Abstract
Objective
To determine the effect of bolus volume on pharyngeal swallowing using high resolution manometry (HRM).
Study design
Repeated measures with subjects serving as own controls.
Methods
Twelve subjects swallowed four bolus volumes in the neutral head position: saliva; 5 ml water; 10 ml water; and 20 ml water. Pressure measurements were taken along the length of the pharynx using a high resolution manometer, with emphasis placed on the velopharynx, tongue base, and upper esophageal sphincter (UES). Variables were analyzed across bolus volumes using three-way repeated measures analysis of co-variance (ANCOVA) investigating the effect of sex, bolus volume, and pharynx length. Pearson’s product moment tests were performed to evaluate how pharyngeal pressure and timing events changed across bolus volume.
Results
Velopharyngeal duration, maximum tongue base pressure, tongue base pressure rise rate, UES opening duration, and total swallow duration varied significantly across bolus volume. Sex did not have an effect, while pharynx length appeared to affect tongue base pressure duration. Maximum velopharyngeal pressure and minimum UES pressure had a direct relationship with bolus volume, while maximum tongue base pressure had an inverse relationship. Velopharyngeal pressure duration, UES opening duration, and total swallow duration increased as bolus volume increased.
Conclusions
Differences in pharyngeal pressures and timing of key pressure events were detected across varying bolus volumes. Knowing the relationships between bolus volume and pharyngeal pressure activity can be valuable when diagnosing and treating dysphagic patients.
Level of evidence
N/A.
Keywords: Pharyngeal pressure, bolus volume, high-resolution manometry, swallowing physiology, deglutition
INTRODUCTION
The pharyngeal swallow is a pressure driven event requiring intricate coordination of muscle contraction to ensure safe swallowing. Traditional methods of quantifying the rapidly changing pressure events used 3–5 unidirectional sensors typically positioned above, at the level of, and below the upper esophageal sphincter (UES).1–6 While such studies provided valuable information regarding the forces underlying bolus propulsion, the limited number and capacity of the sensors may have failed to accurately capture the complex pressure events along the entire length of the pharynx.
Recently, high resolution manometry (HRM) has been used with success to evaluate pharyngeal swallowing.7–10 HRM uses 36 circumferential sensors to measure pressure events. The high number and circumferential nature of the sensors allows for accurate pressure measurement in the asymmetrical pharynx.11 Despite its potential utility, HRM is still in its infancy. Effective clinical application requires establishment of normal and disordered data set across physiological and experimental conditions.
The effect of bolus volume on pharyngeal swallowing has been investigated rather extensively using traditional manometry,12,13 videofluoroscopy, 14–21 electromyography,22 and mathematical modeling.18 The results of these studies, however, have not always been congruous. Disparities in the relationship between swallow duration and bolus volume have been observed when comparing theoretical18 and videofluoroscopic18,19 to manometric data.13 As compared to videofluoroscopic data, manometric data reveal shorter swallow durations with increased bolus volume.13 The reported differences are likely due to the different measurement approaches (videofluoroscopy vs. manometry); however, the type of manometric catheter employed (three sensors) may not have adequately captured all relevant pressure and timing events. The use of high resolution manometry (HRM) may resolve this disparity.
Versions of HRM have been used to evaluate the UES under various bolus conditions. Using a customized water perfusion catheter with ten recording sites, Williams et al found a direct relationship between intrabolus pressure gradient and bolus volume.15 Using solid state HRM, UES opening duration and the minimum pressures during UES opening change with increased bolus volume.23 When accommodating a larger bolus, the UES opens wider and remains open longer.11,15,17,23,24 Little or no data are available with regard to bolus volume effects at the velopharynx. At the tongue base, no change in pressure has been found,12,25 but an increase in duration of activity has been observed.12,19
HRM may provide a more comprehensive picture of how bolus volume affects swallowing physiology. Correctly identifying bolus volume effects is crucial to our understanding of normal and dysfunctional swallow. We anticipated confirmation of prior work showing increased UES opening duration and minimum pressure with increasing bolus volume. We hypothesized that accommodating larger bolus volumes would require prolonged duration of total swallow. Anticipated changes at the level of the tongue base and velopharynx included increased pressure and duration to reflect greater driving force with larger volumes. To test these hypotheses, a solid-state HRM catheter with 36 circumferential sensors was used to record the pressure and timing data along the pharynx of twelve subjects swallowing four different bolus volumes.
