Abstract
Rhinovirus is commonly associated with bronchiolitis - only second to RSV during the first year life. The prevalence of HRV-bronchiolitis may be very high in predisposed infants. HRV diagnosis is almost exclusively based on PCR, which detects respiratory infections with or without symptoms. Two immunologic factors, interferon responses and atopy, have been associated with susceptibility to HRV-bronchiolitis in multiple studies. The current data supports the hypothesis that susceptibility to HRV-bronchiolitis is likely to be an early manifestation of biased immune responses, which are linked to both decreased viral defence and atopic airway inflammation. Prospective studies have consistently shown that early wheezing associated with HRV infection is closely associated with recurrent wheezing and the development of asthma in children. Collectively, these studies suggest that HRV infection in wheezing children could serve as a clinically useful marker for early identification of asthma prone children. The findings to date provide the rationale for future studies to incorporate rhinovirus illnesses into asthma risk indices.
Keywords: Wheezing, bronchiolitis, asthma, rhinovirus, respiratory syncytial virus, prognosis, infant, child
Introduction
Population-based birth cohort studies have shown that wheezing during lower respiratory tract infection is most common during first year of life [1–4]. The prevalence of wheezing has been 18–32%, 9–17% and 4–12% in the first, second and third year of life, respectively [1, 4]. In addition, younger infants are at higher risk than older infants for requiring hospitalization for wheezing [5].
Risk factors for bronchiolitis include premorbid lung function [1, 6–8], innate and acquired immune responses [9–12], genetic factors (limited mainly to RSV induced disease) [11–16], attendance in day-care or number of older siblings [1–3, 17], and environmental tobacco smoke, especially maternal smoking during pregnancy [2, 18–20]. Other environmental factors linked to the risk of bronchiolitis include exposure to air pollution [21], dampness in the home environment [22] and antibiotic exposure in early life [23]; and young maternal age, low maternal occupation level and low household income level are associated with bronchiolitis in some studies [17, 24]. Although acute respiratory tract infections are equally common in males and females [25, 26], acute wheezing is more common in boys [4, 17, 24, 27, 28]. In most studies, breastfeeding has been protective for wheezing illnesses in infancy [2, 17, 29]. Infants born prematurely or those who have congenital heart disease or chronic lung disease are at the highest risk of requiring hospitalization for bronchiolitis [5].
Allergic individuals may have impaired antiviral responses, leading to more severe infections and wheezing [30–36]. It has been proposed that a subgroup of infants with early respiratory allergy and eosinophilic inflammation in the airways is particularly prone to wheeze during rhinovirus infections [11, 37]. In this review, we focus on rhinovirus associated bronchiolitis, and will review its etiology, diagnostics, co-factors and predictive value for the development of asthma.
What is the prevalence of HRV-bronchiolitis?
Wheezing illnesses in young children are almost exclusively (up to 95%) associated with respiratory viral infections [38, 39]. Respiratory syncytial virus (RSV) dominates in bronchiolitis during the winter months. The overall prevalence of RSV-bronchiolitis depends on yearly epidemics, but it may be up to 80% in infants aged less than 3 months and rapidly decreases there after [40, 41]. The first RSV infection usually causes more severe illness in a non-immune infant whereas subsequent infections usually cause milder symptoms.
In older wheezing children, the common cold virus rhinovirus (HRV) is most often detected – the breaking point in dominance between HRV and RSV is around 12 months in hospitalized wheezing children [40]. The prevalence of HRV-associated wheezing increases with age; approximately 20–40% in infantile bronchiolitis, and increasing to about 50% of hospitalized wheezing children by age 36 months, and about 50–85% in older wheezing children or in children with exacerbation of asthma [40–42]. The susceptibility of HRV-bronchiolitis seems to be linked to predisposition, since the prevalence of HRV bronchiolitis has been up to 50–80% during the first year of life in recurrently ill infants of atopic families [43].
One particular feature of HRV is that there are at least 100 circulating HRV serotypes [44, 45]. Moreover, new sensitive molecular typing assays have revealed that there are over 50 distinct HRV strains in the newly described HRV-C species [46–48]. Rhinoviruses elicit serotype specific antibody responses, and recurrent HRV infections are typically caused by different strains [43].
What does positive HRV PCR result mean?
