Abstract
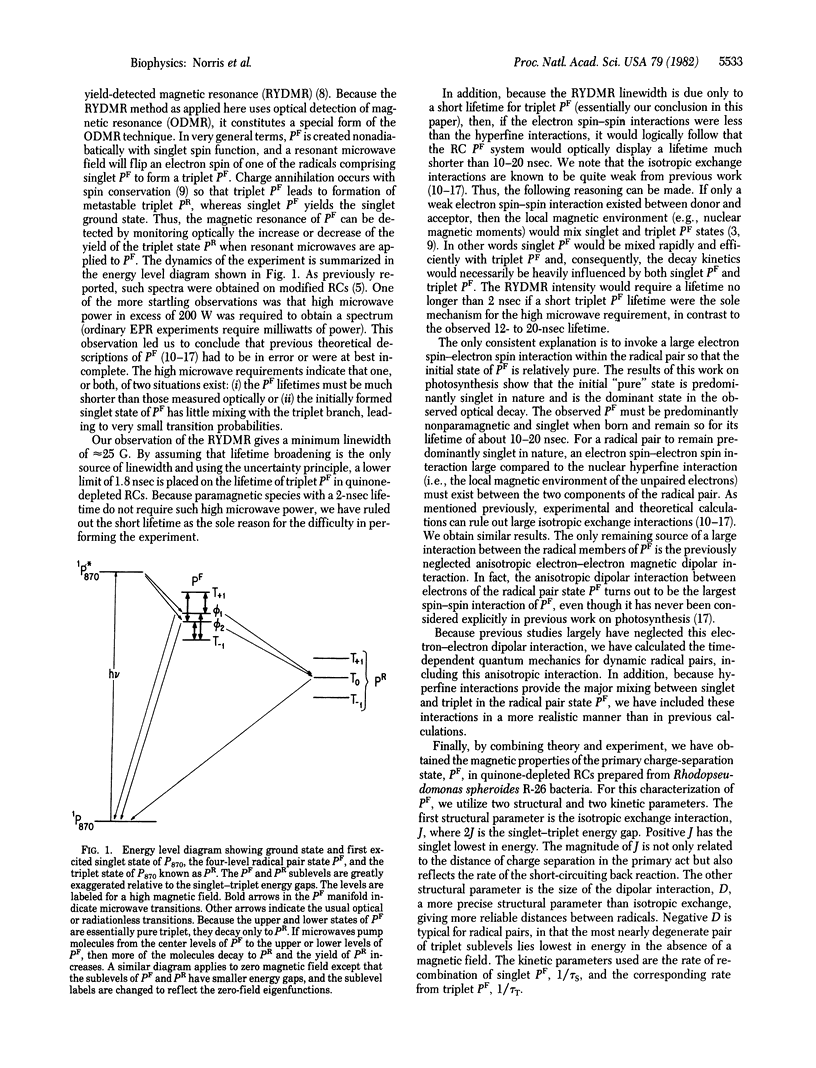
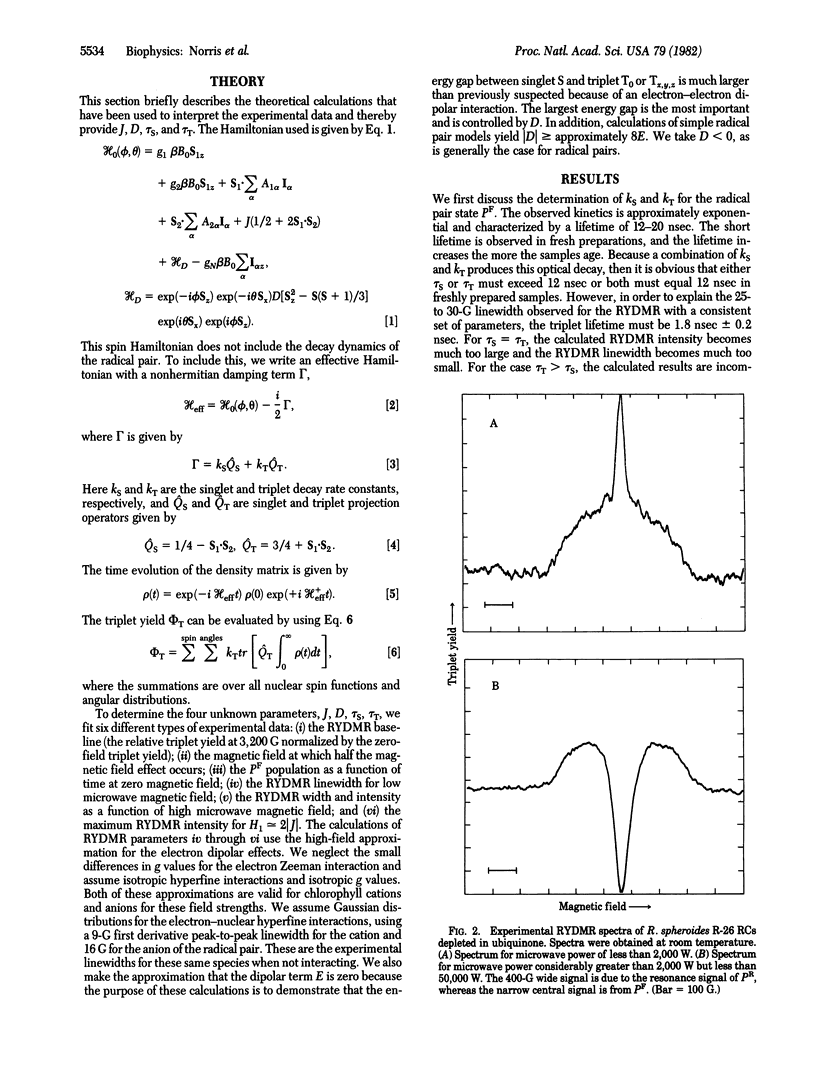
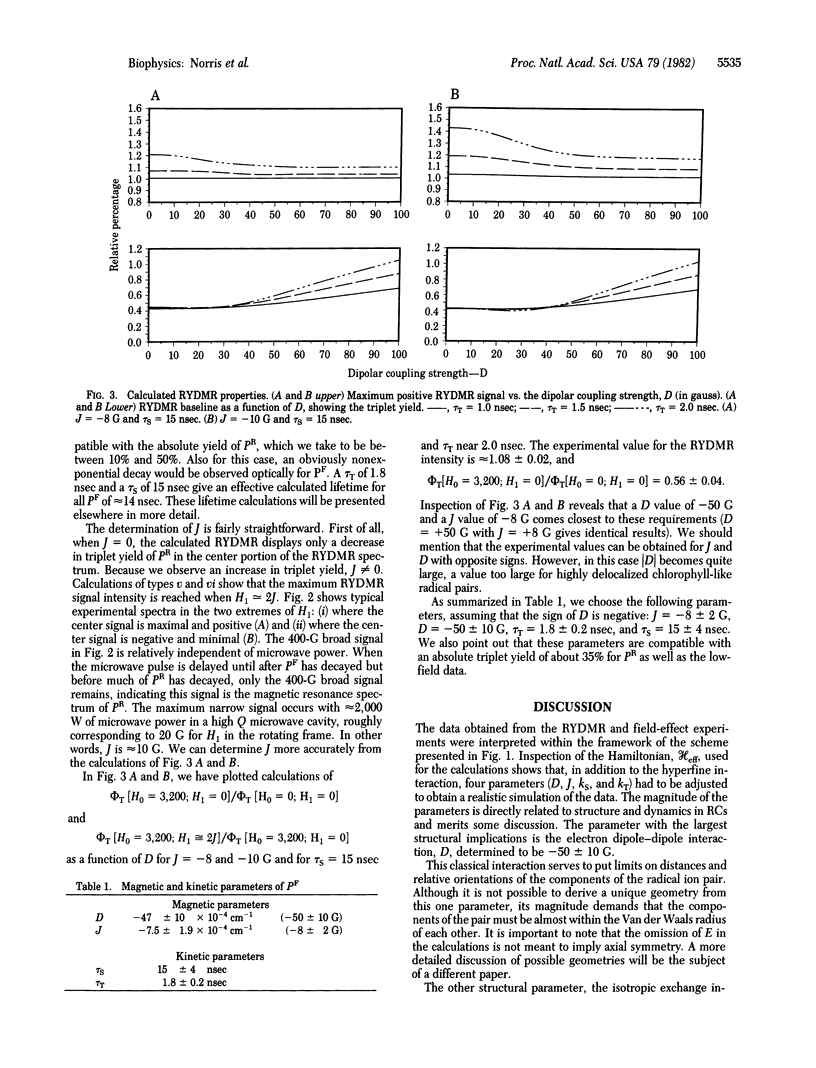
The results of reaction yield-detected magnetic resonance (RYDMR) experiments carried out on modified bacterial photosynthetic reaction centers (RCs) are interpreted in terms of a model that assigns the initial charge-separated radical ion-pair state, PF, as the carrier of the spectrum. The radical pair theory, which has been invoked to explain magnetic field effects in RCs, was significantly expanded to take into consideration the electron dipole-dipole interaction. It is shown that this is the largest interaction between the components of the radical ion pair. Quantum statistical calculations are described simulating the RYDMR spectra and low-field effects in quinone-depleted RCs. The experimental data on which the simulations are based are (i) the magnitude of the field effect at 3,000 G, (ii) the field at which 0.5 of the maximal field effect is observed, (iii) the PF population as a function of time at zero magnetic field, (iv) the RYDMR linewidth for low microwave field strength, (v) the RYDMR intensity and width as a function of microwave field, and (vi) the maximum RYDMR intensity at HI ≃ 2ǀJǀ. With this information