MATERIALS AND METHODS
Equipment
A solid-state high resolution manometer was used for all data collection (ManoScan360 High Resolution Manometry System, Sierra Scientific Instruments, Los Angeles, CA). The manometric catheter has an outer diameter of 4 mm and 36 circumferential pressure sensors spaced 1 cm apart. Each sensor spans 2.5 mm and receives input from 12 circumferential sectors. These inputs are averaged and a mean pressure is recorded as the pressure detected by that individual sensor. The system is calibrated to record pressures between −20 and 600 mmHg with fidelity of 2 mmHg. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments). Prior to calibration, the catheter was covered with a protective sheath to preserve sterility without the need to sterilize the catheter between uses (ManoShield, Sierra Scientific Instruments). The catheter was calibrated before each participant according to manufacturer specifications.
Data collection
Five males and seven females, aged 20.9 ± 1.8 years (range: 19 – 25), participated in this study with the approval of the Institutional Review Board of the University of Wisconsin-Madison. All subjects were without swallowing, neurological, or gastrointestinal disorders. Participants were instructed not to eat for four hours and not to drink liquids for two hours prior to testing to avoid any potential confounding effect of satiety.
Topical 2% viscous lidocaine was applied to the nasal passages with a cotton swab and participants gargled a solution of 4% lidocaine (1 to 2 cc) for several seconds. The manometric catheter was lubricated with 2% viscous lidocaine to ease passage of the catheter through the pharynx. Once the catheter was positioned within the pharynx, participants rested for 5–10 minutes to adjust to the catheter prior to performing the experimental swallows.
The following boluses were swallowed five times with the head in the neutral position: saliva (approximately 1 ml), 5 ml water, 10 ml water, and 20 ml water. Task order was randomized. Each water bolus was delivered to the oral cavity via syringe. Twenty swallows were analyzed for each participant.
Data analysis
Pressure and timing data were extracted using a customized MATLAB program (The MathWorks, Inc., Natick, MA) which locates areas of interest (maximum pressure attained in velopharynx, tongue base, and UES) and then calculates the requisite timing information. The basic workflow is automated, but in cases of anomalous data, the user may override program suggestions and manually select the correct manometric sensors corresponding to the area of interest.
Regions of interest were defined manometrically as in McCulloch et al.8 The velopharynx is the region of swallow-related pressure change just proximal to the area of continuous nasal cavity quiescence and extending two centimeters distally. Maximum velopharyngeal pressure is detected by comparing the peak pressures of the most proximal (rostral) sensors. The peaks continually increase until the maximum velopharyngeal pressure is reached. The tongue base is the area of swallow related pressure change with a high pressure zone approximately midway between the nasopharynx and UES, with its epicenter at the high pressure point and extending two centimeters proximal and distal to that point. Maximum tongue base pressure is less obvious to detect in general, and occasionally requires intervention by the user. A scoring system considers several candidate sensors and their peak pressures, scoring them based on peak pressure, duration, and position relative to the velopharynx. The UES is the midpoint of stable high pressure just proximal (rostral) to the baseline low esophageal pressure zone, extending to a point of low esophageal pressure distally and low baseline pharyngeal pressure proximally. It is detected by computing the average resting pressures of each sensor, and selecting the sensor with the highest value. During swallowing, the UES is mobile along the catheter, moving rostrally as much as 4 cm. Once maximum pressure peaks are found, timing data can be extracted by marking the onset and offset of elevated pressure.
