Abstract
The overall aim of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) is to near-normalize metabolic control in newly diagnosed patients with type 2 diabetes (T2D) using an individualized treatment approach. We hypothesize that this will not only prevent complications and improve quality of life for T2D patients but also result in increased cost efficiency compared with current treatment modalities. This paper provides an overview of the expected outcomes from DD2, focusing on the two main intervention studies. The main data for the DD2 project are collected during patient enrollment and stored using the individual civil registration number. This enables subsequent linking to other national databases where supplemental data can be obtained. All data will be used for designing treatment guidelines and continuously monitoring the development of diabetic complications, thereby obtaining knowledge about predictors for the long-term outcome and identifying targets for new interventions. Further data are being collected from two intervention studies. The aim of the first intervention study is to improve T2D treatment using an individualized treatment modality optimizing medication according to individual metabolic responses and phenotypic characteristics. The aim of the second intervention study is to develop an evidence-based training protocol to be implemented as a treatment modality for T2D and used for initiating lifelong changes in physical activity levels in patients with T2D. An initial pilot study evaluating an interval-based walking protocol is ongoing, and preliminary results indicate that this protocol is an optimal “free-living” training intervention. An initial health-economic analysis will also be performed as a basis for analysis of the data collected during the project. A cost-benefit analysis of the two intervention studies will be conducted. The DD2 project is expected to lead to improved treatment modalities and increased knowledge about existing treatment guidelines, and will also provide a solid base for health-economic decision-making.
Keywords: type 2 diabetes, epidemiological methods, exercise intervention, clinical intervention, individualized treatment, treatment guidelines
Introduction
Type 2 diabetes (T2D) is a rapidly growing disease worldwide. In Denmark, approximately 8% of the adult population is currently affected, with more than 25,000 new patients being diagnosed every year. The new nationwide Danish Centre for Strategic Research in Type 2 Diabetes (DD2) aims to near-normalize the metabolic control in newly diagnosed T2D patients using an individualized treatment approach, with fewer diabetic complications and a higher quality of life as a consequence.
The aim of this paper is to describe the expected outcomes from the DD2 project including the two ongoing intervention studies.
The Danish registry system
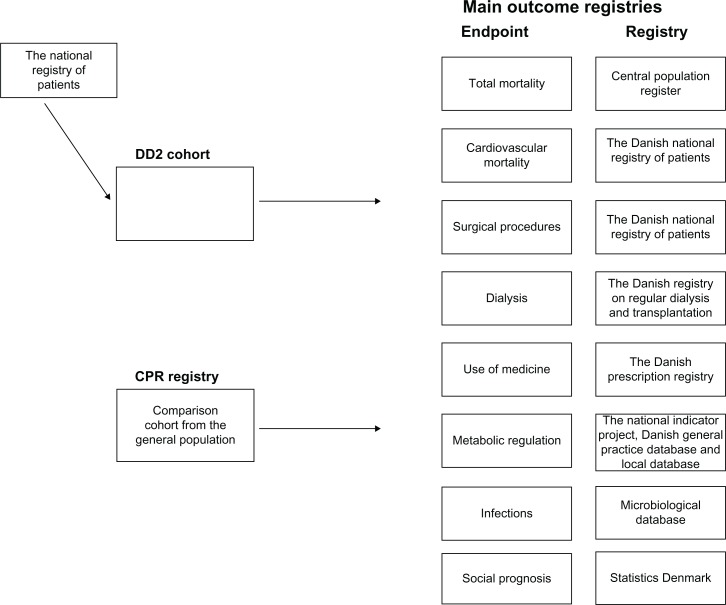
A key characteristic of the Danish health-care system is the access to a wide range of nationwide population-based public registries. Together, they provide a detailed profile of the state of health of individual residents. The Danish Civil Registration System is essential for the usability of the registry. The Civil Registration System maintains electronic records of all Danish residents from 1968 onwards, including daily updated information on vital status. Each record carries a unique ten-digit civil registration number, which is used in all Danish registries and enables unambiguous linkage among these registries. The civil registration number will therefore also be used for storing data obtained in the DD2 project, as described below. Thus, the civil registration number identifies the patient in many health registries, with complete follow-up and history with respect to vital status and migration (from the Civil Registration System), hospital admissions with cardiovascular disease and other morbidities (from the Danish National Registry of Patients), and pharmacological treatment (from the National Prescription Database, containing data of prescription, drugtype, dose, and number of refills). Furthermore, it is possible to conduct linkage to regional laboratory information systems, to clinical registries to ascertain the quality of diabetes care (the National Indicator Project and the Danish General Practice Database), and to other relevant health and demographic databases (Figure 1). A detailed description of the most important registries to be used by DD2 is presented by Thomsen et al.1
Figure 1.