The development of PCR techniques for virus detection in the 1990s has expanded our view of the epidemiology of viral respiratory infections [49]. In cross-sectional studies of subjects with respiratory symptoms, detection rates by PCR have been as high as 85–95% (or even 100% in certain subanalyses) [38, 41, 42, 50–52]. This increase is mainly the result of the development of PCR diagnostics for respiratory picornaviruses, HRV and enteroviruses, and newly discovered viruses such as human bocavirus, human metapneumovirus and new coronaviruses. At the same time, the interpretation of positive PCR results has been made more difficult by multiple co-existing viruses in symptomatic subjects (up to 43%) and by high virus detection rates in asymptomatic subjects (up to 40–68% in young children during high prevalence seasons) [41–43, 53–55]. In a recent comprehensive review of literature from 1965 to present, the mean prevalences of HRV, adenovirus and RSV in respiratory samples of asymptomatic subjects were higher when detected by PCR than those found by conventional methods: HRV 15% (365/2416) vs 1.5% (255/14669, p < 0.0001), adenovirus 5.3% (103/1958) vs 1.8% (40/2175, p < 0.0001) and RSV 2.6% (51/1974) vs. 0.7% (23/3175, p < 0.0001). These findings attest to the increased sensitivity of new molecular diagnostics, but also raise concerns about the clinical significance of these positive viral findings when detected solely by PCR [49].
Rhinovirus is usually the most frequently detected virus regardless of the presence of symptoms. HRV diagnosis relies almost entirely on PCR because this virus is difficult to culture, there is no antigen detection test available and serology is not feasible. Several findings have demonstrated the clinical relevance of PCR-positive HRV findings. First, three recent studies showed HRV to be more prevalent in children with respiratory symptoms (mainly wheezing) when adjusted or compared with the findings in asymptomatic children/phases [43, 56, 57]. Second, it has been shown that HRV can replicate in cells of the lower respiratory tract and persist for more than a year in the lower respiratory tract of immunocompromized patients [58–60]. Third, the prevalence of recurrent or persistent HRV infections has been low (3–4%) in studies that genotyped the virus [43, 60]. Also, findings from studies without genotyping suggest that respiratory picornavirus infections are of rather short duration in immunocompetent subjects whether symptomatic or not [61–63]. Fourth, HRV PCR positive findings correlate with systemic immune responses in young wheezing children [64]. Finally, an immune host may not develop symptoms. Among young children, however, mild symptoms (e.g. sore throat, malaise) may not be verbalized, or easily recognized by the parent or physician [65]. Although it is known that replication of viruses in the respiratory tract can last longer than illness [66], only 20–40% of viruses detected in asymptomatic subjects can be linked to previous (10 days to 4 weeks) or later (7 to 10 days) development of respiratory symptoms [61, 62, 67]. These findings argue against the suggestion that viruses detected by PCR in asymptomatic subjects are residual nucleic acids left over from distant respiratory infections. Instead, the data suggest that PCR detection is likely to reflect true respiratory infections with or without symptoms.
Which factors are linked to HRV-bronchiolitis?
Two immunologic factors, low interferon responses (especially low interferon-gamma [IFN-γ]), and indicators of atopy (eosinophilia, allergen-specific IgE), are closely associated with susceptibility to HRV-bronchiolitis in multiple studies [37]. Experimental and clinical data indicate that interferon responses in early life are inversely associated with the severity of viral respiratory illnesses [31]. For example, there is clinical evidence that babies with low ex-vivo interferon responses in early life are more likely to have frequent viral respiratory illnesses, including those associated with wheezing [9, 34, 35]. In addition, airway epithelial cells cultured from patients with asthma were reported to produce reduced amounts of IFN-β, IFN-γ, and IFN-λ in response to HRV and support enhanced viral replication [31–33, 36]. This concept is controversial, because other studies of isolated epithelial cells have not found asthma-related differences in HRV replication [68–70]. In addition, viral shedding is similar in volunteers with and without asthma after experimental inoculation with a safety tested strain of HRV-16 [36, 71]. Still, these data suggest the possibility that impaired interferon responses, either systemically or in the airways, could increase the risk of more severe viral respiratory infections in infancy and perhaps promote long-term damage to airway structures. Interestingly, reduced IFN-γ responses in infancy are also observed in children with atopic features, which could help to explain why atopy is a risk factor for virus-induced wheezing and the progression to asthma (Figure) [37, 72, 73].