Mean and standard deviation values were recorded for maximum pressure, rate of pressure increase, and duration of pressure above baseline in the regions of the velopharynx and tongue base. Rate of pressure increase was calculated by subtracting baseline pressure from maximum pressure and dividing by the time lapse between these points. Duration of pressure above baseline within a region was defined as the time duration between the onset of pressure escalation and its return to or below baseline using the single senor where maximum pressure was recorded. Minimum pressure during UES opening as well as maximum pressures preceding and succeeding UES opening were also recorded. The time lapse between these pressure peaks is termed UES opening time. Total swallow duration was defined as the time lapse between onset of velopharyngeal pressure rise and the post-swallow UES pressure peak. Length of the pharynx was determined manometrically by finding the distance between the most superior aspect of nasopharyngeal pressure and the most inferior aspect of the UES.
Statistical analysis
SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA) was employed for statistical analyses. Pearson’s product moment correlation tests were used to analyze how each dependent variable (pharyngeal pressure or timing event) changed across bolus volume (independent variable). Three-way repeated measures analysis of co-variance (ANCOVA) was performed to analyze how bolus volume, sex, and pharynx length affected pharyngeal pressure and timing events. Considering sex and pharynx length allowed us to determine if relative bolus volume, rather than absolute bolus volume, is more important for eliciting changes in pharyngeal pressure and timing events. A t-test was used to determine if pharynx length was different between male and female subjects. If data did not meet the assumptions for parametric testing, ANCOVA on ranks and Spearman rank order tests were performed. A significance level of α = 0.05 was determined a priori.
RESULTS
Bar graphs presenting data on maximum pressure and duration are presented in figures 1 and 2, respectively. Pharynx length was significantly greater for males (11.82 ± 0.80 cm) than females (10.14 ± 1.00 cm) (p = 0.018).
Figure 1.
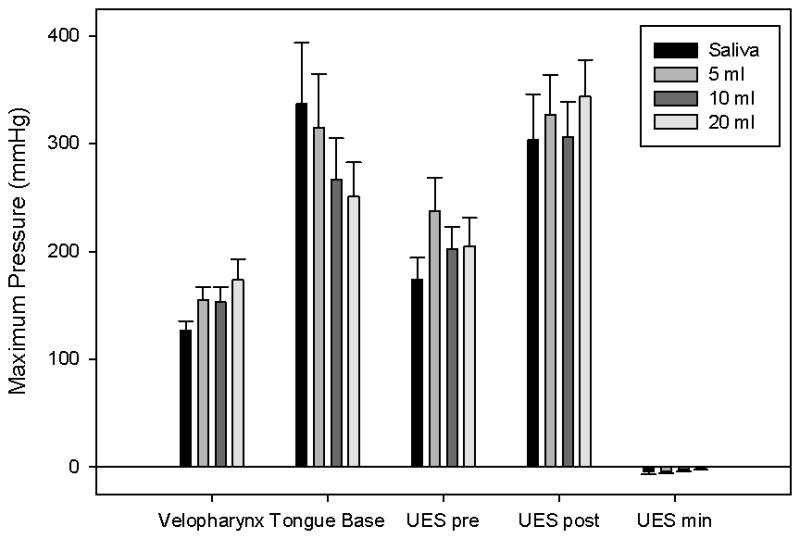
Bar charts displaying average maximum pressure in the three areas of interest. Error bars represent standard error of the mean. Maximum tongue base pressure decreased, while maximum velopharyngeal pressure increased with bolus volume. Upper esophageal sphincter (UES) pressure before and after opening showed no consistent trend.
Figure 2.
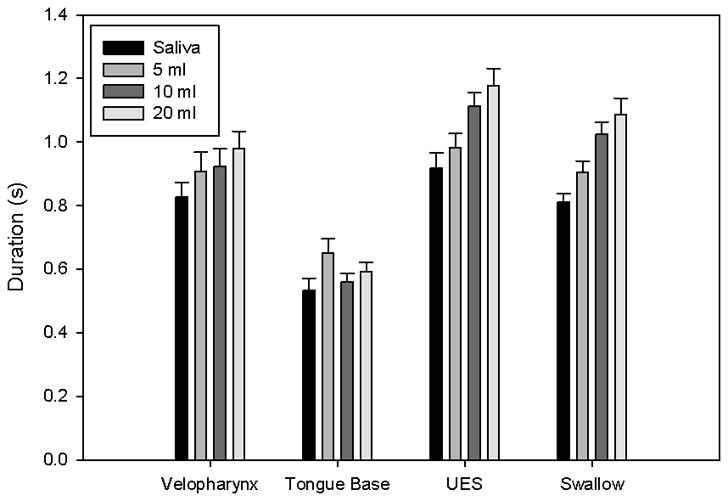
Bar charts displaying average duration for areas of interest and the total swallow. Error bars represent standard error of the mean. Duration for velopharyngeal pressure, upper esophageal sphincter (UES), and total swallow increased with bolus volume. Duration of tongue base pressure above baseline showed no consistent trend.
