Abstract
The glottis is composed of muscular, cartilaginous, and other viscoelastic tissues which perform some of our most important, complex, coordinated, and life-sustaining functions. Dominated by the thyroarytenoid muscles and associated glottic closure muscles, the larynx is involved in respiration, swallowing, voicing, coughing, valsalva, vomiting, laughing, and crying. With respiration continuing in the background, all other “secondary” laryngeal events seamlessly occur. When the delicate balance of coordinating these events is disrupted by disease or disorder, many of these tasks are compromised. Due to the complex innervation of these volitional and reflexive tasks with brainstem central pattern generators, primary sensorimotor areas and importantly, limbic areas, failure can occur due to disease, anatomic compromise, and even emotional state. Understanding the level of sensori-motor control and interaction among systems that share these laryngeal neuromuscular substrates will improve the diagnostic and therapeutic skill of the clinician when treating compromise of laryngeal function.
Keywords: larynx, swallow, respiration, cough, thyroarytenoid
Otolaryngology head and neck surgery is perhaps the sole specialty that will evaluate and treat all of the disorders of the laryngopharynx. Yet we, like most specialists, are ill-prepared to handle the complex and subtle interplay of laryngeal events that occur in concert with background respiration. The larynx and pharynx work to allow for continuous respiration, lung volume control, and safe swallowing, as well as episodic but essential cough, sneeze, vomit, and gag. Coupled with all of these activities are vocalizations that include laughing and crying, as well as phonation associated with speech, a unique human laryngeal use. Most of these laryngeal-based events are occurring in parallel and below the level of our conscious mind, although fine control of the larynx during speech and singing is certainly a skilled and volitional behavior. These unique reflexes, central pattern generators, and laryngeal volitional acts use many common neural pathways, share common motor nuclei, and are acted on by nearly every part of the brain. Many of the articles in this supplement will provide detailed information about these reflexive and central pattern generator-driven behaviors. As such, we will focus our discussion on the thyroarytenoid muscle (musculus thyroarytenoideus [TA]), as this is a well-studied muscle that will allow for reasonable support for our central thesis that the glottis (represented by the TA) is uniquely charged with a diverse set of life-sustaining tasks, but also skilled functions, many of which occur simultaneously and share common neuromuscular substrates. Further, compromise of this muscle at an anatomic or neurologic level can disrupt many, if not all of these functions.
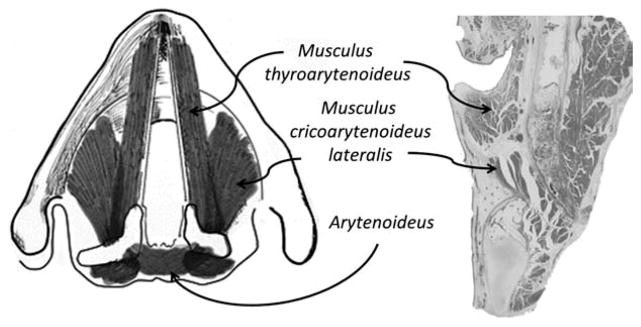
The TA muscle is a very small muscle with its origin on the inner surface of the lower third of the internal aspect of the thyroid cartilage and its insertion on the anterior medial surface of the arytenoid cartilage. It has a very unique medial surface where it interdigitates with a fibroelastic band of nonmuscular tissue, the vocal ligament (Figure 1). Like most muscles, it works in concert with a group of associated, antagonistic, and supportive muscles. The TA motor innervation is the recurrent laryngeal nerve off the vagus nerve, with its motor neurons arising from the nucleus ambiguus. Although studied primarily in animals, the TA is represented dorsally in the nucleus ambiguus, and TA cells can show co-labeling with tracings from the lateral cricoarytenoid (musculus cricoarytenoideus lateralis) and interarytenoid (arytenoideus) muscles.1–4 In humans, these bilateral motor nuclei reside in a region of the mid to upper medulla and receive both excitatory and inhibitory input from the brainstem central pattern generators for respiration, cough, and swallow, as well as the less common gastro-respiratory events of sneezing, gagging, belching, and vomiting.5,6 They are also modulated by motor pathways from the cortex for volitional tasking, such as phonation. These motor nuclei also may have direct and indirect connections to the limbic system.7,8 On the sensory side, along with a variety of pulmonary and pharyngeal regions, the nucleus tractus solitarius receives laryngeal sensory input from both the recurrent and superior laryngeal nerve branch of the vagus.9,10 The sensory innervation includes mucosal mechanoreceptors and chemoreceptors, and deep articular and muscular mechanoreceptors.10–12 During this review, we have given ourselves some latitude to include other muscles that are closely aligned in these functions, such as the lateral cricoarytenoid muscle and interarytenoids, as they are often times targeted in therapies and associated with the functions discussed below.
FIGURE 1.
Basic anatomy of thyroarytenoid and associated laryngeal muscles.
