Abstract
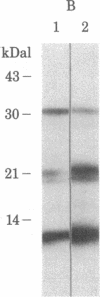
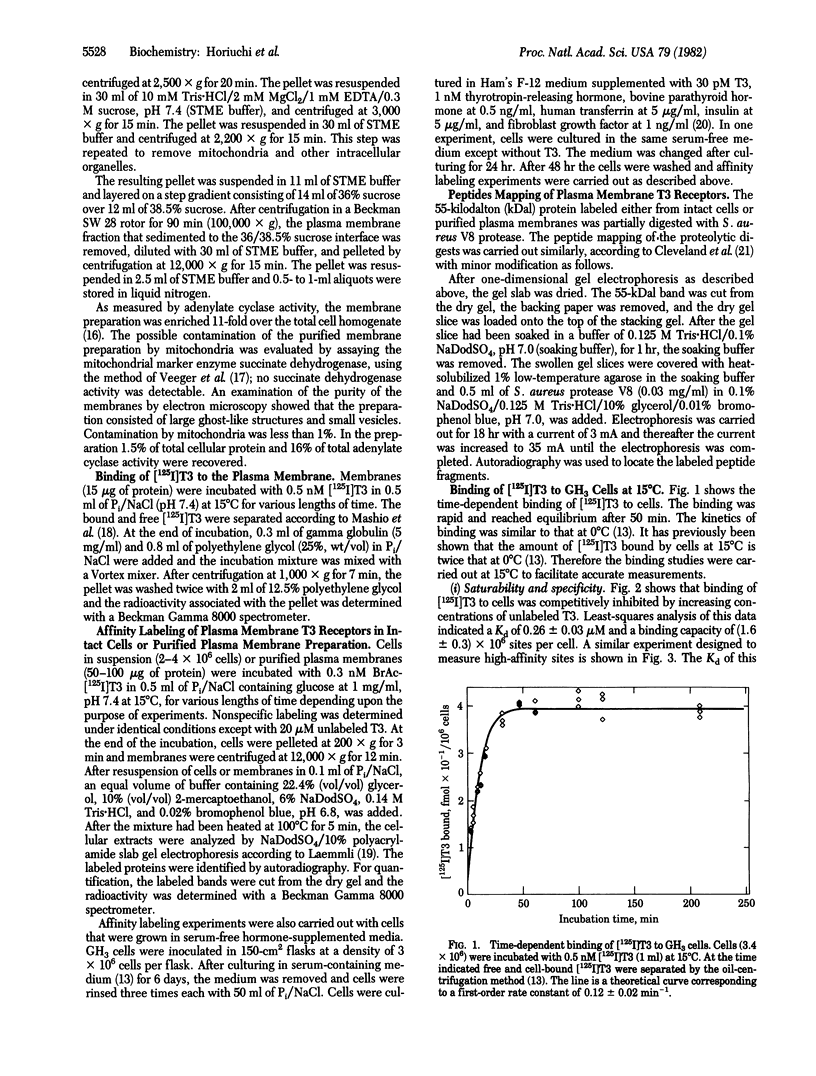
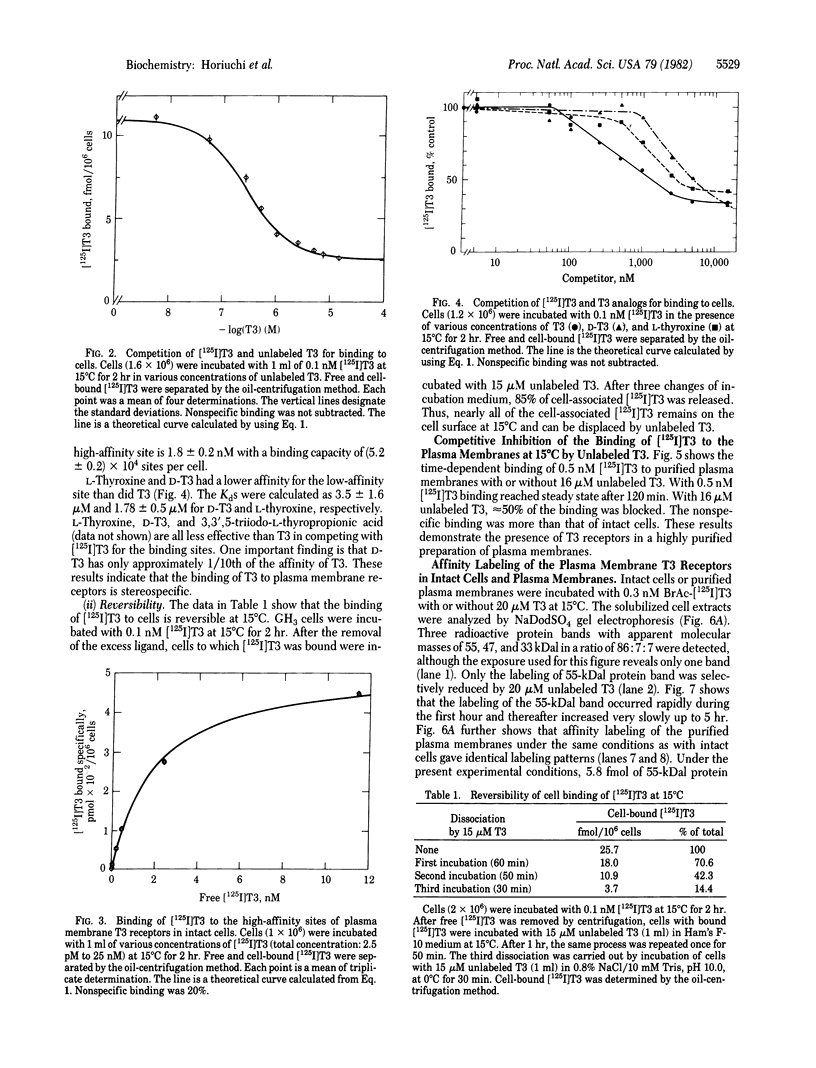
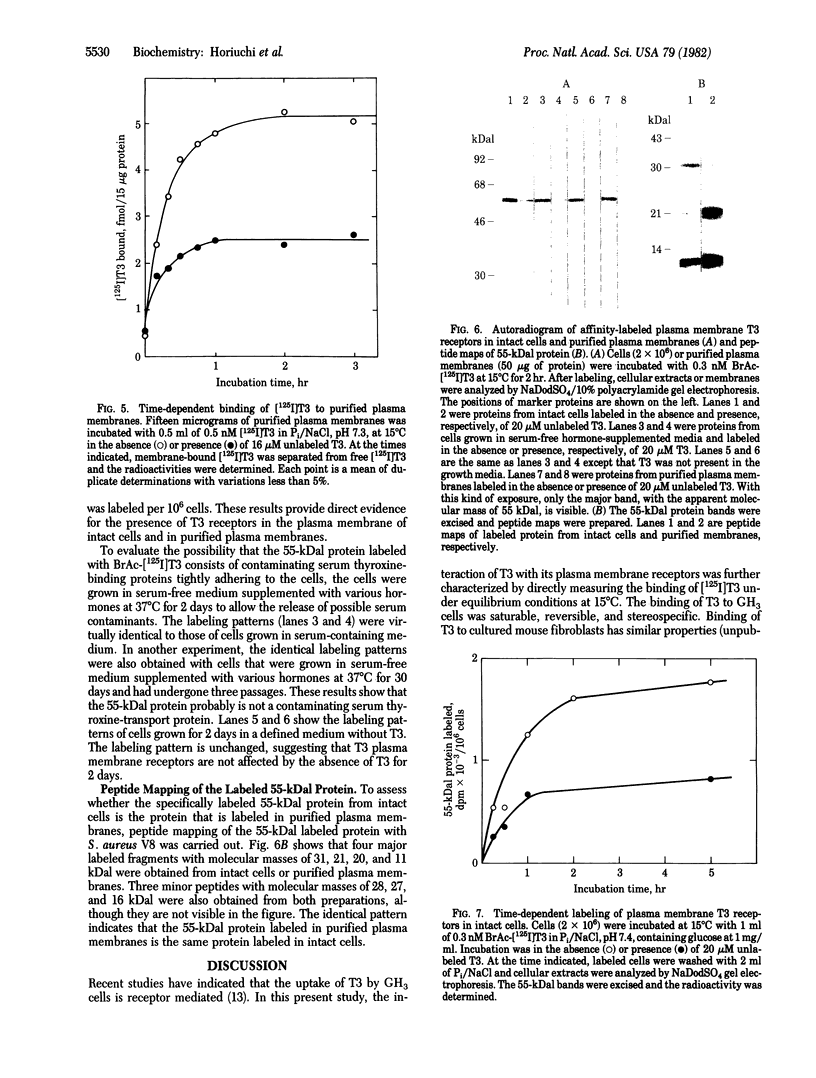
The binding of 3,3',5-triiodo-L-thyronine (T3) to GH3 rat pituitary tumor cells was studied at 15 degrees C and was shown to be saturable, reversible, and stereospecific. Least-squares analysis of the binding data showed two classes of binding sites with dissociation constants of 1.8 +/- 0.2 nM and 260 +/- 30 nM and binding capacities of (5.2 +/- 0.2) X 10(4) and (1.6 +/- 0.2) X 10(6) sites per cell, respectively. Affinity labeling of intact cells was carried out by incubation of cells with 0.3 nM N-bromoacetyl-[125I]T3 at 15 degrees C for 1 hr. Analysis of the cellular extracts by sodium dodecyl sulfate gel electrophoresis showed three labeled protein bands with apparent molecular masses of 55, 47, and 33 kilodaltons (kDal) in a ratio of 86:7:7. The labeling of only the 55-kDal protein band was selectively reduced to 50% by 20 microM unlabeled T3. Highly purified plasma membranes of GH3 cells were prepared and shown to be free of nuclei. Affinity labeling of the purified plasma membranes gave the same labeling pattern as with intact cells. Peptide mapping by Staphylococcus aureus V8 digestion of the 55-kDal protein from cells or plasma membranes gave the identical peptide fragments. Thus the 55-kDal protein labeled from intact cells is the same protein as that from purified plasma membranes. These results together with our earlier findings [Horiuchi, R., Cheng, S.