Velopharynx
Summary data and statistical analyses are provided in tables 1 and 4. There was a significant change in duration across bolus volume (p = 0.0167). A discernible difference was observed for maximum velopharyngeal pressure (p = 0.1413), which increased as bolus volume increased (r = 0.251; p = 0.0855). Pharynx length and sex did not appear to have an effect. Velopharygeal pressure rise time appeared to increase as well (r = 0.219; p = 0.134), but there was no reliable change in velopharyngeal pressure rise rate (r = −0.0754; p = 0.610). Duration of velopharyngeal pressure above baseline also increased as bolus volume increased, though the change did not reach statistical significance (r = 0.256; p = 0.0793).
Table 1.
Summary data for the velopharynx. Pmax = maximum velopharyngeal pressure; r = correlation coefficient. Correlation coefficients (r) and p-values are provided for Pearson product moment tests.
| Variable | 1 ml | 5 ml | 10 ml | 20 ml | r | p-value |
|---|---|---|---|---|---|---|
| Pmax (mmHg) | 127 ± 29 | 154 ± 42 | 153 ± 50 | 174 ± 66 | 0.251 | 0.0855 |
| Rise time (s) | 0.188 ± 0.075 | 0.168 ± 0.064 | 0.197 ± 0.076 | 0.243 ± 0.116 | 0.219 | 0.134 |
| Rise rate (mmHg/s) | 770 ± 322 | 943 ± 301 | 810 ± 250 | 760 ± 188 | −0.0754 | 0.610 |
| Duration (s) | 0.827 ± 0.159 | 0.907 ± 0.208 | 0.923 ± 0.182 | 0.980 ± 0.182 | 0.256 | 0.0793 |
Table 4.
P-values obtained from three-way repeated measures ANCOVA analyzing effect of sex, volume, and pharynx length on velopharyngeal (VP) pressure and timing events.
| Factor | VP max (mmHg) | VP rise (s) | VP rate (mmHg/s) | VP duration (s) |
|---|---|---|---|---|
| Sex | 0.4351 | 0.5896 | 0.9018 | 0.6914 |
| Volume | 0.1413 | 0.4871 | 0.4466 | 0.0167 |
| Pharynx length | 0.5213 | 0.5297 | 0.9847 | 0.2688 |
Tongue base
Summary data and statistical analyses are provided in tables 2 and 5. Significant changes in maximum pressure (p = 0.0402) and rise rate (p = 0.0054) were observed across bolus volume. A discernible change in duration was observed across pharynx length (p = 0.0554). No changes across sex were observed. Maximum tongue base pressure (r = −0.189; p = 0.199) and tongue base pressure rise rate (r = −0.221; p = 0.132) decreased discernibly as bolus volume increased. There was no reliable change in tongue base rise time (r = 0.078; p = 0.599) or duration of tongue base pressure above baseline (r = 0.125; p = 0.396).
Table 2.
Summary data for the tongue base. Pmax = maximum tongue base pressure; r = correlation coefficient. Correlation coefficients (r) and p-values are provided for Pearson product moment tests.
| Variable | 1 ml | 5 ml | 10 ml | 20 ml | r | p-value |
|---|---|---|---|---|---|---|
| Pmax (mmHg) | 337 ± 196 | 315 ± 170 | 267 ± 132 | 251 ± 110 | −0.189 | 0.199 |
| Rise time (s) | 0.184 ± 0.0045 | 0.202 ± 0.042 | 0.205 ± 0.035 | 0.201 ± 0.024 | 0.078 | 0.599 |
| Rise rate (mmHg/s) | 1925 ± 1203 | 1541 ± 713 | 1344 ± 788 | 1259 ± 660 | −0.221 | 0.132 |
| Duration (s) | 0.534 ± 0.129 | 0.650 ± 0.157 | 0.561 ± 0.091 | 0.592 ± 0.100 | 0.125 | 0.396 |
Table 5.