The nucleus ambiguus acts as the final arbitrator of TA movement and interconnections with the medulla and other central nervous system structures are extensive. In a rat model, these were mapped using a pseudorabies virus.8 As shown in Table 1, there is remarkable complexity of the TA in terms of sensorimotor control. These data also demonstrate the relatively early connections to 2 striatal-like limbic structures, the central nucleus of the amygdala and the dysgranular insula.8 The central nucleus of the amygdala is an area of the limbic system believed to be responsible for generating or modulating autonomic and somatic responses to aversive stimuli in humans, and the insula is known to be involved in responses to new aversive stimuli, particularly orofacial stimuli. In humans, limbic connections have been postulated for controlling respiratory muscles,13 and laryngeal functions, such as laugh or cry, can be intact in individuals with ventral pontine infarction or “locked in syndrome,” even with the absence of volitional vocal control for speech or respiratory tasks.14
Table 1.
Transneuronal spread of PRV after inoculation into the thyroarytenoid muscle.
| Day 3 or less | Early day 4 | Late day 4 | Early day 5 | Late day 5 |
|---|---|---|---|---|
| N. ambiguus (loose) central NTS | As previous, plus N. ambiguus (compact) ventral respiratory group Parvocellular reticular formation, (PCRT) N. gigantocellularis (X)1 Probst’s n. Botzinger complex (Area postrema] A7/subceruleus (dorsolateral part of ventrolateral PAG) Dorsal raphe (Perifornical area of hypothalamus) (X) |
As previous, plus N. ambiguus (compact) (X) N. ambiguous semicompact) (X) N. ambiguus (loose) Spinal n. of V Intermediate and medial NTS PCRT (X) Intermediate reticular formation Lateral para-gigantocellularis Ventrolateral PAG (X) Laterodorsal tegmental n. Locus coeruleus (X) Mesencephalic V (X) Substantia nigra, pars reticulata Paraventricular n. of hypothalamus (X) Medial preoptic area (X) Ventrolateral preoptic area (X) Lateral hypothalamus (X) (Posterior hypothalamus) Medial central n. of amygdala (CeM) Granular and dysgranular parietal insular cortex(Infralimbic cortex) entorhinal cortex |
As previous, plus Phrenic n. N. cuneatus Commissural, interstitial and rostral NTS N. Pontis caudalis (X) Pedunculopontine tegmentum Dorsal PAG Median preoptic area (X) Tuberomammillary n. (X) Ventromedial hypothalamic n. Periventricular n. (Subfornical organ) Dorsomedial hypothalamus (X) (Lateral septum) Medial Ce (X) Lateral Ce (CeL) Substantia innominata Bed nucleus of stria terminalis Basolateral n. of amygdala (parvocellular) Granular posterior insular cortex Dysgranular parietal insular cortex (X) Agranular parietal insular cortex Dysgranular anterior insular cortex (X) |
As previous, plus Prelimbic cortex (X) M1 (X) S1 (X) Subiculum (1/2) Hippocampus Perirhinal cortex (X) Entorhinal cortex (X) 1X indicates infected cells were found bilaterally; XO indicates infected cells were found contralaterally only. All other sites are ipsi-lateral to the inoculated muscle. 2Viral clearance from pontomedullary structures is not indicated though both cases exhibited this in the nucleus ambiguus, NTS, and parvocellular reticular formation. |
Abbreviations: PRV, pseudorabies virus; N, Nucleus; NTS, nucleus tractus solitaris; PCRT, parvocellular reticular formation; (X), Indicates infected cells were found bilaterally: (XO) indicated infected cells were found contralaterally only; PAG, periaqueductal gray; Ce, central nucleus of the amygdala; (CeL), central nucleus of the amygdala - lateral; CeM, Medial central nucleus of Amygdala – medial (Modified from Van Daele and Cassell, Neuroscience 2009;162:501–524).