-y., Willingham, M. & Pastan, I. (1982) J. Biol. Chem. 257, 3139-3144] suggest that the 55-kDal protein may be involved in mediating the uptake of T3 in GH3 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng S. Y., Maxfield F. R., Robbins J., Willingham M. C., Pastan I. H. Receptor-mediated uptake of 3,3',5-triiodo-L-thyronine by cultured fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3425–3429. doi: 10.1073/pnas.77.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eckel J., Rao G. S., Rao M. L., Breuer H. Uptake of L-tri-iodothyronine by isolated rat liver cells. A process partially inhibited by metabolic inhibitors; attempts to distinguish between uptake and binding to intracellular proteins. Biochem J. 1979 Aug 15;182(2):473–491. doi: 10.1042/bj1820473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Cheng S. Y., Lippoldt R. E., Lord R. S., Robbins J. Characterization of human thyroxine-binding globulin. Evidence for a single polypeptide chain. J Biol Chem. 1977 Dec 10;252(23):8713–8718. [PubMed] [Google Scholar]
- Hayashi I., Larner J., Sato G. Hormonal growth control of cells in culture. In Vitro. 1978 Jan;14(1):23–30. doi: 10.1007/BF02618171. [DOI] [PubMed] [Google Scholar]
- Horiuchi R., Cheng S. Y., Willingham M., Pastan I. Inhibition of the nuclear entry of 3,3',5'-triiodo-L-thyronine by monodansylcadaverine in GH3 cells. J Biol Chem. 1982 Mar 25;257(6):3139–3144. [PubMed] [Google Scholar]
- INGBAR S. H., FREINKEL N. Regulation of the peripheral metabolism of the thyroid hormones. Recent Prog Horm Res. 1960;16:353–403. [PubMed] [Google Scholar]
- Johnson G. S., Kimura N., Kimura N. Large potentiation of agonist response in intact cells is produced by increases only in GTP-dependent adenylate cyclase activity. J Cyclic Nucleotide Res. 1981;7(2):105–115. [PubMed] [Google Scholar]