P-values obtained from three-way repeated measures ANCOVA analyzing effect of sex, volume, and pharynx length on tongue base (TB) pressure and timing events.
| Factor | TB max (mmHg) | TB rise (s) | TB rate (mmHg/s) | TB duration (s) |
|---|---|---|---|---|
| Sex | 0.644 | 0.3894 | 0.3739 | 0.8182 |
| Volume | 0.0402 | 0.6516 | 0.0054 | 0.1939 |
| Pharynx length | 0.3535 | 0.3918 | 0.1849 | 0.0554 |
Upper esophageal sphincter
Summary data and statistical analyses are provided in tables 3 and 6. There was a significant difference in UES opening time (p < 0.001). Differences in minimum pressure approached significance (p = 0.0566) and differences in pre-opening pressure were discernible (p = 0.112). Pharynx length and sex did not appear to have an effect. Duration increased significantly with increasing bolus volume (r = 0.526; p < 0.001). Maximum pre-opening UES pressure (r = 0.0008; p = 0.996) and maximum post-closure UES pressure (r = 0.113; p = 0.444) did not exhibit reliable trends.
Table 3.
Summary data for the upper esophageal sphincter (UES). Pmax pre = maximum pre-opening pressure; Pmax post = maximum post-closure pressure; total duration = total swallow duration, from onset of velopharyngeal pressure rise to maximum post-closure UES pressure; r = correlation coefficient. Correlation coefficients (r) and p-values are provided for Pearson product moment tests.
| Variable | 1 ml | 5 ml | 10 ml | 20 ml | r | p-value |
|---|---|---|---|---|---|---|
| Pmax pre (mmHg) | 174 ± 70 | 238 ± 105 | 202 ± 71 | 205 ± 90 | 0.0008 | 0.996 |
| Pmax post (mmHg) | 303 ± 147 | 327 ± 127 | 306 ± 111 | 343 ± 118 | 0.113 | 0.444 |
| Pmin (mmHg) | −4 ± 9 | −4 ± 7 | −2 ± 7 | 0 ± 7 | 0.225 | 0.124 |
| UES duration (s) | 0.917 ± 0.172 | 0.981 ± 0.156 | 1.112 ± 0.147 | 1.178 ± 0.186 | 0.526 | <0.001 |
| Total duration (s) | 0.811 ± 0.094 | 0.903 ± 0.126 | 1.025 ± 0.130 | 1.086 ± 0.180 | 0.573 | <0.001 |
Table 6.
P-values obtained from three-way repeated measures ANCOVA analyzing effect of sex, volume, and pharynx length on upper esophageal (UES) pressure and timing events. UES pre = pre-opening maximum pressure; UES post = post-closure maximum pressure; UES min = minimum UES pressure during opening; UES time = time lapse between pre-opening and post-closure pressure peaks.
| Factor | UES pre (mmHg) | UES post (mmHg) | UES min (mmHg) | UES time (s) | Swallow duration |
|---|---|---|---|---|---|
| Sex | 0.8422 | 0.5466 | 0.5497 | 0.3312 | 0.6389 |
| Volume | 0.1112 | 0.2989 | 0.0566 | <0.0001 | <0.0001 |
| Pharynx length | 0.7284 | 0.4651 | 0.7763 | 0.738 | 0.9735 |
Total swallow duration was significantly different across bolus volumes (p < 0.001), increasing as bolus volume increased (r = 0.573; p < 0.001).