Comprehensively describing the functions and neural connections of the TA muscle is well beyond the scope of this article, but its myriad of activities such as respiration, cough, swallowing, and phonation are all represented in its associated neuronal connections. It is equally important to note that this muscle is controlled at various levels, from reflexive to highly skilled. For example, the laryngeal adductor reflex is elicited with sensory stimulation to the laryngopharynx or electrical stimulation of the superior laryngeal sensory nerve and causes contraction to the TA.15–18 Although somewhat controversial, the timing and magnitude of this reflex is often used to inform clinicians about the integrity of airway protection during the swallow,19–22 and is affected by conditions such as Parkinson Disease11 and gastroesophageal reflux,23 and anatomic modifications such as tracheotomy.24 At a more complex level, the TA is a target of several central pattern generators related to swallowing and respiration.25–32 For a review of the seminal work in this area, see Jean et al, 2001.27 At a volitional level, the TA is highly active during phonated segments of speech.33,34 Further, control of the TA is considered a highly skilled fine sensorimotor act for trained singers, such as opera performers.35,36 There are also differences in neural control when moving from a simple reflexive event like spontaneous swallow to a volitional swallow.37
Respiration
The primary task of the TA muscle and its intrinsic laryngeal partners is respiratory. These muscles sit at the gateway to the airway and, despite all of their additional activities, they are continuously active during modulation of airflow and lung volume. The respiratory cycle is driven by several neural receptor end-put variables: circulating pCO2, pO2, and pH, and have both central and peripheral receptors.38 While each respiration is controlled by diaphragmatic and intercostal muscle contractions and continuous monitoring of intrathoracic pressures requiring maintenance of alveolar volumes and thoracic venous blood return, the respiratory glottic configuration is modulated to optimize these functions. During quiet breathing, there are only subtle movements of the larynx, and the vocal folds primarily remain in the paramedian position during both inspiration and expiration. There is a slight inspiratory lateralization and slight early expiratory medialization, which is believed to maintain positive pressure in the alveoli during passive expiration.39,40 In a dog model, it was found that during quiet respiration the TA muscle remains inactive.40 Thus, the unique architecture of the larynx allows for relative laminar flow at all times. With increased respiratory demand, the glottic and supraglottic areas widen to accommodate the increasing flow without significant increases in turbulence. The primary areas of flow turbulence are seen at the subglottic space with a turbulence intensity modeled at 20%.41
The neuronal network or “central pattern generators” responsible for the generation of respiration and respiratory patterns, which guide the motoneurons controlling respiratory and resistance muscles, are located in the lower brainstem. This respiratory network has bilateral representation, particularly in the dorsal respiratory group and ventral respiratory column of the medulla and dorsolateral pons.38,42–44 The ventral respiratory column is the primary location for the generation of respiratory rhythms. It is subdivided into functional compartments, including the retrotrapezoid nucleus/parafacial respiratory group, Botzinger complex, pre-Botzinger complex, and rostral and caudal ventral respiratory groups.38 Using lesioning techniques, the pre-Botzinger complex has been identified as the site of neuronal circuits that produce basic inspiratory activity.45,46 These respiratory centers then must also interact with swallow and phonation centers, as respiratory patterns are modulated during swallowing and phonation, as discussed below.
Swallow
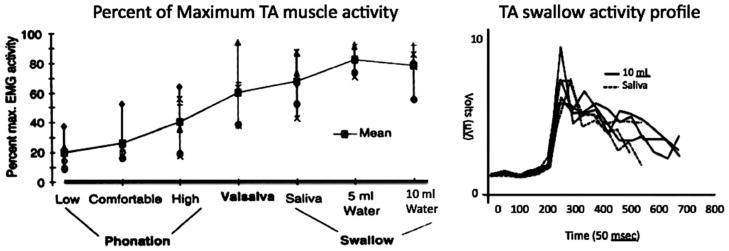
Superimposed on these continuous respiratory events are swallows requiring a brief apnea with vocal fold adduction.47 The laryngeal muscles are essential to the success of both events. From a neuromuscular “effort” point of view, swallow-related TA activity supersedes all other TA functions. Using electromyography, we evaluated TA contractile activity during phonation, swallowing, and valsalvas.48 The magnitude of activity was maximized during all the swallow tasks, with some bolus volume-dependent activity and a characteristic pattern of burst and declination (Figure 2). This event occurs in concert with a pre-swallow respiratory pause in muscular activity and is coupled with a simultaneous activity pause in the posterior cricoarytenoid muscle (Figure 3).49
FIGURE 2.
(A) Thyroarytenoid (TA) activity with vocalization, Valsalva, and swallow (reproduced with permission from Laryngoscope 1996;106:1351–1358). (B) Activity profile from a subject with saliva and 10 mL water swallow. EMG, electromyography.
FIGURE 3.
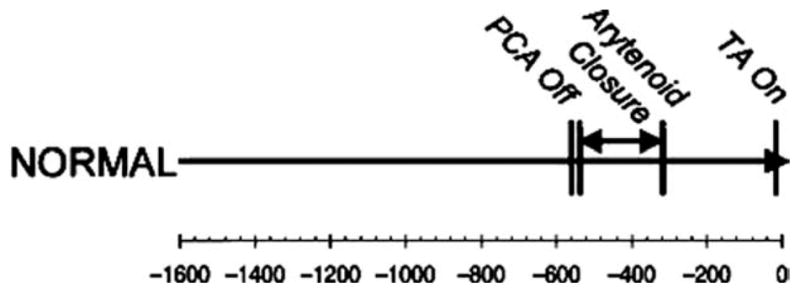
Timing of thyroarytenoid (TA) muscle activity (active glottic closure) relative to posterior cricoarytenoid (PCA) muscle Quiescence (reproduced with permission from Ann Otol Rhinol Laryngol 2005;114:478–487).