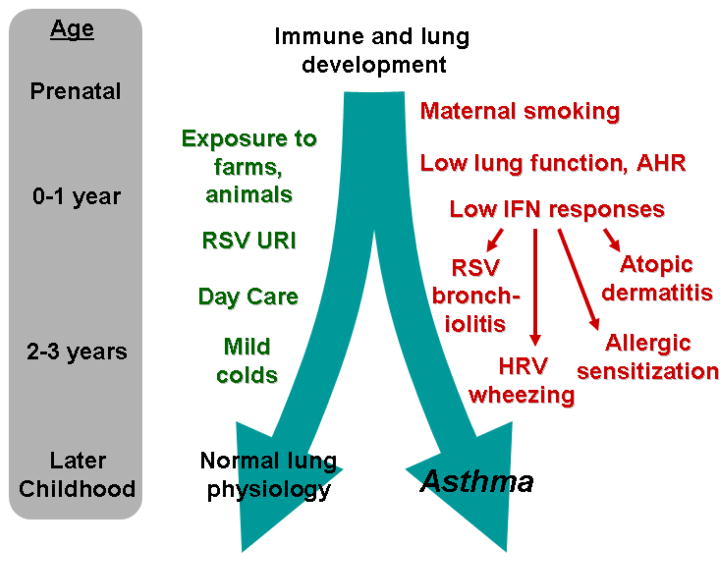
Figure 1.
Early life influences on the development of asthma. Development of the lungs and immune system are initially influenced by genetics and prenatal factors, and later by a number of environmental and lifestyle factors in the first few years of life. Exposure to farm environments, furred animals, and day care in early life can reduce the risk of asthma. In contrast, low interferon responses, wheezing with respiratory viruses, and the development of atopy indicate an increased risk for subsequent childhood asthma.
Four previous studies have linked HRV-induced wheezing in infancy to allergic sensitization, nasal and systemic eosinophilia, and clinically diagnosed atopic eczema [41, 72, 74, 75]. The presence of respiratory allergen-specific IgE and high total IgE is a risk factor for viral wheeze in children presenting for emergency care [76, 77]. These findings are supported by a case-controlled study of children admitted for asthma exacerbations in which detectable virus, allergic sensitization, and high allergen exposure were synergistic risk factors for asthma hospitalization in children [6]. The exacerbation group also had fewer subjects using inhaled corticosteroids compared to children with stable asthma. These data are not entirely consistent since Rakes et al. (1999) reported a link between eosinophils and HRV wheezing illnesses, but they also reported that HRV-negative wheezing children had higher rates of allergic sensitization than HRV-positive wheezing children [41]. Finally, a post hoc analysis of experimental HRV infections in adult subjects with mild asthma revealed that those with high levels of total IgE had greater lower respiratory tract symptom scores than the low IgE group [78].
In the long-term follow-up studies assessing the development of asthma, the interaction between atopy and susceptibility to HRV-bronchiolitis has been less clear. Hyvärinen et al. (2005) and Kusel et al. (2007) found that the relationship between HRV wheezing and subsequent childhood asthma was dependent on early onset of atopy [72, 79]. On the contrary, Lehtinen et al. (2007) and Jackson et al (2008) found HRV associated wheezing to be independent risk factor for asthma at ages 2–6 years [73, 80].
How does allergic sensitization increase risk for HRV associated wheezing?
There are several mechanisms that could explain why allergic sensitization increases the severity of HRV infection in the lower airways. First, atopic inflammation may increase the expression of the major HRV receptor, ICAM-1 (intercellular adhesion molecule 1) [81]. For example, IL-13 and other T-helper2 cytokines have been shown to increase expression of ICAM-1 on an epithelial cell line in vitro, which resulted in increased viral titers after HRV infection [81]. Moreover, epithelial cells recovered from nasal brushings of atopic subjects express significantly higher ICAM-1 levels than those from healthy controls, and increased ICAM-1 expression with allergen exposure was inducible only in the epithelial cells from atopic subjects [82]. In agreement with these findings, atopic individuals have shown more severe illnesses after experimental HRV inoculation[41, 83], although there are also contradictory reports [30, 84]. This mechanism does not apply to minor HRV group, or HRV-C species strains that presumably bind to unique cellular receptors.
Second, T helper2 (Th2) cell polarized immune responses can counteract Th1 responses such as interferon- γ, which potentiates innate antiviral responses [9, 31–36]; subjects with low interferon responses have more severe vral respiratory illnesses as mentioned above. Third, disrupted airway epithelium may favor HRV replication as shown in recent in vitro studies [85]. Interestingly, damaging airway epithelium allowed HRV to access deeper cell layers that express increased amounts of ICAM-1 and support greater HRV replication. Also, poorly differentiated epithelial cells may be more susceptible to HRV infections than intact layers of well-differentiated epithelial cells [86]. Airway epithelium could be damaged by allergic inflammation, repeated respiratory infections, and/or by air pollution. Finally, HRV associated wheezing increases with age as does atopy[40]. Thus, virus/allergen interactions are likely to be stronger as childhood progresses. Overall, atopy is a common condition in asthma, with allergic rhinitis occurring in as many as 80% of older children and young adults with asthma [77].
