Abstract
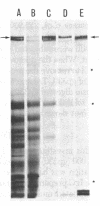
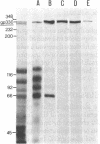
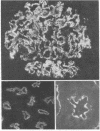
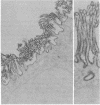
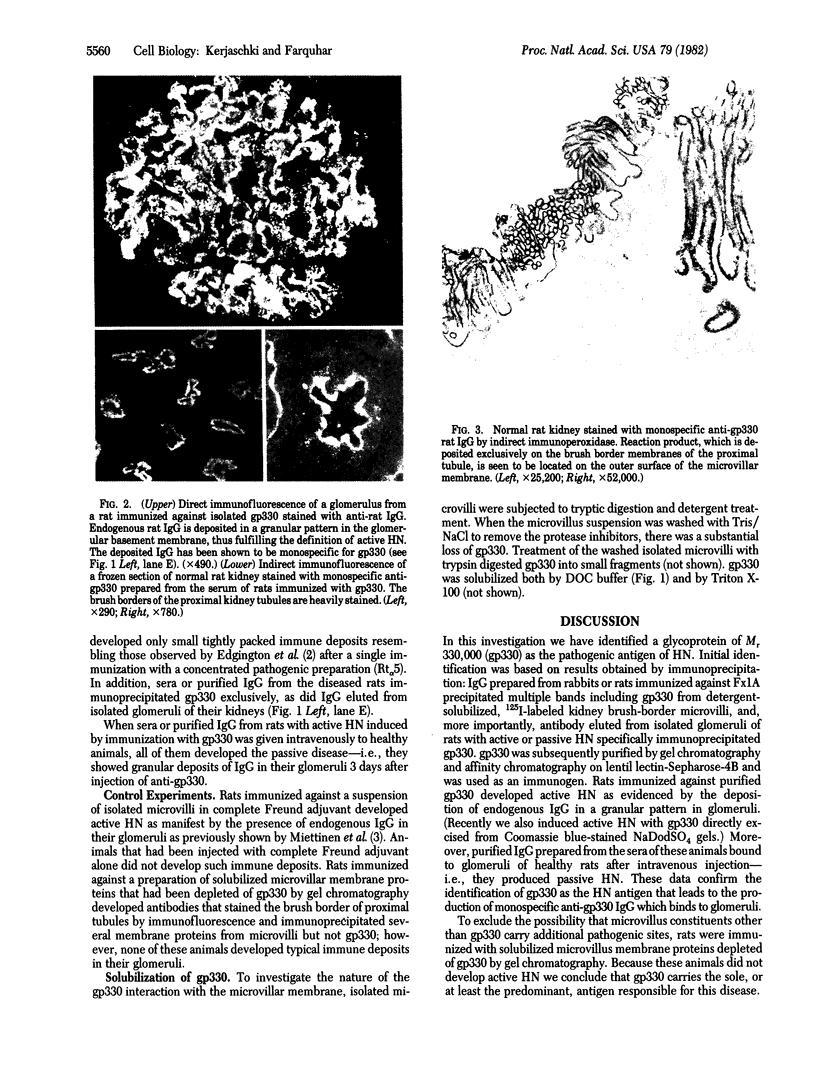
Purified brush border fractions prepared from rat kidneys were solubilized in detergent, iodinated, and subjected to immunoprecipitation to identify the pathogenic antigen present in brush border membranes that is responsible for the production of Heymann nephritis (HN). Purified IgG prepared from the sera of rabbits or rats immunized with a crude cortical preparation, known as Fx1A, precipitated multiple peptides, whereas IgG eluted from glomeruli of rats with active or passive HN specifically immunoprecipitated a single large glycoprotein (Mr = 330,000). This protein (gp330) was subsequently purified by gel filtration and lentil lectin affinity chromatography from detergent-solubilized brush border membranes. When rats were immunized with purified gp330, they developed anti-brush border antibodies and active HN. IgG prepared from the serum of rats with active HN caused passive HN when injected into normal recipients. Rats immunized against brush border membrane proteins depleted of gp330 developed anti-brush border antibodies but did not develop HN. Further analysis of gp330 indicated that it is solubilized by detergent treatment of isolated brush border microvilli, and its antigenic component is released from intact microvilli by trypsin. By immunoperoxidase staining it was localized to the luminal side of the brush border membranes. These results indicate that (i) gp330 is the pathogenic antigen of HN; (ii) the antigen is a glycoprotein of the brush border membrane; and (iii) it is disposed with its pathogenic domain(s) facing the tubule lumen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabas A. Z., Lannigan R. Induction of an autologous immune-complex glomerulonephritis in the rat by intravenous injection of heterologous anti-rat kidney tubular antibody. I. Production of chronic progressive immune-complex glomerulonephritis. Br J Exp Pathol. 1974 Feb;55(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Nolin L., Foidart J., Vandewalle A., Verroust P. The effect of puromycin on subepithelial deposits induced by antibodies directed against tubular antigens: a quantitative study. Eur J Clin Invest. 1979 Dec;9(6):465–472. doi: 10.1111/j.1365-2362.1979.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. B., Andres G. A., McCluskey R. T. Lack of evidence for a role of renal tubular antigen in human membranous glomerulonephritis. Nephron. 1981;27(6):297–301. doi: 10.1159/000182074. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Kanwar Y. S., Hynes R. O., Farquhar M. G. Fibronectin localization in the rat glomerulus. J Cell Biol. 1980 Dec;87(3 Pt 1):691–696. doi: 10.1083/jcb.87.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science. 1967 Mar 17;155(3768):1432–1434. doi: 10.1126/science.155.3768.1432. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. II. K+-dependent p-nitrophenyl phosphatase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jun 29;121(2):375–385. [PubMed] [Google Scholar]