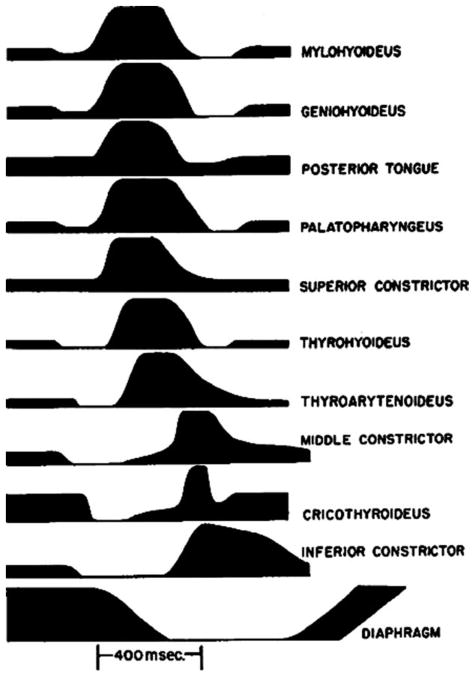
Akin to respiration, there exists a well-identified group of neurons in the medulla which can be defined as a pharyngeal swallow central pattern generator.28,50,51 In 1956, Doty and Bosma50 identified a “swallowing center” in the “internuncial” region around the nucleus solitarius and reticular formation, which interact with sensory input from the pharynx and larynx and output through the motor nuclei of the fifth, seventh, ninth, 10th, and 12th cranial nerves.28,50 Within the nucleus solitarius are inter-neurons which largely account for a portion of the network which initiates and programs swallowing patterns. From these neurons, the swallow pattern command is relayed by the interneurons within the ventrolateral reticular formation before reaching the different groups of motoneurons.28 This interplay consistently produced a pattern of laryngopharyngeal muscle activity which creates a swallow, including TA activity centrally located within the 800 to 1000 millisecond pharyngeal swallow event (Figure 4).50 Similar patterns are found with human swallow.49 Further, other subcortical and cortical structures beyond the brainstem central pattern generators can modulate the pharyngeal swallow (Mosier and Bereznaya, 2001).52–54
FIGURE 4.
Electromyographic activity in deglutition for unanesthetized dog medulla. Height of line for each muscle indicated intensity of action observed (reproduced with permission from Neurophysiology 1956;19:44–60).
This critical timing and importance of TA activity accounts for the common occurrence of aspiration with vocal fold paralysis.55 Vocal fold paralysis has been shown to be a process of denervation/reinnervation. It is now believed that reinnervation is the rule, and in nearly all cases there may be some degree of nondirected or “inappropriate” reinnervation.56–58 With TA neural pathways now directed toward the posterior cricoarytenoid, the swallow event could be uncoupled even beyond the static lateralized vocal fold. This may potentially lead to active swallow-related lateralization of the vocal fold during bolus transit, which is the high-risk period of a swallow, when swallow apnea and glottis closure should occur. This is best seen when TA closure activity is superimposed on bolus transport. Figure 5 shows this in 4 normal subjects when data was collected with simultaneous TA electromyography and fiber-optic swallowing evaluation of 10 mL water boluses. Figure 5 demonstrates the burst of TA activity centered within the period of bolus movement past the larynx.
FIGURE 5.
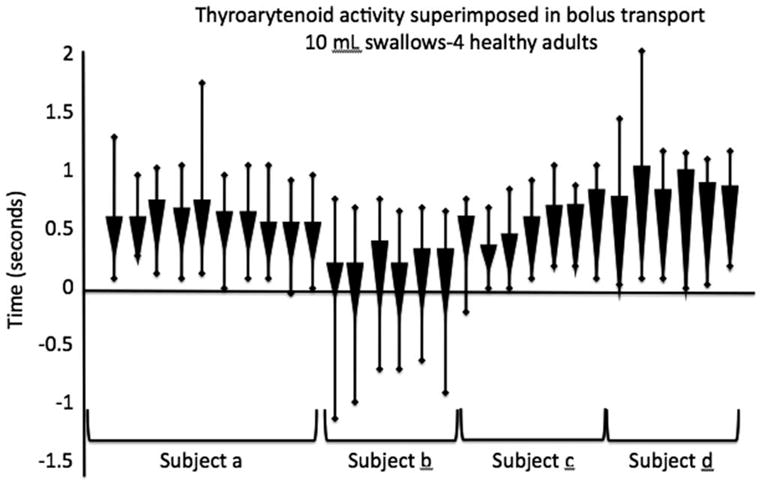
Timing of thyroarytenoid (TA) activity relative to bolus transport measured with simultaneous TA electromyography and fiber-optic examination of swallow with bolus transport recorded as video “whiteout”.
