources of chemical exposures are seasonal (exposure related to heating systems, outdoor products, etc.).
Temporal changes in episodic variables are modeled by a series of binomial decisions (the variable either changes or remains the same). The decision is made on a daily basis (or at some other appropriate frequency). The probability of change and the selection of new values can be determined from studies of populations that are consistent with the person’s age and other assigned characteristics. Once a change has been made (change in residence, etc.), all affected variables are modified.
Selection of transitory inputs is based on a random or constrained random model. These models may take several forms. One method is to randomly sample from records that are constrained to be consistent with relevant inputs such as the day of the week, season, age of the person, gender, residence type, and region. This approach has been used for selecting activity patterns and dietary records. A second method is to use a binomial model where the probability of an input changing is contingent on relevant inputs such as season, region, prior use, and residence.
The temporal patterns of change for characteristics are determined independently. Changes in values are never automatically linked, unless there is a sound reason for predicting a correlation. For example, moving to a new home does not change a person’s height but does change room sizes.
In summary, the above categories and rules provide a useful starting point for the design of simulation models that address the needs of cumulative risk assessment for chemical and nonchemical stressors. Given an almost infinite number of exposure scenarios, simulation modeling will be vital to our ability to not only model available exposure data but to provide reasonable estimation/simulation of exposure for the large number of situations where adequate data do not exist.
A PROPOSED FRAMEWORK FOR QUANTIFICATION OF THE JOINT IMPACTS OF CHEMICAL AND NONCHEMICAL STRESSORS FOR RISK ASSESSMENT
As mentioned previously, a lack of both consistent exposure metrics and quantitative exposure-response relationships complicates quantification of the joint impact of chemical and nonchemical stressor exposures. However, the U.S. EPA (2007) has eased the process somewhat with different tiers of guidance for risk assessment: general frameworks, scenario-specific procedural guidelines, and legally enforceable policy. For quantitative cumulative risk assessment, the natural starting place is a framework.
Quantitative risk assessment involves data, models, and decision trees that complement each other and result in a number that can aid risk management decisions. With cumulative risk assessment, quantifying risk is usually impeded by the lack of data on the specific exposure combinations and the similar lack of verified models that might fill in the data gaps. The framework suggested here for the combined assessment of chemical and nonchemical stressors is loosely derived from two successful approaches: the conceptual models used in the U.S. EPA (2007) metals framework and the binary weight of evidence (WOE) scheme used in the interaction profiles of the Agency for Toxic Substances Disease Registry (ATSDR) (2004). In the quantitative framework for cumulative risk of chemical and nonchemical stressors presented here, the emphasis is on predictive quality of models and evidence of interaction and on relevance to human health risk.
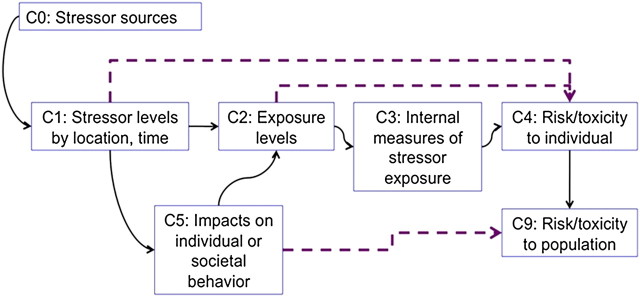
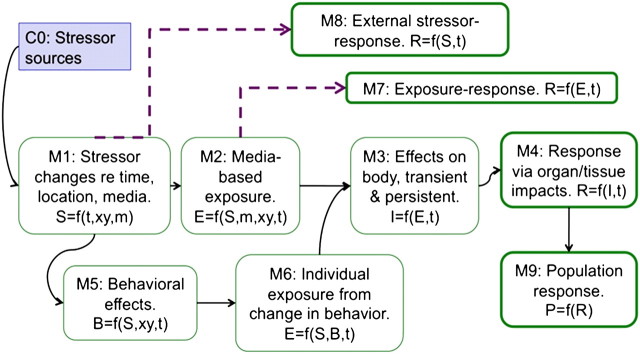
The U.S. EPA framework for risk assessment of metals has two useful conceptual models that we have adapted here for cumulative risk assessment: one of compartments (for data and for derived quantities) and one for the models that connect the quantities. Because metals, like nonchemical stressors, are ubiquitous with several known toxicological interactions, the framework is a good example of how to conduct a complex risk assessment. The counterpart charts (Figs. 1 and 2) for cumulative risk are shown for chemical and psychological stressors and include time-varying exposure models (e.g., fate and transport) as well as behavioral influences (personal and societal response). Here, psychological stress is treated as an intermediate internal effect of some external stressor, such as living in a high-crime area or suffering trauma of a recent hurricane strike. In this example (Fig. 2), the population response is calculated for a specified time period, e.g., lifetime or one year, so time is no longer a model variable. Note that the models can be more complex if information is available. For example, socioeconomic statue (e.g., income) can be included in the model M4 to reflect the influence of that factor on the internal psychological stress effect.