Schematic overview of main outcomes obtained by linking the Danish Centre for Strategic Research in Type 2 Diabetes cohort with data from local and national registry all using the civil registration number as personal identification.
Abbreviations: DD2, Danish Centre for Strategic Research in Type 2 Diabetes; CPR, civil registration number.
The DD2 database and registry-based outcome
As part of DD2 patient enrollment, information about the patients’ lifestyle, physical activity level, and family history of T2D is recorded and stored – see Nielsen et al.2 The data are identified using the civil registration number, making it possible to retrieve supplemental data from other Danish databases, as described above and in details by Thomsen et al1 in brief, DD2 data are linked with data from the Danish Diabetes Database for Adults (DDDA), a nationwide public clinical quality-improvement registry to which hospital physicians and a growing number of general practitioners routinely transfer diabetes data. For general practitioners, the Danish General Practice Database acts as an important feeding database for the DDDA and thereby also the DD2 project. The newly diagnosed type 2 diabetic subjects in DD2 will be followed by yearly extraction of data from the DDDA and other registries outlined in Figure 1. Based on collected and registry data, the following results will be used for evaluation of the metabolic profile of the cohort: mortality rate, cardiovascular mortality, mortality due to cancer, cardiovascular events, development of microvascular complications, metabolic status (hemoglobin A1c [HbA1c] and lipid profile) and current medication. Furthermore, scores for psychosocial well-being, as estimated by the 12-Item Short Form Health Survey, will be assessed and used to evaluate the quality of life together with health economic estimation of cost per quality-adjusted life years. The objective is to use the retrieved data to improve treatment guidelines and continuously monitor the development of diabetic complications.
The DD2 data will be linked to the Statistics Denmark database, where data on income, individual economy, job, and education can be retrieved with regular intervals. These data will be used for determination of the social and economic status for the diabetic subjects included, and afterwards will be related to the outcome of interventions described below.
In addition to the above, the DD2 biobank – a biobank established specifically for the DD2 project – will be incorporated in the project and used for testing and developing new antidiabetic drugs. This will be conducted in collaboration with the pharmaceutical industry, and this collaboration will also facilitate effective large-scale post-marketing studies on the effectiveness and safety of antidiabetic drugs.
DD2 intervention studies
Further data for the DD2 project will be collected through two main intervention studies, as described below. In order to evaluate the data retrieved from these studies and from the DD2 database, a baseline health-economic analysis will be performed.
Intervention study I: individually tailored treatment in T2D
The first intervention study will focus on individualizing T2D treatment. Today, patients are treated according to algorithms based on the “treat-to-target” approach and polypharmacy, as described in the general clinical guidelines. The results obtained with polypharmacy and the treat-to-target approach aiming at near-normalization of HbA1c (often to <6.5%) have failed, as shown in three recent studies: Action to Control Cardiovascular Risk in Diabetes (ACCORD),3 the Veterans Affairs Diabetes Trial,4 and Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation.5 These studies showed only minimal effects on the risk of diabetic complications, whereas mortality was increased in the ACCORD study.3 Thus, polypharmacy is not only ineffective and expensive but may also be dangerous. Furthermore, patient compliance was shown to be low when using these approaches. The reaction to the polypharmaceutical approach has been a nihilistic attitude to treatment of blood glucose in T2D subjects, whereas more focus has been put on treatment of blood pressure and high cholesterol values.
Our concept is that T2D is a heterogeneous disease affecting patients of all ages and patients with several confounding diseases. The latter must be taken into consideration due to treatment with several other drugs besides antidiabetic compounds. We therefore hypothesize that an individually tailored treatment will be more effective, will increase patient compliance, and result in fewer side effects, such as hypoglycemia. The long-term effects of individually tailored treatment modalities, however, remain unknown.