What is the prevalence of asthma symptoms after early childhood wheezing?
According to the International Study of Asthma and Allergies in Childhood (ISAAC), the global prevalence of wheezing is 11.5 %, and frequent or severe asthma symptoms occur in 4.9 % of children at the age of 6–7 years [87]. The respective numbers are 14.1 % and 6.9 % for 13–14 years old children [87]. The reported prevalence rates of asthma symptoms vary considerably; the rates of frequent asthma symptoms in the developed countries are highest in Australia and New Zealand (8.4–12.1%), and lower in Eastern Europe (3.2–6.2%).
Depending on the study design and length of follow-up, approximately one-third of infants who wheeze in the first three years of life continue to wheeze after the age of three years [4, 88, 89]. Even a mild wheezing episode during infancy is a significant risk factor for wheezing and asthma later in life. Prospective birth-cohort-studies, have shown that about 30% of infants who wheeze in early life continue to report wheezing symptoms in the pre-school years [3, 4, 88, 90, 91]. A retrospective population-based study, which applied a health-care specialist-confirmed diagnosis of bronchiolitis, revealed that 16% of infants with an outpatient visit for bronchiolitis at less than one year of age had frequent wheezing symptoms or asthma at age 4–5.5 years [92]. In a prospective high-risk birth cohort study, which included only children with an atopic predisposition, the risk of frequent symptoms or asthma at age 6 was 30–60 %, depending on the viral aetiology of early wheeze [73]. After hospital admission for wheezing in infancy 18–53 % of pre-school children experience frequent symptoms or asthma, and the prevalence of school-age asthma, at 7.6–9.5 years of age, has been 15–40 % [93–97].
What are the early predictive factors for asthma?
Early predictive factors of asthma mainly include atopic eczema, parental asthma, allergic sensitization and eosinophilic inflammation [91, 98, 99], but also low lung function in infancy [8], bronchial hyperreactivity at least in atopic infants during early life [100, 101] and higher age when wheezing occurs (Figure) [1]. Exposure to tobacco smoke in utero and in infancy reduces lung function in infancy and impairs postnatal lung function development [102–106]. Birth cohort studies evaluating effects of early pet exposure on subsequent asthma have yielded inconsistent results [3, 107–110]. It has been proposed that exposure to microbes associated with barn dust, furred animals and livestock early in life may protect agiainst the development of allergies and asthma [109, 110, 111].
Asthma is a heterogeneous condition, and currently there is no single predictive marker available that can identify those wheezing infants who are at high risk of developing asthma. Therefore, a combination of clinical characteristics and simple biological markers has been created to form algorithms, which may identify, at an early stage of the disease, high risk for asthma. Castro-Rodriquez et al. introduced the first algorithm known as the “asthma predictive index” (API) [97]. The API was later modified for the use in a pharmacological trial for secondary asthma prevention (mAPI) and to be applied to hospitalized wheezing children (hAPI) [98, 112]. In general, the API and mAPI have good specificity, but relatively low sensitivity. Aeroallergen sensitization is not feasible as an early risk factor for asthma in infants since it is rare during early life even in wheezing children [40, 91]. Improved predictive algorithms for infants are therefore needed.
What is the long-term outcome of HRV-bronchioltis?
Several recent observations highlight HRV wheezing illnesses in young wheezing children as an important predictive factor for recurrent wheezing or asthma [39, 73, 80, 113]. In population based studies on young hospitalized children with acute wheezing, HRV infection has been associated with recurrent wheezing (≥3 physician confirmed episodes) during a 12-month follow-up period after the first episode (hazard ratio 5.1, 95% confidence interval 1.0–25, vs. RSV positive cases) and with the development of asthma at school-age (odds ratio 4.1, respectively 1.0–17, vs. HRV negative cases) [80, 113]. In outpatient populations at increased risk for atopy based on family history, more than one wheezing HRV illness during infancy increased the risk for third year wheezing (odds ratio 10, 4.1–26) and modestly increased the risk for asthma at age 6 years (odds ratio 2.8, 1.1–7.5) [39, 73]. Interestingly at the third year of life, wheezing with HRV was markedly associated with asthma at age 6 years (odds ratio 26, 8.2–80) [73]. Nearly 90% of children who wheezed with HRV in year 3 had asthma at age 6 years. In the same study, aeroallergen sensitization during infancy and at age 3 years only modestly increased the risk for asthma at age 6 years (odds ratios 3.4 and 3.6 respectively) [73]. In another study on a high risk cohort, wheezing with HRV during the first year of life was associated with wheezing at age 5 years (odds ratio 3.2, 1.1–9.5) [72]. Comparable findings were made for current asthma. Strikingly, these associations were restricted to children who displayed early sensitization (≤2 years old).