DISCUSSION
Changes in both pressure and timing events were observed as bolus volume was varied. Discernible effects were seen at all three areas of interest, demonstrating the widespread effects that bolus conditions have on pharyngeal pressure generation. Generally, as bolus volume increased, the maximum pressure and pressure rise time increased at the velopharynx and in the UES, there was a rise in the minimum pressure during bolus transport. Conversely, pressures at the tongue base decreased with increased bolus volume. With regard to timing, pressure duration above baseline in the velopharynx, UES opening duration, and the total swallow duration increased with larger bolus volumes (figure 3). No durational changes were observed at the tongue base. However, despite the overall trend in those directions, not all findings were statistically significant. Statistically significant differences were observed for total swallow duration, UES opening time, and minimum UES pressure. Additional variables such as maximum velopharyngeal pressure, duration of velopharyngeal pressure above baseline, and maximum tongue base pressure showed a correlation with bolus volume and approached, but did not reach significance at the 0.05 level.
Figure 3.
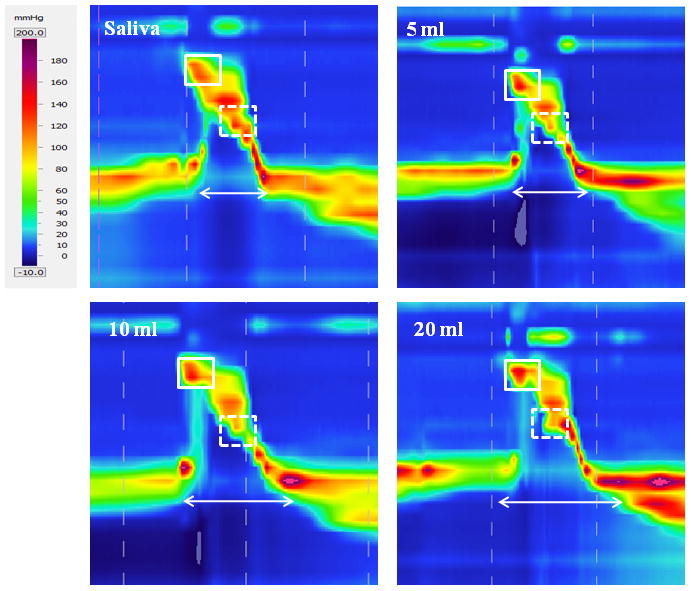
Spatiotemporal plots displaying swallows at each volume from one subject. As bolus volume increased, velopharyngeal pressure (box with solid lines) and upper esophageal sphincter activity time (arrow) increased while tongue base pressure decreased (box with dashed lines).
Increased maximum pressure and duration of pressure above baseline in the velopharynx likely achieves two main goals. First, this reflects the need to ensure a tight seal at the velopharynx when swallowing a large bolus to prevent nasal regurgitation. Second, these changes were predictably accompanied by an increase in UES opening duration as bolus volume increased. Elevated velopharyngeal pressure, combined with a relaxed cricopharyngeal muscle and consequently open UES creates a large pressure gradient favoring bolus propulsion toward the esophagus. No change in velopharyngeal pressure rise rate was detected, attributable to offsetting increases in both maximum pressure and rise time.
Contrary to previous studies that did not observe a change in tongue base pressure,12,25 we found tongue base pressure had an inverse relationship with bolus volume. Both previous investigations used a pressure catheter with a single sensor. It is possible that this sensor did not capture relevant pressure data which could have revealed the negative trend found in this study using HRM. A discernible decrease in tongue base pressure rise rate was also observed, likely due to a decrease in maximum pressure while pressure rise time stayed relatively constant. Lower pressures may be due to larger boluses capitalizing on their gravitational force,13 decreasing the necessary muscular force required for successful swallowing. Probably the most influential physiologic change involves movement of the hyoid bone. Degree of hyoid bone movement has been strongly associated with bolus volume.16,17,21,26 Increases in the anterosuperior excursion of the hyoid certainly affects the shape and position of the tongue base (and at the same time, UES opening). This creates a larger cavity during bolus flow, which may be measurable in the oropharynx as lower pressures with larger volumes.