Swallow and Respiratory Interaction
The interactions between respiratory and swallow-related events are complex. When a swallow is superimposed on the respiratory system, a “reset” occurs. The system pauses momentary creating a pressure neutral environment for the swallow.59–62 The coordination respiratory pause and bolus movement has been documented by many authors including Doty and Bosma.50 The respiratory pause certainly occurs in concert with vocal fold closure but seems to be an independent, centrally mediated event, as this apnea persists even in individuals with laryngectomy.63,64 The swallow apnea will begin often times long before the active laryngeal closure occurs and in normal subjects the apnea can last for 3 seconds or longer.60,62 In most cases, the swallow occurs during the expiratory phase, in at least healthy adults,65 and is commonly observed as an inspiration-expiration-swallow-expiration. However, the swallow can occur at the end of inspiration in about 20% of swallows in an inspiration-swallow-expiration pattern.59 These patterns are believed to be most favorable for safe swallowing, as it establishes a positive subglottic pressure at the time of the bolus passage, discouraging content movement toward the airway. It seems that there is some effort to swallow when there is a condition of low elastic resistance in the lung (final phase of expiration).64 However, this pattern is not hard-wired and can be modulated volitionally and spontaneously.37 It is also modified by disease, such as chronic obstructive pulmonary disease66 and amyotrophic lateral sclerosis.67 Although well beyond the scope of this review, these patterns are often times inconsistent in premature infants and neonates with stabilization during maturation.68,69
Cough
Cough is another important sensorimotor task of the larynx. Cough requires coordination with the respiratory system, strongly incorporating abdominal and diaphragmatic muscle, but its effectiveness squarely lies on glottic closure. An effect cough requires a pre-cough high subglottic pressure produced by vocal fold approximation and then a rapid glottic release coupled with contraction of abdominal muscles and respiratory recoil. Cough, in most cases, is the result of a respiratory track irritant. However, it can be induced by medications (angiotensin-converting enzyme inhibitors),70 viral illness (pertussis),71 and as a variant of reactive airways disease.72 Clinical cough disorders are difficult to treat and interfere with patient quality of life and social interactions.73 Therapies have centered on superseding the reflexive cough with other volitional laryngeal activities like voicing, swallowing, and respiration.74 This takes advantage of the ability of central processes to commandeer the motor output to the larynx, suppressing the reflex with a volitional task. Cough, like respiration and swallow, has an identifiable medullary central pattern generator with significant neuronal overlap with respiration and sneeze.6 Cough also has strong limbic connections which manifest as urge-to-cough and post-cough clearance satisfaction.75
There is an association between cough and laryngospasm, as well threshold changes in laryngeal sensation.23 Chronic cough could, in part, be due to sensory threshold shifts which preclude the limbic system from perceiving the clearance “reward.” Stress and other emotional states could interfere with these limbic interactions. It is believed that there is a neural pathway “strengthening” with repeated, traumatic, and stressful events as the amygdala contains much of the neurocircuitry involved in Pavlovian conditioning (learning).76 In many cases, these limbic-associated pathways will trigger a laryngeal dysfunctional behavior. However, it is also possible to “unlearn” or more accurately learn anew and reinstate more normal patterns, again using behavioral interventions that include repetition, reinforcement, modified sensory awareness, and volitional control.77 Cough is certainly a complex clinical issue, however, the potential for cough to exist as a byproduct of stress-induced limbic reactions and associated learning event needs to be considered when managing chronic cough.
Voice
Vocal control of phonation during speech is a learned behavior and is a volitional and skilled act. With changes in respiratory demand, the larynx will reconfigure itself to produce sufficient vocal power at the level of the vocal folds by converting air pressure (subglottic pressure) to the mechanical work of vocal fold vibration, much of which is released as sound pressure and heard as the voiced output. This is coupled with continuous modification of the vocal fold configuration during speech and requires a fair amount of cortical and brainstem control.78 The complexities of voice production, control, and failure are well beyond the scope of this review. The control of the TA muscle is certainly important in the human voice, but the neuromuscular demands on the TA are not high in normal voice use.48 The phrase “it is not what you said but how you said it” also speaks to the amazing level of control we have when it comes to our use of the larynx for communication. We modulate our larynx to produce language, coupling thought with our language centers, sensorimotor cortex, and feedback loops involves learned vocal patterns.78 This is also modulated through auditory and proprioceptive monitoring and shows individual levels of control in terms of skill.79 Communication can be a highly charged personal task. It can be emotional, stressful, beautiful, and unfortunately is also vulnerable to neural pathology. Further, it is highly susceptible to our emotional state, and like chronic cough can become dysfunctional due to limbic interference and disruptive “learning.”