Therefore, we have developed a long-term study, as part of the DD2 project, comparing individually tailored treatment based on the aforementioned risk factors with a treat-to-target approach based on the published and national algorithms.6 The individually tailored treatment will be based on phenotypical characterization, including measurements of glutamic acid decarboxylase antibodies, fasting C-peptide, body weight, the degree of insulin resistance, and obesity – and may also build on genotyping. Furthermore, the individually tailored treatment will set an individualized goal for HbA1c, blood pressure, and cholesterol values in each subject. The number of drugs used will be minimized. This will be achieved by discontinuing drugs that have not proven to be effective within a 3-month period or have been proven to have side effects.
This intervention study will run for at least 5 years and will be geared to find differences in both surrogate end points and hard end points. The focus will be on the development of retinopathy and nephropathy with regard to microvascular complications and on the development of cardiovascular events such as myocardial infarction and heart failure with regard to macrovascular complications. Furthermore, mortality will be determined. Finally, the quality of life associated with the two approaches will also be assessed, as will the economic costs associated with the treatment modalities.
The final end point used for power calculations will be the composite end points of cardiovascular mortality, cardiovascular events, development of cancer, and development of hypoglycemia. Based on these end points, we have calculated that a maximum of 2000 patients will need to be included in order to reach a statistically significant reduction of 30% in the composite end point. In a randomized clinical controlled trial, patients will be randomized into two groups of 1000 newly diagnosed subjects, which will be followed for 5 years. Based on the phenotype, an individually tailored pharmacological treatment will be decided upon, and an individual HbA1c goal will be set up by the general practitioner following the patient. It is possible to aim for three levels of HbA1c: optimal control (HbA1c < 6.5%), acceptable control (HbA1c < 7.5%), and relief of diabetic symptoms (HbA1c > 7.5%). The HbA1c target will also be chosen on the basis of age, motivation, skills, risk of hypoglycemic events, therapy resistance, initial HbA1c, and concomitant illness. Furthermore, the aim is to reduce the number of pharmacological drugs used to less than five individual compounds per patient, including statin treatment, in all subjects. The plan is to get as close as possible to the goal with a minimal use of pharmacological treatment. However, an escape algorithm is set up if HbA1c and blood pressure values reach a critical level. We will put maximum effort into lifestyle changes and to treat basically with metformin in combination with glucagon-like peptide-1 analogs and/or insulin. Hypertension will primarily be treated with an angiotensin-converting-enzyme inhibitor in combination with a diabetic and/or a calcium antagonist. The treatment will be instituted by general practitioners but supervised by a project manager.
This study is slated to start in 2012.
Intervention study II: exercise intervention program
Physical activity is the focus of the second intervention study. The evidence for the beneficial effects of physical activity in T2D patients is strong, and regular physical activity is recommended for anyone with T2D.7 Compliance is often, however, limited, and reported drop-out rates in exercise studies are often high.8 Fully supervised training may improve compliance, but this is a costly strategy to implement in primary care, particularly when considering the large number of patients with T2D. A need seems to exist for new training protocols that simultaneously fit the patient group and are easily implemented and maintained in a large and ever-increasing group of patients. Furthermore, increased physical activity has never been shown to improve the quality of diabetes care. The aim of the exercise intervention program of the DD2 project is therefore to develop and evaluate a free-living training protocol and to subsequently implement this training protocol nationwide in a large-scale observational intervention study. In the broader perspective, the aim is to develop a strategy for initiating lifelong changes in physical activity in newly diagnosed T2D patients and to test the impact of such changes in physical activity on the quality of diabetes care.