FIG. 1.
Example compartments for cumulative risk assessment showing key measured or estimated quantities. Dashed line connections represent models that bypass some steps, e.g., estimating individual risk directly from ambient air concentrations.
FIG. 2.
Example models for cumulative risk assessment to connect the compartments in Figure 1. Dashed lines show models that bypass key quantities and so must include critical assumptions. S = stressor; B = behavioral change; E = exposure; I = internal chemical concentration or intermediate physiological change; R = individual response; P = population response; t = time; xy = geographic location; m = medium.
There are two advantages in using these conceptual models. First, they show clearly what parts are deemed most important to the risk assessor, at least those parts that have enough information to allow inclusion. They also show how uncertainties in one quantity or model can be propagated, affecting the quality of all “downstream” estimates. Having the final estimate (individual or population risk) at the end of the conceptual model visually shows how that risk estimate reflects the combination of uncertainties in all preceding compartments and models.
Once the key information values and formulas are identified, they must be evaluated for overall quality as well as relevance to the scenario being assessed. The WOE approach of both ATSDR and the U.S. EPA, which was designed to denote quality and relevance of information on two-chemical interactions with regard to human risk from mixtures of chemicals, can be readily adapted for consideration of the impact of a nonchemical stressor on a health outcome. Each WOE category and its associated numerical score reflect a judgment of relevance, usually the extent of extrapolation required when using the available interaction information to modify the risk assessment. In the cumulative risk example shown in Table 2, a nonchemical stressor (hurricane strike) has been shown to contribute to psychological stress in pregnant women with resultant increase in incidence of fetal distress (e.g., measurable decrease in oxygen reaching the fetus) (Zahran et al., 2010), a connection strengthened by the link of stress to increased fetal cortisol, which is linked to fetal distress. In the same study, race is also significant, combining with hurricane occurrence to produce even higher incidence of fetal distress. The evidence of the impact from this combination of nonchemical physical stressor (hurricane occurrence) with the variable of race is then categorized regarding the models being used (Table 2). With this type of WOE notation for causality, one can ask whether Hurricane Katrina impacted non-whites more than whites regarding incidence of fetal distress. Table 2 suggests the connections are fairly strong so that the available quantitative indicators (e.g., change in odds ratios) can be used with confidence to estimate fetal distress risk in that exposed subpopulation. This example highlights the need both for causal models with combination exposures and for human data, the latter to reflect direct toxicity and behavioral/societal responses.
TABLE 2.
Example WOE Categories for Models Used to Assess Risk of Fetal Distress After Hurricane Katrina Landfall
| Modela | WOE categoryb |
| M1: Hurricane strength by location, time | A2 |
| M3: Hurricane → psychological stress | A1 |
| M4a: Psychological stress → fetal distress | B1 |
| M4b: Race as factor → fetal distress | B2 |
Denotes “causes,” e.g., M3 assumes hurricane landfall causes psychological stress.
A = direct evidence for scenario being assessed (Katrina); B = evidence from similar scenario (e.g., results for Andrew, not Katrina); 1 = strong model (accuracy and reliability); 2 = moderate model (important variation, unsure causality, and other unknowns).
CASE STUDY: A COMPARATIVE DIETARY RISK FRAMEWORK
Frameworks for incorporating nonchemical stressors into proposed exposure and risk assessment models discussed above represent tools that can inform the data-gathering process and provide a view to the future possibilities in cumulative risk assessments. In this section, a risk-benefit analysis of fish consumption is presented to illustrate a practical adaptation of a risk assessment approach to include nonchemical parameters. Analyses of fish throughout the United States confirm the presence of chemical contaminants. In many cases, the concentrations of these contaminants have been high enough to warrant the posting of fish consumption advisories. Typically, these advisories are based on the potential adverse effects posed by the contaminants in fish. Yet, medical practice and recent publications suggest that the health benefits of eating (even contaminated) fish may outweigh the potential risks caused by the contaminants. Furthermore, cultural and personal perceptions affect the choice of fish in the diet. A framework for comparing the risks from chemical contaminants in fish, the health benefits from fish consumption, and the cultural and personal perceptions in fish consumption choices is proposed. Data limitations and assumptions used to develop the framework are highlighted.