- Feenstra K., van den Lee R., Greben H. A., Arends A., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. I. The natural history: a histologic and immunohistologic study at the light microscopic and the ultrastructural level. Lab Invest. 1975 Feb;32(2):235–242. [PubMed] [Google Scholar]
- Glassock R. J., Edgington T. S., Watson J. I., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. II. The pathogenetic mechanism. J Exp Med. 1968 Mar 1;127(3):573–588. doi: 10.1084/jem.127.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Grupe W. E., Kaplan M. H. Demonstration of an antibody to proximal tubular antigen in the pathogenesis of experimental autoimmune nephrosis in rats. J Lab Clin Med. 1969 Sep;74(3):400–409. [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makker S. P. Evidence that the antigen of autologous immune complex glomerulonephritis of rats is a mannose- or glucose-containing glycoprotein. Proc Soc Exp Biol Med. 1980 Jan;163(1):95–99. doi: 10.3181/00379727-163-40729. [DOI] [PubMed] [Google Scholar]
- Makker S. P., Moorthy B. In situ immune complex formation in isolated perfused kidney using homologous antibody. Lab Invest. 1981 Jan;44(1):1–5. [PubMed] [Google Scholar]
- Malathi P., Preiser H., Fairclough P., Mallett P., Crane R. K. A rapid method for the isolation of kidney brush border membranes. Biochim Biophys Acta. 1979 Jun 13;554(1):259–263. doi: 10.1016/0005-2736(79)90023-3. [DOI] [PubMed] [Google Scholar]
- Miettinen A., Törnroth T., Tikkanen I., Virtanen I., Linder E. Heymann nephritis induced by kidney brush border glycoproteins. Lab Invest. 1980 Dec;43(6):547–555. [PubMed] [Google Scholar]
- Naruse T., Fukasawa T., Hirokawa N., Oike S., Miyakawa Y. The pathogenesis of experimental membranous glomerulonephritis induced with homologous nephritogenic tubular antigen. J Exp Med. 1976 Nov 2;144(5):1347–1362. doi: 10.1084/jem.144.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse T., Fukasawa T., Miyakawa Y. Laboratory model of membranous glomerulonephritis in rats induced by pronase-digested homologous renal tubular epithelial antigen. Lab Invest. 1975 Aug;33(2):141–146. [PubMed] [Google Scholar]
- Neale T. J., Couser W. G., Salant D. J., Lowenstein L. M., Wilson C. B. Specific uptake of Heymann's nephritic kidney eluate by rat kidney: studies in vivo and in isolated perfused kidneys. Lab Invest. 1982 Apr;46(4):450–453. [PubMed] [Google Scholar]
- Neale T. J., Wilson C. B. Glomerular antigens in Heymann's nephritis: reactivity of eluted and circulating antibody. J Immunol. 1982 Jan;128(1):323–330. [PubMed] [Google Scholar]
- Payne J. W. Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem J. 1973 Dec;135(4):867–873. doi: 10.1042/bj1350867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Darby C., Couser W. G. Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest. 1980 Jul;66(1):71–81. doi: 10.1172/JCI109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki T., Klassen J., Andres G. A., Milgrom F., McCluskey R. T. Passive transfer of Heymann nephritis with serum. Kidney Int. 1973 Feb;3(2):66–73. doi: 10.1038/ki.1973.13. [DOI] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]