it was found possible to characterize PF in terms of four parameters, two containing structural information and two with kinetic implications. These are the dipole-dipole interaction, D = -47 ± 10 × 10-4 cm-1; the exchange interaction, J = -7.5 ± 1.9 × 10-4 cm-1; and the inverse rate constants of the decay of the radical pair states with singlet and triplet spin functions, respectively, kS-1 = 15 ± 4 nsec and kT-1 = 1.8 ± 0.2 nsec. The structural and dynamic implications of these parameters are discussed.
Keywords: primary radical pair, reaction yield-detected magnetic resonance, optically detected magnetic resonance, electron-electron dipolar interaction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman M. K., Budil D. E., Closs G. L., Kostka A. G., Wraight C. A., Norris J. R. Magnetic resonance spectroscopy of the primary state, P, of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3305–3307. doi: 10.1073/pnas.78.6.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E., Marcus R. A. On spin-exchange and electron-transfer rates in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4185–4188. doi: 10.1073/pnas.76.9.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E. On the mechanism of magnetic field effects in bacterial photosynthesis. Biophys J. 1979 Jun;26(3):489–498. doi: 10.1016/S0006-3495(79)85266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Michel-Beyerle M. E., Scheer H., Seidlitz H., Tempus D., Haberkorn R. Time-resolved magnetic field effect on triplet formation in photosynthetic reaction centers of Rhodopseudomonas sphaeroides R-26. FEBS Lett. 1979 Apr 1;100(1):9–12. doi: 10.1016/0014-5793(79)81120-5. [DOI] [PubMed] [Google Scholar]
- Ogrodnik A., Krüger H. W., Orthuber H., Haberkorn R., Michel-Beyerle M. E., Scheer H. Recombination dynamics in bacterial photosynthetic reaction centers. Biophys J. 1982 Jul;39(1):91–99. doi: 10.1016/S0006-3495(82)84494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnauer M. C., Katz J. J., Norris J. R. The triplet state in bacterial photosynthesis: Possible mechanisms of the primary photo-act. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3270–3274. doi: 10.1073/pnas.72.9.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H. J., Schulten K., Weller A. Electron transfer and spin exchange contributing to the magnetic field dependence of the primary photochemical reaction of bacterial photosynthesis. Biochim Biophys Acta. 1978 May 10;502(2):255–268. doi: 10.1016/0005-2728(78)90047-6. [DOI] [PubMed] [Google Scholar]