Clinical Interplay
The integrative function of the larynx can be deprioritized when evaluating a patient with a complex presentation. Often, we are concerned with airway closure during a swallow or the vibratory pattern of the vocal fold. However, addressing problems at the periphery does not necessarily mitigate a problem that may be central in nature. For example, with adductor-type spasmodic dysphonia, we inject Botox (quite successfully) in the TA to create a muscle weakness.80–83 However, spasmodic dysphonia is likely a focal dystonia with aberrant neuroplasticity at the level of the basal ganglia and cortex.84,85 As such, we are controlling symptoms, but not addressing the central cause. Further, the side effects of this treatment, the voice disorder, may comprise vocal fold closure during swallow and cough as the TA and neighboring lateral cricoarytenoid are crucial for laryngeal closure. The voice disorders of many known diseases are explained via the neurocircuitry control of laryngeal functions. Misdirected neural pathways can account for the pathology seen with peripheral nerve injury and “recovery.”
We have also reinforced the importance of the limbic system in laryngeal control and failure. There is evidence that this emotional portion of our brain has functional connections to the sensorimotor control centers of the larynx. These identified neuronal pathways and clinical experience teaches us to consider the possibility of maladaptive learning when faced with difficult to diagnose and difficult to treat laryngeal (and pharyngeal) issues: idiopathic voice loss, chronic cough, muscle tension dysphonia, globus sensation, and many others.
In this article, we discussed several continuous and life-preserving functions that center on our complex sensorimotor control of the larynx, with an emphasis on laryngeal adductors and specifically the TA muscle. Our TA muscles support successful respiration, eating, and communication, but this control goes well beyond this muscle. The ability to preserve, protect, and restore these complex, intertwined, and essential functions will be improved when all of the interactions are considered, understood, and targeted when dysfunction is assessed and therapies are designed. It is the complex connections in the nervous system that account for much of what we see clinically and certainly if we fail to address these neural issues we will consistently fail our patients.
Acknowledgments
Contract grant sponsor: This Head & Neck Supplement was jointly supported by funds from the National Institutes for Health (NIDCD –National Institute on Deafness and Other Communication Disorders, Grant #1R13DC009556-01A1S1) and the Division of Otolaryngology –Head and Neck Surgery, University of Wisconsin School of Medicine and Public Health.
References
- 1.Saito Y, Tanaka I, Ezure K. Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res. 2002;44:141–153. doi: 10.1016/s0168-0102(02)00095-0. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida Y, Miyazaki T, Hirano M, Shin T, Totoki T, Kanaseki T. Localization of efferent neurons innervating the pharyngeal constrictor muscles and the cervical esophagus muscle in the cat by means of the horseradish peroxidase method. Neurosci Lett. 1981;22:91–95. doi: 10.1016/0304-3940(81)90069-0. [DOI] [PubMed] [Google Scholar]
- 3.Ciucci MR, Ahrens AM, Ma ST, et al. Reduction of dopamine synaptic activity: degradation of 50-khz ultrasonic vocalization in rats. Behav Neurosci. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida Y, Miyazaki T, Hirano M, Shin T, Kanaseki T. Arrangement of motoneurons innervating the intrinsic laryngeal muscles of cats as demonstrated by horseradish peroxidase. Acta Otolaryngol. 1982;94:329–334. doi: 10.3109/00016488209128920. [DOI] [PubMed] [Google Scholar]
- 5.Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J Neurosci. 1999;19:2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci. 2007;27:5156–5162. doi: 10.1523/JNEUROSCI.0001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Daele DJ, Fazan VP, Agassandian K, Cassell MD. Amygdala connections with jaw, tongue and laryngo-pharyngeal premotor neurons. Neuroscience. 2011;177:93–113. doi: 10.1016/j.neuroscience.2010.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience. 2009;162:501–524. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altschuler SM. Laryngeal and respiratory protective reflexes. Am J Med. 2001;111(Suppl 8A):90S–94S. doi: 10.1016/s0002-9343(01)00862-2. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Ezure K, Tanaka I. Intracellular activity of superior laryngeal nerve motoneurons during fictive swallowing in decerebrate rats. Brain Res. 2002;956:262–267. doi: 10.1016/s0006-8993(02)03549-7. [DOI] [PubMed] [Google Scholar]
- 11.Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. J Neurol. 2010;257:1692–1702. doi: 10.1007/s00415-010-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchner J, Wyke B. Electromyographic analysis of laryngeal articular reflexes. Nature. 1964;203:1243–1245. doi: 10.1038/2031243a0. [DOI] [PubMed] [Google Scholar]
- 13.Straus C, Zelter M, Derenne JP, Pidoux B, Willer JC, Similowski T. Putative projection of phrenic afferents to the limbic cortex in humans studied with cerebral-evoked potentials. J Appl Physiol. 1997;82:480–490. doi: 10.1152/jappl.1997.82.2.480. [DOI] [PubMed] [Google Scholar]