Short courses of systemic corticosteroids are one of the cornerstones of the management of acute asthma in children, but their efficacy among young wheezing children has remained obscure. RSV bronchiolitis does not respond to systemic corticosteroids [114–116], but few studies have tested for specific subgroups of patients who do respond. One study evaluated the efficacy of systemic corticosteroid in relation to HRV etiology among young first-time wheezers [75, 80]. A 3-day course of oral prednisolone decreased the probability of recurrent wheezing (≥3 physician-confirmed episodes) in children with eczema (hazard ratio 0.2, 95% confidence interval 0.0–0.6) and HRV (respectively, 0.2, 0.1–0.7) in this post hoc analysis. Prednisolone decreased recurrent wheezing by 48% over a 12-month study period in these first-time wheezers affected by HRV. The authors speculated that the efficacy of prednisolone may be related to pre-existing airway inflammation that may predispose to HRV infection and is associated with eczema.
Conclusions
Rhinovirus is commonly associated with bronchiolitis, and is second only to RSV during the first year of life. The prevalence of HRV-bronchiolitis may be even higher in predisposed infants. HRV diagnosis is almost exclusively based on PCR, which is likely to detect true respiratory infections with or without symptoms. Two immunologic factors, interferon responses and atopy, have been associated with susceptibility to HRV-bronchiolitis in multiple studies. These findings supports the hypothesis that susceptibility to HRV-bronchiolitis is likely to be an early manifestation of biased immune responses, which could be linked to both decreased viral defence and atopic airway inflammation. Accordingly, prospective studies have consistently shown that early wheezing associated with HRV infection together with early onset of atopy are closely associated with recurrent wheezing and the development of childhood asthma. Collectively, these studies suggest that HRV infection in wheezing children could serve as a tool for early identification of asthma prone children. The findings to date provide the rationale for future studies to incorporate early HRV wheezing episodes into an improved asthma risk index [117].
Acknowledgments
Supported by the Academy of Finland (Grant numbers114034 and 132595), the Foundation for Pediatric Research, both in Helsinki, Finland, and NIH grants.
Footnotes
The authors have no conflict of interest in connection with this paper.
References
- 1.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–75. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 2.Polk S, Sunyer J, Muñoz-Ortiz L, et al. A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am J Respir Crit Care Med. 2004;170:273–8. doi: 10.1164/rccm.200310-1348OC. [DOI] [PubMed] [Google Scholar]
- 3.Sandin A, Bjorksten B, Braback L. Development of atopy and wheezing symptoms in relation to heredity and early pet keeping in a Swedish birth cohort. Pediatr Allergy Immunol. 2004;15:316–22. doi: 10.1111/j.1399-3038.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Matricardi PM, Illi S, Gruber C, et al. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J. 2008;32:585–92. doi: 10.1183/09031936.00066307. [DOI] [PubMed] [Google Scholar]
- 5.Scottish Intercollegiate Guidelines Network. Bronchiolitis in children. A national clinical guideline. 2006 Nov; http://www.sign.ac.uk/pdf/sign91.pdf.