This research developed a mechanism to evaluate the comparative risks posed by dietary changes and the effects of fish consumption advisories on diet and public health. An approach for considering severity of risks and magnitude of benefits while addressing chemical mixtures as well as cultural and social considerations is presented. The results of this research have been previously published (Dourson, 2002), and all results are available at: http://www.tera.org/Publications/Publications.html#tera_reports.
The target risks were considered to be the noncancer and cancer effects from eating contaminated fish, which were quantified by either standard modeling for cancer end points or bootstrap methods for noncancer end points. The countervailing risks associated with ceasing fish consumption were considered to be: social and cultural impacts, religious or ceremonial importance, traditions disrupted, quality of life impacts, and lack of health benefits from eating fish, the latter of which were obtained from published epidemiology data. These elements were combined in a simple series of algorithms to compare the potential benefits and potential risks of eating contaminated fish through the use of a multiplier for both severity of health risk and medical impact of the health benefit, referred to as the comparative dietary risk framework (CDRF). Although a quantitative representation of the net risk or benefit is made, these results should not be interpreted as an absolute measure of risk or benefit. Rather, the algorithms resulted in the development of a fish consumption index (FCI), which has the units of risk, relative to both the risk and benefit and can only be seen as a crude measure of relative risk as per the following equation:
where Benefiti is the benefit for health end point “i” associated with eating a given amount of fish, as determined from published studies. Benefiti is calculated using the following equation:
where Bi is the background incidence of health end point “i,” RRi is the epidemiological relative risk “decrease” of “i” at the given consumption rate, and Si is the biological “severity,” or more appropriately, positive medical impact of “i.” Riski is the increase in risk of “i” associated with eating a given amount of fish as determined from standard toxicological bioassays and appropriate modeling and is calculated according to
where, Ri is the “increased” risk of health end point “i” associated with a particular fish consumption rate and Si is the biological “severity” of the toxicological end point being modeled, as shown in Table 3.
TABLE 3.
Severity Ranking of Effects and Benefits and Multipliers to the Frameworka
| Multiplier to the incidence of effect/benefit | |
| EPA severity ranking of effects | |
| NOEL (no effect) | 0 |
| NOAEL (nonadverse effect) | 1 |
| (LO)AEL (adverse effect) | 2 |
| FEL (frank toxicity) | 3 |
| “Severity” ranking of benefits | |
| None | 0 |
| Minimal (decreased arthritis) | 1 |
| Moderate (lower blood pressure) | 2 |
| Maximum (decreased CHD) | 3 |
Note. CHD, coronary heart disease; FEL, frank effect level; LOAEL, lowest observed adverse effect level; NOAEL, no observed adverse effect level; NOEL, no observed effect level.
Please note the intended association of the term “severity” with “benefits.” In order to balance risks with benefits, matching schemes, and terminology, seem appropriate.
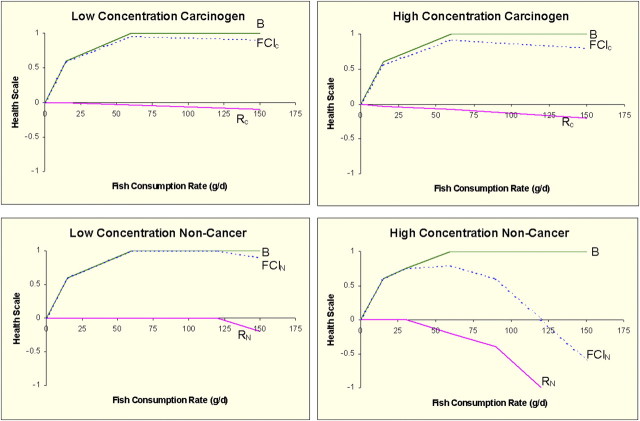
The FCI is an estimate of relative risk (Fig. 3). It does not provide users of the CDRF with an estimate of their increased or decreased incidence of a particular health outcome. It simply provides a mechanism by which users can weigh the possible health risks versus the possible health benefits of eating contaminated fish. Cultural benefits of catching and eating fish (or detriments of not being able to catch or consume fish), or the addition of other chemicals or stressors, may also be considered; however, the current version of the framework does not attempt to precisely quantify these benefits or detriments, other than through a simple algorithm that modulates the values of B or R and FCI.