Interval training has for a long time been a cornerstone in training among top athletes. The high intensity and subsequently high physiological stress associated with interval training has been regarded as too demanding and potentially dangerous for chronically ill patients; thus, interval training has been discouraged for patients with T2D.9 In recent years, however, an increasing number of interval-training studies have shown remarkable improvements in disease status in various patient groups, including patients suffering from metabolic syndrome and T2D.10,11
To initiate a physical activity program in primary care, the exercise model used must be achievable in a free-living environment and effective in terms of health outcome. A novel free-living, interval-based training called “interval walking” has been developed by a Japanese group.12 Interval walking has proven superior to standard walking on improving a number of classical cardiovascular risk factors and has, with limited resources, been implemented and maintained in a cohort of several thousand subjects. Interval walking consists of repeated cycles of fast and slow walking, 3 minutes each. The training is controlled by a portable accelerometer (the JD Mate) that can be individually set, taking the physical abilities of a given subject into account. By providing the wearer with audible feedback, the JD Mate indicates when to switch from fast to slow walking and vice versa, and whether the pace is high enough. Furthermore, the JD Mate stores all training data, which subsequently can be uploaded to a computer, thereby allowing monitoring of compliance. Interval walking has only been tested in nondiabetic cohorts, but is a potentially good model for a free-living exercise intervention in patients with T2D. Therefore, we have initiated a pilot study to evaluate the qualities of interval walking in a Danish group of patients with T2D. In a randomized controlled trail, subjects with well-controlled T2D were randomized into three groups: interval walking training, continuous walking training, and a control group (no training). The study period was 4 months, and subjects in the training groups were instructed to train five times a week, 1 hour per session. Participants in the control group were told to continue their present lifestyle. The pilot study is ongoing, but both training groups describe the JD Mate device as “motivating in relation to maintenance of training,” and compliance in both training groups is high. Participants in the interval walking training group describe the training in itself as “motivating,” “interesting,” and “addictive.” Furthermore, self-reported health is better after the intervention as compared with before in the interval walking training group. Thus, preliminary data indicate that interval walking training can be implemented as a free-living training intervention in a larger population of T2D patients.
Based on the outcomes of the pilot study, DD2 will from 2012 implement the interval walking training intervention in a larger group of newly diagnosed patients with T2D. The implementation will be mediated using a community-based approach using observational study with community-based matched controls. Newly diagnosed T2D patients who have been referred to local health-care centers will be offered inclusion in the study. If they accept, a JD Mate will be handed out and instructions on how to perform interval walking will be given. Training will be monitored and controlled by the local health-care centre. Questionnaires assessing training compliance, quality of life, and motivational factors regarding training maintenance will be collected. Furthermore, health outcome will be assessed by extracting registry information regarding glycemic control (fasting glucose and HbA1c) and cardiovascular risk factors (blood pressure, lipid status, weight) from the DD2 database. Finally, cardiovascular morbidity and mortality, and all-cause mortality will be assessed using Danish national registries. Outcomes will be analyzed after 1 year and after additional follow-up periods of 1, 5, and 10 years. The study will continue to be evaluated, and an increasing number of local health-care centers will be invited to participate in the intervention program as the study progresses. The long-term goal of the DD2 exercise program is to implement interval walking training in all newly diagnosed T2D patients nationwide.
This interval walking intervention started in the first Danish municipality in January 2012, and we aim to enroll 250 subjects in 2012.
Health economics
Prior to analyzing DD2 data, a baseline economic analysis will be conducted using existing patient cohort data, data from the Danish National Hospital Register, and data from the Danish Quality Unit of General Practice.13 The costs of T2D in the Danish health-care system will be explored in order to provide knowledge concerning which components of T2D treatment, which complication types, and which patient groups are the greatest cost drivers in Danish T2D treatment. Using multilevel models, cost variability will be sought and explained from patients, clinical, and socioeconomic characteristics, and from characteristics of the health-care provider. Such knowledge will indicate areas to focus on in future prevention and treatment strategies, where costs might be reduced. Further, it will throw light on inequalities in the use of health services within T2D treatment.
These initial analyses are intended to serve as a basis for the evaluation of the outcomes of the DD2 project. This evaluation will primarily be based on cost-benefit analyses comparing the costs incurred by the new treatments with the benefits obtained, measured as improved length of life and quality of life and the savings obtained from reduced costs in the health-care system due to fewer patients with severe complications. Several analyses may be undertaken, whereof two are planned at the moment. First, cost effectiveness and cost-utility analyses will be undertaken comparing the effect of the intervention aimed at physical exercise habits and the intervention aimed at individualized treatment algorithms to standard treatment in Danish counties. Thus, the costs of attaining an extra life year or quality-adjusted life year in each of the interventions can be quantified. These analyses will be conducted using data on costs and outcomes from the DD2 randomized clinical trials combined with data from the baseline analyses and external sources such as diagnosis-related groupings. Second, by combining the results of the baseline analyses with outcome data from the DD2 randomized clinical trials, the extent to which the DD2 treatments are succeeding in attaining effects within the identified areas with cost-saving potential can be explored. This will indicate implications of the DD2 treatments as well Danish health-care costs on equity in health and health-service use within T2D.