- 6.Murray CS, Pipis SD, McArdle EC, et al. Lung function at one month of age as a risk factor for infant respiratory symptoms in a high risk population. Thorax. 2002;57:388–92. doi: 10.1136/thorax.57.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner SW, Palmer LJ, Rye PJ, et al. Infants with flow limitation at 4 weeks: outcome at 6 and 11 years. Am J Respir Crit Care Med. 2002;165:1294–8. doi: 10.1164/rccm.200110-018OC. [DOI] [PubMed] [Google Scholar]
- 8.Turner SW, Palmer LJ, Rye PJ, et al. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am J Respir Crit Care Med. 2004;169:921–7. doi: 10.1164/rccm.200307-891OC. [DOI] [PubMed] [Google Scholar]
- 9.Copenhaver CC, Gern JE, Li Z, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–80. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 10.Heymann PW, Platts-Mills TA, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24:S217–22. doi: 10.1097/01.inf.0000188164.33856.f9. discussion S220–1. [DOI] [PubMed] [Google Scholar]
- 11.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–19. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 12.Juntti H, Osterlund P, Kokkonen J, et al. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J Allergy Clin Immunol. 2009;124:52–8. doi: 10.1016/j.jaci.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Scagnolari C, Midulla F, Trombetti S, et al. Upregulation of interferon-induced genes in infants with virus-associated acute bronchiolitis. Exp Biol Med (Maywood) 2007;232:1355–9. doi: 10.3181/0705-BC-124. [DOI] [PubMed] [Google Scholar]
- 14.Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helminen M, Nuolivirta K, Virta M, et al. IL-10 gene polymorphism at -1082 A/G is associated with severe rhinovirus bronchiolitis in infants. Pediatr Pulmonol. 2008;43:391–5. doi: 10.1002/ppul.20793. [DOI] [PubMed] [Google Scholar]
- 16.Forton JT, Rowlands K, Rockett K, et al. Genetic association study for RSV bronchiolitis in infancy at the 5q31 cytokine cluster. Thorax. 2009;64:345–52. doi: 10.1136/thx.2008.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehoorn M, Karr CJ, Demers PA, Lencar C, Tamburic L, Brauer M. Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics. 2008;122:1196–203. doi: 10.1542/peds.2007-2231. [DOI] [PubMed] [Google Scholar]
- 18.Wright AL, Holberg C, Martinez FD, Taussig LM. Relationship of parental smoking to wheezing and nonwheezing lower respiratory tract illnesses in infancy. Group Health Medical Associates. J Pediatr. 1991;118:207–14. doi: 10.1016/s0022-3476(05)80484-6. [DOI] [PubMed] [Google Scholar]
- 19.Carroll KN, Gebretsadik T, Griffin MR, et al. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119:1104–12. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- 20.Håberg SE, Stigum H, Nystad W, Nafstad P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am J Epidemiol. 2007;166:679–86. doi: 10.1093/aje/kwm134. [DOI] [PubMed] [Google Scholar]
- 21.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Emenius G, Svartengren M, Korsgaard J, et al. Indoor exposures and recurrent wheezing in infants: a study in the BAMSE cohort. Acta Paediatr. 2004;93:899–905. [PubMed] [Google Scholar]
- 23.Kummeling I, Stelma FF, Dagnelie PC, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2007;119:e225–31. doi: 10.1542/peds.2006-0896. [DOI] [PubMed] [Google Scholar]
- 24.Linneberg A, Simonsen JB, Petersen J, et al. Differential effects of risk factors on infant wheeze and atopic dermatitis emphasize a different etiology. J Allergy Clin Immunol. 2006;117:184–89. doi: 10.1016/j.jaci.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–46. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 26.von Linstow ML, Holst KK, Larsen K, Koch A, Andersen PK, Høgh B. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study. Pediatr Pulmonol. 2008;43:584–93. doi: 10.1002/ppul.20828. [DOI] [PubMed] [Google Scholar]
- 27.Korppi M, Halonen P, Kleemola M, Launiala K. Viral findings in children under the age of two years with expiratory difficulties. Acta Paediatr Scand. 1986;75:457–64. doi: 10.1111/j.1651-2227.1986.tb10230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reijonen T, Korppi M, Pitkakangas S, Tenhola S, Remes K. The clinical efficacy of nebulized racemic epinephrine and albuterol in acute bronchiolitis. Arch Pediatr Adolesc Med. 1995;149:686–92. doi: 10.1001/archpedi.1995.02170190096017. [DOI] [PubMed] [Google Scholar]
- 29.Oddy WH, Sly PD, de Klerk NH, et al. Breast feeding and respiratory morbidity in infancy: a birth cohort study. Arch Dis Child. 2003;88:224–8. doi: 10.1136/adc.88.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardin PG, Fraenkel DJ, Sanderson G, et al. Amplified rhinovirus colds in atopic subjects. Clin Exp Allergy. 1994;24:457–64. doi: 10.1111/j.1365-2222.1994.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–32. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 34.Gern JE, Brooks GD, Meyer P, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 36.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9:73–8. doi: 10.1097/ACI.0b013e32831f8f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Jartti T, Lehtinen P, Vuorinen T, Ruuskanen O. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28:311–7. doi: 10.1097/INF.0b013e31818ee0c1. [DOI] [PubMed] [Google Scholar]
- 41.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 42.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32:314–20. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987;156:43–9. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Hamparian VV, Colonno RJ, Cooney MK, et al. A collaborative report: rhinoviruses--extension of the numbering system from 89 to 100. Virology. 1987;159:191–2. doi: 10.1016/0042-6822(87)90367-9. [DOI] [PubMed] [Google Scholar]