FIG. 3.
Fish consumption index measured on a relative health scale as a function of fish consumption in grams per day. Low and high concentrations refer to levels of chemical in fish tissue. “B” is the estimated benefit from fish consumption, “R” is the estimated risk from contaminants in fish, and FCI is the net benefit/risk, all as a function of fish consumption in grams per day.
The CDRF is designed to provide information for a range of fish consumption rates, allowing a user to roughly estimate the range of consumption rates at which people may have a net benefit, a net risk, and the consumption rate at which no net change in the health index would be likely. However, the suggested CDRF has a number of significant data gaps as discussed more extensively by Dourson (2002). Further study is needed to confirm and extend the preliminary findings.
Use of the CDRF and FCI does not imply the proper choice is simply achieving a situation in which the net risks and benefits are zero. Nor is it a justification for accepting fish consumption risks as long as there is a net benefit. Rather, the CDRF helps make the risks and benefits transparent. Where the individual has both the opportunity and the means to take actions that directly affect their personal risk and their personal benefit, a full accounting of all associated risks and benefits will enable informed decisions. However, many decisions are made at the level of the society (determining the acceptable level of contamination of water or fish or the level of poverty or neglect that is acceptable without intervention), and an accurate accounting of all risks and all benefits and which segments of society bear risks and which receive benefits will inform such decisions. That the FCI may demonstrate cases in which fish consumption benefits may outweigh the risks is not a license to pollute. Rather, society must determine policy about long-term goals for minimizing environmental pollution based on a range of ethical, economic, social, and other criteria. Again, the purpose of this text is to discuss the underlying scientific issues associated with comparing the risks and benefits of fish consumption. It does not address the social, economic, or ethical considerations.
Consuming uncontaminated fish (i.e., smaller, younger) or reducing fish contamination by reducing hazardous chemical levels in freshwater and marine environments may provide health benefits but without the potential health risks associated with contamination. The eating of such “cleaner” fish, rather than more contaminated fish, would maximize the net benefit of fish consumption. This framework is an initial attempt to evaluate risks and benefits (qualitatively and quantitatively) on a common scale. Constructing this framework has identified numerous areas that need further research and development. Two needs seem paramount. First, better estimations of benefits are needed for the general population and its sensitive subgroups. Although information in this text is highly suggestive of the protective effects of eating fish and allows some quantification, more definitive work is needed to support or modify our chosen quantitative values. Second, better risk information is needed on the chemicals that commonly contaminate fish. Sufficient knowledge on the toxicity of most of these pollutants exists, on which noncancer risks could be quantified. Both sets of information are essential for this framework to be most effective.
CONCLUSION
The frameworks provided here illustrate how risk assessors can initially address additional risk modifications associated with exposure to nonchemical stressors using the limited tools and scant human data currently available. Inclusion of nonchemical stressors in cumulative risk assessments may enable risk assessors to identify those segments of the population who are more susceptible or vulnerable to the effects of chemical stressors. Furthermore, putting nonchemical stressors in the same risk-based language as is typically used with chemical stressors will aid in the prioritization of public health resources. Steps that would facilitate quantitative estimation of cumulative risk include: Clearly articulated definitions and standardization of the terminology used in cumulative risk assessment, identification of information sources that include levels of nonchemical stressors and contributing factors, and compilation of a list of causal models with good quality (e.g., good fit and biologically based) that relate stressors to human health effects and that reflect joint exposures to chemical and nonchemical stressors. Additionally, a better understanding of the long-term effects associated with nonchemical stressors (e.g., long-term effects of natural disasters) is needed, and a shift from a focus on single values (i.e., reference doses and no observed effect levels) to value ranges should be considered. It is not necessary that fully biomathematical risk models be used. Semiquantitative methods should also be considered, such as a hazard index informed by WOE categories for chemical-nonchemical interactions. The immediate goal is to have nonchemical stressors included in the risk characterization.
In conclusion, as we move toward making risk assessments more accurate by accounting for the complete exposure scenario, we increase the complexity of calculating the associated risk. This does not, however, mean that the risk is concomitantly increased. It is important to recognize that risk assessors from different regions may have site-specific stressors of concern, which require flexible cumulative risk assessment approaches. Sorting through the complexities associated with identifying and quantifying nonchemical stressors and incorporating them into predictive models of cumulative risk will require a concerted effort with contributions from multiple disciplines, including, among others, risk assessment, toxicology, exposure sciences, biology, psychology/neuroscience, sociology, epidemiology, economics, and biomathematics.