- 14.Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159:115–126. doi: 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Murakkami Y, Kirshner J. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81:59–71. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- 16.Boushey H, Richardons P, Wieddicombe J, Wise J. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol. 1974;240:153–175. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludlow CL, Van Pelt F, Koda J. Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol. 1992;101:127–134. doi: 10.1177/000348949210100204. [DOI] [PubMed] [Google Scholar]
- 18.Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112:834–840. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- 19.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102:777–780. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 21.Aviv JE, Liu H, Parides M, Kaplan ST, Close LG. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000–1006. doi: 10.1177/000348940010901103. [DOI] [PubMed] [Google Scholar]
- 22.Aviv JE, Parides M, Fellowes J, Close LG. Endoscopic evaluation of swallowing as an alternative to 24-hour pH monitoring for diagnosis of extraesophageal reflux. Ann Otol Rhinol Laryngol Suppl. 2000;184:25–27. doi: 10.1177/0003489400109s1006. [DOI] [PubMed] [Google Scholar]
- 23.Murry T, Branski R, Yu K, Cukier–Blaj S, Duflo S, Aviv JE. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope. 2010;120:1576–1581. doi: 10.1002/lary.20985. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki C, Suzuki M, Horiuchi M, Kirshner J. The effect of tracheostomy on the laryngeal closure reflex. Laryngoscope. 1977;87:1428–1433. doi: 10.1288/00005537-197709000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Sumi T. The activity of brainstem respiratory neurons and spinal respiratory motoneurons during swallowing. J Neurophysiol. 1963;26:466–477. doi: 10.1152/jn.1963.26.3.466. [DOI] [PubMed] [Google Scholar]
- 26.Sumi T. Modification of cortically evoked rhythmic chewing and swallowing from midbrain and pons. Jpn J Physiol. 1971;21:489–506. doi: 10.2170/jjphysiol.21.489. [DOI] [PubMed] [Google Scholar]
- 27.Jean J. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 28.Jean A. Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25:109–116. doi: 10.1159/000118856. [DOI] [PubMed] [Google Scholar]
- 29.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review J Auton Nerv Syst. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 30.Jean A. Brainstem control of swallowing: localization and organization of the central pattern generator for swallowing. In: Taylor A, editor. Neurophysiology of the Jaws and Teeth. London: MacMillan; 1990. pp. 294–321. [Google Scholar]
- 31.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol. 1995;74:1509–1517. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 32.McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. J Neurophysiol. 1993;69:95–108. doi: 10.1152/jn.1993.69.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: common volitional control for two upper airway functions. Neuroimage. 2007;36:131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreatta RD, Stemple JC, Joshi A, Jiang Y. Task-related differences in temporo-parietal cortical activation during human phonatory behaviors. Neurosci Lett. 2010;484:51–55. doi: 10.1016/j.neulet.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Herbst CT, Ternström S, Svec JG. Investigation of four distinct glottal configurations in classical singing—a pilot study. J Acoust Soc Am. 2009;125:EL104–EL109. doi: 10.1121/1.3057860. [DOI] [PubMed] [Google Scholar]
- 36.Kochis–Jennings KA, Finnegan EM, Hoffman HT, Jaiswal S. Laryngeal muscle activity and vocal fold adduction during chest, chestmix, headmix, and head registers in females. J Voice. 2011 doi: 10.1016/j.jvoice.2010.11.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26:183–192. doi: 10.1007/s00455-010-9319-8. [DOI] [PubMed] [Google Scholar]
- 38.Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pressman JJ, Kelemen G. Physiology of the larynx. Physiol Rev. 1955;35:506–554. doi: 10.1152/physrev.1955.35.3.506. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura F, Uyeda Y, Sonada Y. Electromyographic study on respiratory movements of the intrinsic laryngeal muscles. Laryngoscope. 1958;68:109–119. doi: 10.1288/00005537-195802000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Lin CL, Tawhai MH, McLennan G, Hoffman EA. Characteristics of the turbulent laryngeal jet and its effect on airflow in the human intra-thoracic airways. Respir Physiol Neurobiol. 2007;157:295–309. doi: 10.1016/j.resp.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchi AL, Denavit–Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 44.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardran GM, Kemp FH. Radiologic investigation of pharyngeal and laryngeal palsy. Acta Radiologica. 1956;46:446–455. doi: 10.1177/028418515604600156. [DOI] [PubMed] [Google Scholar]
- 48.McCulloch TM, Perlman AL, Palmer PM, Van Daele DJ. Laryngeal activity during swallow, phonation, and the Valsalva maneuver: an electromyographic analysis. Laryngoscope. 1996;106:1351–1358. doi: 10.1097/00005537-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114:478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 50.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 51.Umezaki T, Matsuse T, Shin T. Medullary swallowing-related neurons in the anesthetized cat. Neuroreport. 1998;9:1793–1798. doi: 10.1097/00001756-199806010-00022. [DOI] [PubMed] [Google Scholar]