The study started in February 2012.
Perspectives
The longitudinal cohort design of DD2 and the possibility of obtaining complete and inexpensive data on treatment, demography, and other types of clinical information will provide new knowledge within a relatively short time span. This knowledge may be used for continuous improvement of the guidelines. The follow-up design, supported by the structure of the database setup, and the expected large number of participants will enable a global assessment. At the same time, it will also be possible to examine closely individual treatment effects, treatment complications, genotype, biomarkers, or other phenotypic characteristics. This unique capability to collect data will help evaluate existing treatment algorithms and provide evidence-based knowledge to improve and develop new algorithms.
DD2 can also be used for improving and developing treatment modalities. The study will provide a platform for testing and developing new antidiabetic drugs in collaboration with the pharmaceutical industry, and enable long-term evaluation and post-marketing surveys of pharmacological treatment. The DD2 biobank will offer a unique possibility for identifying new biomarkers, and thus will be of potential interest for both scientists and the pharmaceutical industry.
The construction of the DD2 database and the biobank will be a natural platform and forum for scientific partners to work together at different levels in order to optimize treatment.
Conclusion
Using the DD2 setup to enroll newly diagnosed T2D patients and further describing them by linking data from registries will, for the first time, give a detailed description of the newly diagnosed T2D patients on a national scale. This cohort will provide a unique possibility for longitudinal and follow-up studies. The benefits of achieving the aims and objectives of the DD2 project will be improvement of treatment modalities, increased knowledge about existing treatment guidelines, and a reduced distance between clinic and research. This will provide a solid base for health-economic decisions, which will bring economic benefits for society as a consequence.
The outcomes from the intervention studies will arise from data generated during intervention and subsequently linking information from the DD2 database. This will provide evidence for designing an individually tailored treatment and training regime for each individual patient with T2D, not only in Denmark but worldwide.
Acknowledgments
DD2 is the acronym of ‘The Danish Centre for Strategic Research in Type 2 Diabetes’ supported by the Danish Agency for Science (grant no. 09-067009 and 09-075724). DD2 is also supported by The Danish Health and Medicines Authority, The Danish Diabetes Association and a unrestricted donation from Novo Nordisk A/S. The partner’s of the project are listed on the web-site of the project, www.DD2.nu.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Thomsen RW, Friborg S, Nielsen JS, Schroll H, Johnsen SP. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) – Organization of diabetes care in Denmark and supplementary data sources for data collection among DD2 study participants. Clin Epidemiol. doi: 10.2147/CLEP.S30082. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen JS, Thomsen RW, Steffensen C, Christiansen JS. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) – Implementation of a nationwide patient enrollment system. Clin Epidemiol. doi: 10.2147/CLEP.S30838. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.De nationale algoritmer, Dansk Endokrinologisk Selskab behandlingsvejledning. Behandling og kontrol af type 2 diabetes 2011 (The national algorithms, Danish Endocrine Society, treatment guidelines. Treatment and control of Type 2 Diabetes 2011. Danish Endocrine Society) Dansk Endokrinologisk Selskab. 2012. [serial on the Internet]. http://www.endocrinology.dk. Accessed January 27, 2012. Danish.
- 7.Colberg SR, Sigal RJ. Prescribing exercise for individuals with type 2 diabetes: recommendations and precautions. Phys Sportsmed. 2011;39:13–26. doi: 10.3810/psm.2011.05.1909. [DOI] [PubMed] [Google Scholar]
- 8.Praet SF, van Loon LJ. Optimizing the therapeutic benefits of exercise in Type 2 diabetes. J Appl Physiol. 2007;103:1113–1120. doi: 10.1152/japplphysiol.00566.2007. [DOI] [PubMed] [Google Scholar]
- 9.Albright A, Franz M, Hornsby G, et al. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32:1345–1360. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little JP, Gillen JB, Percival M, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 12.Nose H, Morikawa M, Yamazaki T, et al. Beyond epidemiology: field studies and the physiology laboratory as the whole world. J Physiol. 2009;587:5569–5575. doi: 10.1113/jphysiol.2009.179499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hangaard J, Basset B, Hansen C, Henriksen JE, Vagner H. Diabetesbehandling – den fynske model (Diabetes treatment – The Funen model) Manedsskr Prakt Laegegern. 2006;1:33. Danish. [Google Scholar]