- 46.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kistler A, Avila PC, Rouskin S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–25. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27:1103–7. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 50.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garbino J, Gerbase MW, Wunderli W, et al. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170:1197–203. doi: 10.1164/rccm.200406-781OC. [DOI] [PubMed] [Google Scholar]
- 52.Arnold JC, Singh KK, Spector SA, Sawyer MH. Undiagnosed respiratory viruses in children. Pediatrics. 2008;121:e631–7. doi: 10.1542/peds.2006-3073. [DOI] [PubMed] [Google Scholar]
- 53.van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, et al. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis. 2005;41:490–7. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malmström K, Pitkäranta A, Carpen O, et al. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol. 2006;118:591–6. doi: 10.1016/j.jaci.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Jartti T, Waris M, Niesters HG, Allander T, Ruuskanen O. Respiratory viruses and acute asthma in children. J Allergy Clin Immunol. 2007;120:216. doi: 10.1016/j.jaci.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–6. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 57.Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–61. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 59.Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 60.Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174:1392–9. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 61.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 62.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–50. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 63.Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–9. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 64.Jartti T, Paul-Anttila M, Lehtinen P, et al. Systemic T-helper and T-regulatory cell type cytokine responses in rhinovirus vs. respiratory syncytial virus induced early wheezing: an observational study. Respir Res. 2009;10:85. doi: 10.1186/1465-9921-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–75. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 66.Winther B, Gwaltney JM, Jr, Mygind N, Turner RB, Hendley JO. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–7. [PubMed] [Google Scholar]
- 67.Hyypiä T, Puhakka T, Ruuskanen O, Mäkelä M, Arola A, Arstila P. Molecular diagnosis of human rhinovirus infections: comparison with virus isolation. J Clin Microbiol. 1998;36:2081–3. doi: 10.1128/jcm.36.7.2081-2083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Souza N, Favoreto S, Wong H, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–90. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13 Induced Mucous Metaplasia Increases Susceptibility of Human Airway Epithelium to Rhinovirus Infection. Am J Respir Cell Mol Biol. 2010 Jan 15; doi: 10.1165/rcmb.2009-0244OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeMore JP, Weisshaar EH, Vrtis RF, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–52. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korppi M, Kotaniemi-Syrjänen A, Waris M, Vainionpää R, Reijonen TM. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995–9. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 75.Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–8. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 76.Heymann PW, Rakes GP, Hogan AD, Ingram JM, Hoover GE, Platts-Mills TA. Assessment of eosinophils, viruses and IgE antibody in wheezing infants and children. Int Arch Allergy Immunol. 1995;107:380–2. doi: 10.1159/000237043. [DOI] [PubMed] [Google Scholar]
- 77.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:S8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 78.Zambrano JC, Carper HT, Rakes GP, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 79.Hyvärinen MK, Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Korppi MO. Teenage asthma after severe early childhood wheezing: an 11-year prospective follow-up. Pediatr Pulmonol. 2005;40:316–23. doi: 10.1002/ppul.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–5. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bianco A, Sethi SK, Allen JT, Knight RA, Spiteri MA. Th2 cytokines exert a dominant influence on epithelial cell expression of the major group human rhinovirus receptor, ICAM-1. Eur Respir J. 1998;12:619–26. doi: 10.1183/09031936.98.12030619. [DOI] [PubMed] [Google Scholar]
- 82.Bianco A, Whiteman SC, Sethi SK, Allen JT, Knight RA, Spiteri MA. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin Exp Immunol. 2000;121:339–45. doi: 10.1046/j.1365-2249.2000.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim WK, Olenec JP, Lee WM, Vang F, Pappas TE, Salazar LEP, Evans MD, Bork J, Roberg K, Lemanske RF, Jr, Gern JE. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.01.059. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Kluijver J, Evertse CE, Sont JK, et al. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med. 2003;168:1174–80. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- 85.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–23. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Souza N, Dolganov G, Dubin R, et al. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L373–81. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 87.Lai CK, Beasley R, Crane J, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64:476–83. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 88.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 89.Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Korppi M. Wheezing requiring hospitalization in early childhood: Predictive factors for asthma in a six-year follow-up. Pediatr Allergy Immunol. 2002;13:418–25. doi: 10.1034/j.1399-3038.2002.02091.x. [DOI] [PubMed] [Google Scholar]
