Abstract
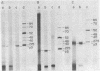
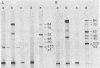
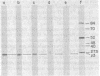
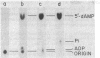
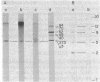
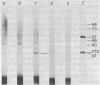
Incubation of extracts of phi 29-infected Bacillus subtilis with [alpha-32P]dATP produced a labeled protein having the electrophoretic mobility of p3, the 5'-terminal protein of phi 29 DNA. The reaction product was resistant to treatment with micrococcal nuclease, phosphatase, and RNases A and T1 and sensitive to proteinase K. Incubation of the 32P-labeled protein with piperidine under conditions in which the phi 29 DNA-protein p3 linkage is hydrolyzed released 5'-dAMP. The reaction with [alpha-32P]dATP was strongly inhibited by anti-p3 serum and required the preence of phi 29 DNA-protein p3 complex; no reaction took place with proteinase K-treated phi29 DNA. These results, together with those of acid hydrolysis and partial proteolysis, indicated that a covalent complex between protein p3 and 5'-dAMP is formed in vitro. The initiation complex (protein p3-dAMP) formed in the presence of 0.5 microM [alpha-32P]dATP can be elongated by addition of 40 microM dNTPs. Treatment with piperidine of the product elongated in the presence of 2',3'-dideoxycytidine 5'-triphosphate released the expected oligonucleotides, 9 and 12 bases long, taking into account the sequence at the left and right DNA ends, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Avila J., Hermoso J. M., Vinuela E., Salas M. Purification and properties of DNA-dependent RNA polymerase from Bacillus subtilis vegetative cells. Eur J Biochem. 1971 Aug 25;21(4):526–535. doi: 10.1111/j.1432-1033.1971.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Ostrove J. M., Kelly T. J., Jr Initiation of adenovirus DNA replication: detection of covalent complexes between nucleotide and the 80-kilodalton terminal protein. J Virol. 1982 Jan;41(1):265–270. doi: 10.1128/jvi.41.1.265-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M. The inverted terminal repetition of the DNA of weakly oncogenic adenovirus type 7. Gene. 1979 Dec;8(1):7–15. doi: 10.1016/0378-1119(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence at the termini of the DNA of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1446–1450. doi: 10.1073/pnas.78.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA replication of bacteriophage phi 29: characterization of the intermediates and location of the termini of replication. Virology. 1980 Jul 30;104(2):323–338. doi: 10.1016/0042-6822(80)90337-2. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Inciarte M. R., Salas M., Sogo J. M. Structure of replicating DNA molecules of Bacillus subtilis bacteriophage phi 29. J Virol. 1980 Apr;34(1):187–199. doi: 10.1128/jvi.34.1.187-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lechner R. L. The structure of replicating adenovirus DNA molecules: characterization of DNA-protein complexes from infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):721–728. doi: 10.1101/sqb.1979.043.01.080. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Peñalva M. A., Inciarte M. R., Salas M. The protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi 29 is involved in the initiation of DNA replication. Virology. 1980 Jul 15;104(1):84–96. doi: 10.1016/0042-6822(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Pincus S., Robertson W., Rekosh D. Characterization of the effect of aphidicolin on adenovirus DNA replication: evidence in support of a protein primer model of initiation. Nucleic Acids Res. 1981 Oct 10;9(19):4919–4938. doi: 10.1093/nar/9.19.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bodnar J. W., Coombs D. H., Pearson G. D. Replicating adenovirus 2 DNA molecules contain terminal protein. Virology. 1979 Jul 15;96(1):143–158. doi: 10.1016/0042-6822(79)90180-6. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Shinagawa M., Padmanabhan R. Comparative sequence analysis of the inverted terminal repetitions from different adenoviruses. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3831–3835. doi: 10.1073/pnas.77.7.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., García J. A., Peñalva M. A., Salas M. Structure of protein-containing replicative intermediates of Bacillus subtilis phage phi 29 DNA. Virology. 1982 Jan 15;116(1):1–18. doi: 10.1016/0042-6822(82)90398-1. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. An adenovirus protein associated with the ends of replicating DNA molecules. Virology. 1979 Feb;93(1):69–79. doi: 10.1016/0042-6822(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Yehle C. O. Genome-linked protein associated with the 5' termini of bacteriophage phi29 DNA. J Virol. 1978 Sep;27(3):776–783. doi: 10.1128/jvi.27.3.776-783.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Friedmann T., Ito J. Nucleotide sequences at the termini of phi 29 DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1336–1340. doi: 10.1073/pnas.78.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wielink P. S., Naaktgeboren N., Sussenbach J. S. Presence of protein at the termini of intracellular adenovirus type 5 DNA. Biochim Biophys Acta. 1979 Jun 20;563(1):89–99. doi: 10.1016/0005-2787(79)90010-8. [DOI] [PubMed] [Google Scholar]