- 52.Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999;109:1417–1423. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Humbert IA, Fitzgerald ME, McLaren DG, et al. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44:982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharyya N, Kotz T, Shapiro J. The effect of bolus consistency on dysphagia in unilateral vocal cord paralysis. Otolaryngol Head Neck Surg. 2003;129:632–636. doi: 10.1016/S0194-59980300633-8. [DOI] [PubMed] [Google Scholar]
- 56.Maronian NC, Robinson L, Waugh P, Hillel AD. A new electromyographic definition of laryngeal synkinesis. Ann Otol Rhinol Laryngol. 2004;113:877–886. doi: 10.1177/000348940411301106. [DOI] [PubMed] [Google Scholar]
- 57.Statham MM, Rosen CA, Smith LJ, Munin MC. Electromyographic laryngeal synkinesis alters prognosis in vocal fold paralysis. Laryngoscope. 2010;120:285–290. doi: 10.1002/lary.20629. [DOI] [PubMed] [Google Scholar]
- 58.Flint PW, Downs DH, Coltrera MD. Laryngeal synkinesis following reinnervation in the rat. Neuroanatomic and physiologic study using retrograde fluorescent tracers and electromyography. Ann Otol Rhinol Laryngol. 1991;100:797–806. doi: 10.1177/000348949110001003. [DOI] [PubMed] [Google Scholar]
- 59.Martin–Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94:1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 60.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol. 1994;76:714–723. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 61.Hirst LJ, Ford GA, Gibson GJ, Wilson JA. Swallow-induced alterations in breathing in normal older people. Dysphagia. 2002;17:152–161. doi: 10.1007/s00455-001-0115-3. [DOI] [PubMed] [Google Scholar]
- 62.Ha °rdemark Cedborg AI, Sundman E, Bodén K, et al. Co-ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Exp Physiol. 2009;94:459–468. doi: 10.1113/expphysiol.2008.045724. [DOI] [PubMed] [Google Scholar]
- 63.Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Swallowing apnea as a function of airway closure. Dysphagia. 2003;18:293–300. doi: 10.1007/s00455-003-0021-y. [DOI] [PubMed] [Google Scholar]
- 64.Costa MM, Lemme EM. Coordination of respiration and swallowing: functional pattern and relevance of vocal folds closure. Arq Gastroenterol. 2010;47:42–48. doi: 10.1590/s0004-28032010000100008. [DOI] [PubMed] [Google Scholar]
- 65.Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483:273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gross RD, Atwood CW, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:559–565. doi: 10.1164/rccm.200807-1139OC. [DOI] [PubMed] [Google Scholar]
- 67.Nozaki S, Sugishita S, Saito T, Umaki Y, Adachi K, Shinno S. Prolonged apnea/hypopnea during water swallowing in patients with amyotrophic lateral sclerosis. Rinsho Shinkeigaku. 2008;48:634–639. doi: 10.5692/clinicalneurol.48.634. [DOI] [PubMed] [Google Scholar]
- 68.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr. 2003;92:721–727. [PubMed] [Google Scholar]
- 69.Nixon GM, Charbonneau I, Kermack AS, Brouillette RT, McFarland DH. Respiratory-swallowing interactions during sleep in premature infants at term. Respir Physiol Neurobiol. 2008;160:76–82. doi: 10.1016/j.resp.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med. 2010;123:1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 71.von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–750. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 72.Turcotte SE, Lougheed MD. Cough in asthma. Curr Opin Pharmacol. 2011;11:231–237. doi: 10.1016/j.coph.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Altman KW, Irwin RS. Cough: a new frontier in otolaryngology. Otolaryngol Head Neck Surg. 2011;144:348–352. doi: 10.1177/0194599810396136. [DOI] [PubMed] [Google Scholar]
- 74.Gibson PG, Vertigan AE. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Ther. 2009;22:159–162. doi: 10.1016/j.pupt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186 (Suppl 1):S107–S111. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 76.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 77.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 78.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 79.Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist. 2011;17:197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17 (Suppl 1):28–30. doi: 10.1111/j.1468-1331.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 81.Hallett M, Benecke R, Blitzer A, Comella CL. Treatment of focal dystonias with botulinum neurotoxin. Toxicon. 2009;54:628–633. doi: 10.1016/j.toxicon.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young N, Blitzer A. Management of supraglottic squeeze in adductor spasmodic dysphonia: a new technique. Laryngoscope. 2007;117:2082–2084. doi: 10.1097/MLG.0b013e318124a97b. [DOI] [PubMed] [Google Scholar]
- 83.Gibbs SR, Blitzer A. Botulinum toxin for the treatment of spasmodic dysphonia. Otolaryngol Clin North Am. 2000;33:879–894. doi: 10.1016/s0030-6665(05)70249-8. [DOI] [PubMed] [Google Scholar]
- 84.Simonyan K, Tovar–Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludlow CL. Spasmodic dysphonia: a laryngeal control disorder specific to speech. J Neurosci. 2011;31:793–797. doi: 10.1523/JNEUROSCI.2758-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]