- 90.Sherriff A, Peters TJ, Henderson J, Strachan D ALSPAC Study Team. Avon Longitudinal Study of Parents and Children. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. Int J Epidemiol. 2001;30:1473–84. doi: 10.1093/ije/30.6.1473. [DOI] [PubMed] [Google Scholar]
- 91.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 92.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–61. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Korppi M, Kuikka L, Reijonen T, et al. Bronchial asthma and hyperreactivity after early childhood bronchiolitis or pneumonia. An 8-year follow-up study. Arch Pediatr Adolesc Med. 1994;148:1079–84. doi: 10.1001/archpedi.1994.02170100077015. [DOI] [PubMed] [Google Scholar]
- 94.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 95.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 96.Kotaniemi-Syrjänen A, Laatikainen A, Waris M, Reijonen TM, Vainionpää R, Korppi M. Respiratory syncytial virus infection in children hospitalized for wheezing: virus-specific studies from infancy to preschool years. Acta Paediatr. 2005;94:159–65. doi: 10.1111/j.1651-2227.2005.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 97.Valkonen H, Waris M, Ruohola A, Ruuskanen O, Heikkinen T. Recurrent wheezing after respiratory syncytial virus or non-respiratory syncytial virus bronchiolitis in infancy: a 3-year follow-up. Allergy. 2009;64:1359–65. doi: 10.1111/j.1398-9995.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 98.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 99.Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 100.Saga R, Mochizuki H, Tokuyama K, Morikawa A. Relationship between bronchial hyperresponsiveness and development of asthma in wheezy infants. Chest. 2001;119:685–90. doi: 10.1378/chest.119.3.685. [DOI] [PubMed] [Google Scholar]
- 101.Turner SW, Young S, Goldblatt J, Landau LI, Le Souëf PN. Childhood asthma and increased airway responsiveness: a relationship that begins in infancy. Am J Respir Crit Care Med. 2009;179:98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
- 102.Wennergren G, Amark M, Amark K, Oskarsdottir S, Sten G, Redfors S. Wheezing bronchitis reinvestigated at the age of 10 years. Acta Paediatr. 1997;86:351–5. doi: 10.1111/j.1651-2227.1997.tb09021.x. [DOI] [PubMed] [Google Scholar]
- 103.Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. Thorax. 1999;54:469. [PMC free article] [PubMed] [Google Scholar]
- 104.Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–63. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 105.Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Early predictors for adult asthma and lung function abnormalities in infants hospitalized for bronchiolitis: a prospective 18- to 20-year follow-up. Allergy Asthma Proc. 2006;27:341–9. doi: 10.2500/aap.2006.27.2912. [DOI] [PubMed] [Google Scholar]
- 106.Goksör E, Amark M, Alm B, Gustafsson PM, Wennergren G. The impact of pre- and post-natal smoke exposure on future asthma and bronchial hyper-responsiveness. Acta Paediatr. 2007;96:1030–5. doi: 10.1111/j.1651-2227.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 107.Apelberg BJ, Aoki Y, Jaakkola JJ. Systematic review: Exposure to pets and risk of asthma and asthma-like symptoms. J Allergy Clin Immunol. 2001;107:455–60. doi: 10.1067/mai.2001.113240. [DOI] [PubMed] [Google Scholar]
- 108.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol. 2001;108:509–15. doi: 10.1067/mai.2001.117797. [DOI] [PubMed] [Google Scholar]
- 109.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 110.Ownby DR, Johnson CC. Does exposure to dogs and cats in the first year of life influence the development of allergic sensitization? Curr Opin Allergy Clin Immunol. 2003;3:517–22. doi: 10.1097/00130832-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 111.von Mutius E. Asthma and allergies in rural areas of Europe. Proc Am Thorac Soc. 2007;4:212–6. doi: 10.1513/pats.200701-028AW. [DOI] [PubMed] [Google Scholar]
- 112.Piippo-Savolainen E, Korppi M. Wheezy babies--wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr. 2008;97:5–11. doi: 10.1111/j.1651-2227.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 113.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roosevelt G, Sheehan K, Grupp Phelan J, Tanz RR, Listernick R. Dexamethasone in bronchiolitis: a randomised controlled trial. Lancet. 1996;348:292–5. doi: 10.1016/s0140-6736(96)02285-4. [DOI] [PubMed] [Google Scholar]
- 115.De Boeck K, Van der Aa N, Van Lierde S, Corbeel L, Eeckels R. Respiratory syncytial virus bronchiolitis: a double-blind dexamethasone efficacy study. J Pediatr. 1997;131:919–21. doi: 10.1016/s0022-3476(97)70044-1. [DOI] [PubMed] [Google Scholar]
- 116.Bülow SM, Nir M, Levin E, et al. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics. 1999;104:e77. doi: 10.1542/peds.104.6.e77. [DOI] [PubMed] [Google Scholar]
- 117.Jackson DL, Guilbert TW, Evans MD, et al. Inclusion of rhinovirus wheezing history in early life improves the sensiticity of the modified asthma predictive index (mAPI) J Allergy Clin Immunol. 2009;123:S82. [Google Scholar]