- Johnson M. L., Correia J. J., Yphantis D. A., Halvorson H. R. Analysis of data from the analytical ultracentrifuge by nonlinear least-squares techniques. Biophys J. 1981 Dec;36(3):575–588. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Krenning E. P., Docter R., Bernard B., Visser T., Hennemann G. Decreased transport of thyroxine (T4), 3,3',5-triiodothyronine (T3) and 3,3',5'-triiodothyronine (rT3) into rat hepatocytes in primary culture due to a decrease of cellular ATP content and various drugs. FEBS Lett. 1982 Apr 19;140(2):229–233. doi: 10.1016/0014-5793(82)80900-9. [DOI] [PubMed] [Google Scholar]
- Krenning E. P., Docter R., Bernard H. F., Visser T. J., Hennemann G. Active transport of triiodothyronine (T3) into isolated rat liver cells. FEBS Lett. 1978 Jul 1;91(1):113–116. doi: 10.1016/0014-5793(78)80029-5. [DOI] [PubMed] [Google Scholar]
- Krenning E., Docter R., Bernard B., Visser T., Hennemann G. Regulation of the active transport of 3,3',5-triiodothyronine (T3) into primary cultured rat hepatocytes by ATP. FEBS Lett. 1980 Oct 6;119(2):279–282. doi: 10.1016/0014-5793(80)80271-7. [DOI] [PubMed] [Google Scholar]
- LEIN A., DOWBEN R. M. Uptake and binding of thyroxine and triiodothyronine by rat diaphragm in vitro. Am J Physiol. 1961 May;200:1029–1031. doi: 10.1152/ajplegacy.1961.200.5.1029. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- Mashio Y., Inada M., Tanaka K., Ishii H., Naito K., Nishikawa M., Takahashi K., Imura H. High affinity 3,5,3'-L-triiodothyronine binding to synaptosomes in rat cerebral cortex. Endocrinology. 