Both UES opening duration and total swallow duration had a direct relationship with bolus volume. Longer and wider opening of the UES accommodates a larger bolus, as reported in previous videofluoroscopic studies.14,15,17,19 While the effect of bolus volume on UES opening duration is consistently reported, the effect on total swallow duration has been disputed. Previous manometric studies using only three sensors reported a decrease in swallow duration, although the small number of sensors may not have captured all relevant pressure data. The increased number of sensors employed with HRM (up to 36) provides a more comprehensive assessment of both timing and pressure events, eliminating potential “blind spots” which may occur if using a manometric catheter with one to three sensors. Not all 36 sensors were used in our analysis, as maximum pharynx length was 12.6 cm which would require only 13 sensors to span the pharynx. The high number of sensors ensures that when structures such as the UES move during swallowing, they do not move beyond the catheter. As total swallow duration can reliably be determined using videofluoroscopy, it would be interesting to evaluate the effect of increasing bolus volume using simultaneous HRM and videofluoroscopy. Previous videofluoroscopic findings are not consistent, as some studies report an increase in total swallow duration,18 while others did not find a significant change.14,20 Coupling HRM with videofluoroscopy may help clarify this issue.
Sex and pharynx length were incorporated into our analysis as covaraiates, as changes in pharyngeal pressures may be dependent on pharynx size. No differences in pressure or timing events were observed between males and females, similar to the findings from Takasaki et al.7 Pharynx length was significantly greater in males, and of the three regions of interest, differences were most evident at the tongue base. Increased duration of tongue base pressure above baseline approached significance for the male participants and a similar trend occurred for rise rate.
A notable distinction between our study and previous investigations of bolus volume is the measurement of UES opening time, defined manometrically as the time lapse between pre-opening and post-closure maximum UES pressures. Previous studies have recorded UES opening duration11,13,15,24 or cricopharyngeal relaxation time,22 for which similar values have been reported. Our UES opening duration, however, is significantly longer. Others used points along the declining and then rising pressure slopes which results in shorter measured durations,13,15,24 yet revealed similar increasing duration with increasing bolus volume. When recording cricopharyngeal EMG signals, though, no bolus effect was seen between one, five, and ten ml water volumes.22 Cricopharyngeal EMG identifies a robust post-closure electromyographic signal burst, but there is no pre-opening parallel muscle activity signal burst. Only loss of the continuous, low baseline muscle activity can be observed. While the post-closure EMG signal likely coincides with UES post-closure peak pressure pattern, the pre-opening pressure and pressure events are possibly due in part to non-cricopharyngeal muscle events such as laryngeal positioning adjacent to cervical spine soft tissues and laryngopharyngeal posturing prior to bolus delivery. The variation before cricopharyngeal relaxation measurable with EMG and consequent UES opening measured with manometry may account for the differences.
Interestingly, minimum UES pressure increased as bolus volume increased. It is important to note that this increase only approached zero, with all average minimum UES pressures recorded below atmospheric pressure and thus as a negative number. Dantas et al. found a similar positive correlation between minimum UES pressure and bolus volume, though all pressures were greater than zero.14 The negative UES pressure is thought to be generated in part by laryngeal elevation and serves to move a bolus into the esophagus. It is possible that with larger bolus volumes, less negative pressure at the bolus head is needed as the positive pressure at the bolus tail and increased opening duration are sufficient for the bolus to traverse the pharynx. However, we are recording a complex event in which bolus volume changes appear to be accommodated for primarily with prolonged duration of low pressure at the UES, yet complete accommodation is lacking, leading to the measured increase in minimum pressure during bolus transport through the UES.
Two aspects of this study will be the subject of future investigations seeking to improve HRM analysis. First, while the measurement of regional maximum pressure can provide valuable information on swallowing physiology and can readily be compared to previous studies, it does not utilize the full potential of the HRM multi-sensory array. Analyzing pressure patterns, rather than simply pressure values, may provide a more comprehensive evaluation of the pharyngeal swallow. Second, the pressures measured in this study were likely a result of luminal closure and may not necessarily represent bolus driving forces. Measuring bolus driving forces would require implementation of a bulb sensor as in Pouderoux et al.,25 simultaneous HRM and videofluoroscopy, and further evaluation of pressure gradients, which are the force underlying bolus propulsion.