Decisions about levels of acceptable risks and appropriate benefits that offset accompanying risks are ultimately made by society as a whole. Frequently, these decisions are made with limited understanding of all the risks and benefits. Furthermore, there is incomplete knowledge of how the risks and benefits are distributed across society, yet, such information is required so that decisions are made based on knowledge of all benefits, all risks, and an understanding of those who receive the benefits and those who bear the risks. Improvements in incorporation of nonchemical stressors into cumulative risk assessments will enhance the knowledge base from which such decisions are made, and an improved understanding of the impact of nonchemical stressors on chemical risk will allow individuals to make personal decisions that influence their risk level and their benefit level.
Acknowledgments
The authors would like to thank Dr Glenn Rice and Dr Michael DeVito for their thoughtful reviews of this manuscript.
References
- Agency for Toxic Substances Disease Registry (ATSDR) Guidance Manual for the Assessment of Joint Toxic Action of Chemical Mixtures. Atlanta, GA: Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service; 2004. p. 107. [Google Scholar]
- Borrell B. Every bite you take. Nature. 2011;470:319–321. doi: 10.1038/470320a. [DOI] [PubMed] [Google Scholar]
- Chai C-P. The Assessment of the Ability of Various Heat Stress Indices to Predict Safe Work Behavior. Lubbock, TX: Texas Tech University; 1981. p. 117. [Google Scholar]
- Clougherty JE, Rossi CA, Lawrence J, Long MS, Diaz EA, Lim RH, McEwen B, Koutrakis P, Godleski JJ. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010;118:769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2011;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- deFur PL, Evans GW, Hubal EAC, Kyle AD, Morello-Frosch RA, Williams DR. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environ. Health Perspect. 2007;115:817–824. doi: 10.1289/ehp.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourson M. Comparative dietary risk: Balance the risk and benefits of fish consumption. Comments Toxicol. 2002;8:335–536. [Google Scholar]
- Eder K, Köhler H, Werner I. Pesticide and pathogen: Heat shock protein expression and acetylcholinesterase inhibition in juvenile Chinook salmon in response to multiple stressors. Environ. Toxicol. Chem. 2007;26:1233. doi: 10.1897/05-462r2.1. [DOI] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ. Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos PG, Wang SW, Yang YC, Xue JP, Zartarian VG, McCurdy T, Ozkaynak HK. Biologically based modeling of multimedia, multipathway, multiroute population exposures to arsenic. J. Expo. Sci. Environ. Epidemiol. 2008;18:462–476. doi: 10.1038/sj.jes.7500637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ. Role of environmental stress in the physiological response to chemical toxicants. Environ. Res. 2003;92:1–7. doi: 10.1016/s0013-9351(02)00008-7. [DOI] [PubMed] [Google Scholar]
- Lifeline. Technical Manual LifelineTM Version 4.3 Software. 2006. The Lifeline Group Inc. Available at: http://www.thelifelinegroup.org/lifeline/documents/v4.3_UserManual.pdf. Accessed March 26, 2012. [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabol. Clin. Exp. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, et al. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res. Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Morata TC. Interaction between noise and asphyxiants: A concern for toxicology and occupational health. Toxicol. Sci. 2002;66:1–3. doi: 10.1093/toxsci/66.1.1. [DOI] [PubMed] [Google Scholar]
- Price PS, Chaisson CF. A conceptual framework for modeling aggregate and cumulative exposures to chemicals. J. Expo. Anal. Environ. Epidemiol. 2005;15:473–481. doi: 10.1038/sj.jea.7500425. [DOI] [PubMed] [Google Scholar]
- Price PS, Young JS, Chaisson CF. Assessing aggregate and cumulative pesticide risks using a probabilistic model. Ann. Occup. Hyg. 2001;45:S131–S142. doi: 10.1016/s0003-4878(00)00103-4. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Framework for Metals Risk Assessment. Washington, DC: Office of Research and Development, Risk Assessment Forum; 2007. [Google Scholar]
- Yue Z, Mester J. On the cardiovascular effects of whole-body vibration part I. Longitudinal effects: Hydrodynamic analysis. Stud. Appl. Math. 2007;119:95–109. [Google Scholar]
- Zahran S, Snodgrass JG, Peek L, Weiler S. Maternal hurricane exposure and fetal distress risk. Risk Anal. 2010;30:1590–1601. doi: 10.1111/j.1539-6924.2010.01453.x. [DOI] [PubMed] [Google Scholar]