1982 Apr;110(4):1257–1261. doi: 10.1210/endo-110-4-1257. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Pastan I., Dragsten P., Cheng S. Y. Binding and mobility of the cell surface receptors for 3,3',5-triiodo-L-thyronine. Science. 1981 Jan 2;211(4477):63–65. doi: 10.1126/science.6255563. [DOI] [PubMed] [Google Scholar]
- Nikodem V. M., Cheng S. Y., Rall J. E. Affinity labeling of rat liver thyroid hormone nuclear receptor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7064–7068. doi: 10.1073/pnas.77.12.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parl F., Korcek L., Siegel J. S., Tabachnick M. Uptake of triiodothyronine and thyroxine by isolated rabbit adipocytes. FEBS Lett. 1977 Nov 1;83(1):145–147. doi: 10.1016/0014-5793(77)80660-1. [DOI] [PubMed] [Google Scholar]
- Pliam N. B., Goldfine I. D. High affinity thyroid hormone binding sites on purified rat liver plasma membranes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):166–172. doi: 10.1016/0006-291x(77)90075-4. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Eckel J., Rao M. L., Breuer H. Uptake of thyroid hormone by isolated rat liver cells. Biochem Biophys Res Commun. 1976 Nov 8;73(1):98–104. doi: 10.1016/0006-291x(76)90502-7. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Sakurada T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science. 1980 Oct 17;210(4467):340–342. doi: 10.1126/science.7423197. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O. Thyroid hormone binding by a component of mitochondrial membrane. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3225–3229. doi: 10.1073/pnas.72.8.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]