Knowledge of how swallowing physiology changes with varying bolus conditions can be used during swallowing therapy and rehabilitation. At larger volumes, the pattern of greater velopharyngeal pressure, less tongue base pressure, and increased duration of UES opening is characteristic of typical swallowing. Therefore, changing bolus size may alter physiology in ways that can compensate for or exacerbate deficits. Although this strategy is already employed in routine therapy, these data provide further evidence for its use. This preliminary HRM study provides some new insights and confirms results of prior studies yet leaves many questions unanswered. Additional studies using simultaneous videofluoroscopy or electromyography could address many of these basic and clinically important questions.
CONCLUSION
This is the first investigation analyzing the effect of bolus volume on pharyngeal pressures using high resolution manometry. Changes in pressure and timing variables were detected along the length of the pharynx, including the key areas of the velopharynx, tongue base, and upper esophageal sphincter. Several key changes across bolus volume, including decreased tongue base pressure and prolonged UES opening, may be attributed to increased anterosuperior hyoid excursion changing the volume of the pharynx. Knowledge of the relationships between pharyngeal pressure and bolus volume can be used clinically in both the diagnosis and management of swallowing disorders, as different patterns of activity are required at different volumes to ensure safe swallowing. Future studies could determine if these relationships are upheld in dysphagic patients.
Acknowledgments
This study was supported by a grant from the Department of Surgery of the University of Wisconsin School of Medicine and Public Health. The authors thank Dr. Glen Leverson for his help with the statistical analysis.
Footnotes
Conflicts of interest: None.
References
- 1.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989;70:767–771. [PubMed] [Google Scholar]
- 2.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 3.Boden K, Hallgren A, Witt Hedstrom H. Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. 2006;47:628–633. doi: 10.1080/02841850600774043. [DOI] [PubMed] [Google Scholar]
- 4.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82:1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 5.Bulow M, Olsson R, Ekkberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17:197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 6.Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- 7.Takasaki K, Umeki H, Enatsu K, et al. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729–1732. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch T, Hoffman MR, Ciucci MR. High resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010 doi: 10.1177/000348941011900602. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umeki H, Takasaki K, Enatsu K, Tanaka F, Kumagami H, Takahashi H. Effects of a tongue-holding maneuver during swallowing evaluated by high-resolution manometry. Otolaryngol Head Neck Surg. 2009;141:119–122. doi: 10.1016/j.otohns.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Takasaki K, Umeki H, Kumagami H, Takahashi H. Influence of head rotation on upper esophageal sphincter pressure evaluated by high-resolution manometry system. Otolaryngol Head Neck Surg. 142:214–217. doi: 10.1016/j.otohns.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 12.Perlman AL, Schultz JG, VanDaele DJ. Effects of age, gender, bolus volume, and bolus viscosity on oropharyngeal pressure during swallowing. J Appl Physiol. 1993;75:33–37. doi: 10.1152/jappl.1993.75.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res. 2009;52:240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 14.Dantas RO, Kern MK, Massey BT, et al. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol. 1990;258:G675–681. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- 15.Williams RB, Pal A, Brasseur JG, Cook IJ. Space-time pressure structure of pharyngo-esophageal segment during swallowing. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1290–1300. doi: 10.1152/ajpgi.2001.281.5.G1290. [DOI] [PubMed] [Google Scholar]
- 16.Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume on swallow-induced hyoid movement in normal subjects. AJR Am J Roentgenol. 1988;150:1307–1309. doi: 10.2214/ajr.150.6.1307. [DOI] [PubMed] [Google Scholar]
- 17.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Lin S, Chen J, Logemann JA. Oropharyngeal accommodation to swallow volume. Gastroenterology. 1996;111:297–306. doi: 10.1053/gast.1996.v111.pm8690194. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 20.Chi-Fishman G, Sonies BC. Motor strategy in rapid sequential swallowing: new insights. J Speech Lang Hear Res. 2000;43:1481–1492. doi: 10.1044/jslhr.4306.1481. [DOI] [PubMed] [Google Scholar]
- 21.Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 22.Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–1669. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291:G525–531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 24.Pal A, Williams RB, Cook IJ, Brasseur JG. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1037–1048. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]
- 25.Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108:1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- 26.Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–287. doi: 10.1007/s00455-002-0070-7. [DOI] [PubMed] [Google Scholar]


