Abstract
The creation of sexual dimorphism in the gonads is essential for producing the male and female gametes required for sexual reproduction. Sexual development of the gonads involves both somatic cells and germ cells, which often undergo sex determination by different mechanisms. While many sex-specific characteristics evolve rapidly and are very different between animal species, gonad function and the formation of sperm and eggs appear more similar and may be more conserved. Consistent with this, the doublesex/mab3 Related Transcription factors (DMRTs) are important for gonad sexual dimorphism in a wide range of animals, including flies, worms and mammals. Here we explore how sexual dimorphism is regulated in the Drosophila gonad, focusing on recent discoveries relating to testis development. We will discuss how sex determination in both the germline and the soma are utilized to create a testis, including the role of the key somatic sex determination factor doublesex.
Keywords: DMRT, Drosophila, doublesex, germ cell, germline stem cell, gonad, sexual dimorphism, stem cell niche, testis
The Drosophila testis is an attractive model for understanding how sexual identity impacts the processes of organogenesis to create sexual dimorphism. Embryogenesis is only 24 h in this species and the currently known cell types of the adult testis form during this time, including a fully functional gonad stem cell niche. The pathway that initiates sexual identity in the soma is largely understood, and we are making progress on understanding how sexual identity is specified in the germline. The challenge now is to elucidate the mechanisms by which sexual identity is used by these cell types to control sex-specific development and organogenesis. This review will focus on the creation of sexual dimorphism in the gonad and the development of the testis. We will focus on more recent work rather than on describing important groundwork laid by older studies, for which we will refer to previous reviews.
Formation of the Embryonic Gonad
In most animals, the somatic support cells that will house and nurture the germ cells during gametogenesis are formed from the mesoderm during development. In Drosophila, the somatic gonadal precursors (SGPs) are specified using the anterior/posterior (A/P) and dorsal/ventral (D/V) patterning systems that divide the mesoderm into distinct cell types (reviewed in ref. 1). Three clusters of ≈12 SGPs each will form on either side of the embryo in parasegments (PSs) 10–12 (ref. 2, Figure 1) (“parasegments” are the units of segmental identity along the A/P axis). Each mesodermal PS is divided into an anterior (“even skipped (eve) domain”) and posterior (“sloppy paired domain”). SGPs form within the eve domain while in other PSs this domain gives rise to the fat body.3,4 The D/V axis is also divided into distinct domains, and the SGPs in PS10–12 form within the dorso-lateral domain, below the dorsal domain that gives rise to the heart and visceral mesoderm. The transcription factors Eyes Absent (EYA) and Zn Finger Homeodomain 1 (ZFH-1) are expressed in this domain and are critical for SGP and fat body specification (see refs. 3–5, reviewed in ref. 1).
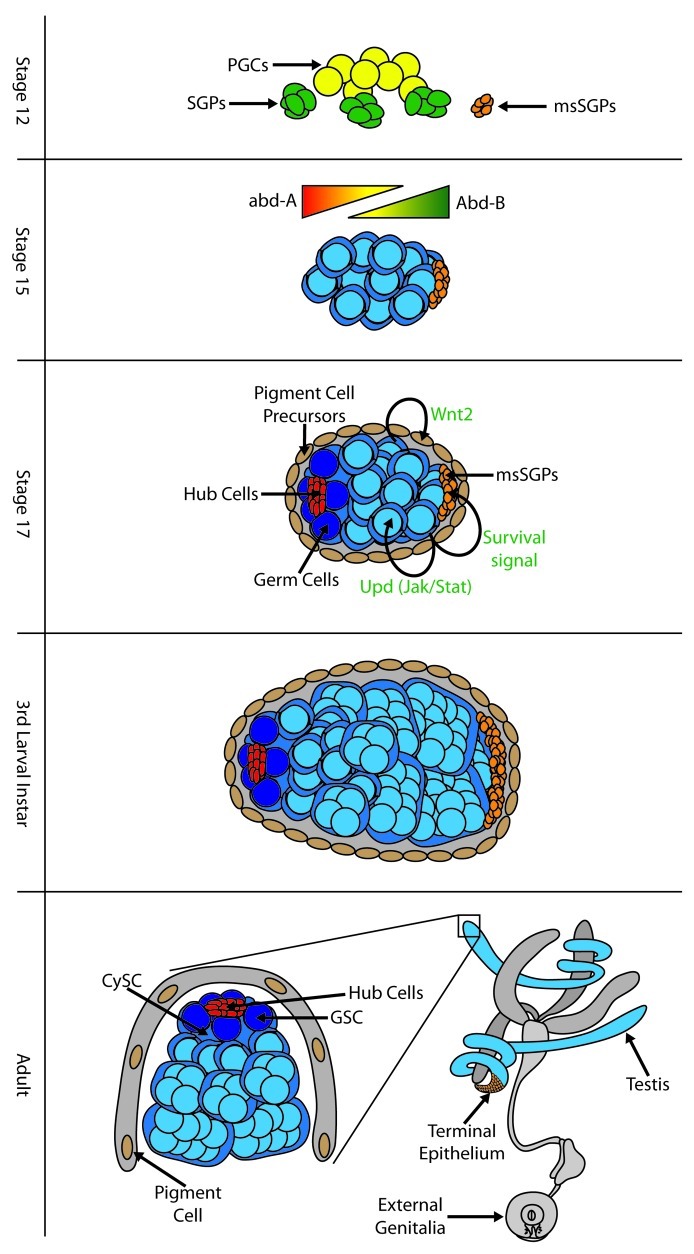
An additional cluster of SGPs forms in PS13, but is located more ventrally in the mesoderm and does not depend on the genes that form the dorso-lateral domain.6 However, these cells do share characteristics of SGP identity, such as expression of EYA, Six4 and Doublesex.6-8 These are known as “male-specific SGPs” (msSGPs) since they will only survive in males (see below). Thus, each gonad in males is initially formed from 4 separate clusters of SGPs.
The homeotic (HOX) genes provide different parasegments with their unique identities (Fig. 1). During SGP specification, the homeotic gene abdominal A (abd-A) determines which parasegments of the dorso-lateral mesoderm will form SGPs, and which will form fat body.3,4,9 In addition, the individual SGP clusters have distinct identities depending on their pattern of homeotic gene expression; anterior SGPs (PS 10) express ABD-A, posterior SGPs (PS 12) express both ABD-A and Abdominal B (ABD-B), while msSGPs (PS13) express only ABD-B10,11 (the exact A/P identity of the PS 11 SGPs has not been defined). Correspondingly, anterior and posterior SGPs express different molecular markers (e.g., anterior SGPs express escargot while posterior SGPs express Wnt2 and higher levels of EYA),10,11 indicating their distinct identities.
Figure 1. A summary of testis development in Drosophila.
The SGPs must join with one another and incorporate the germ cells to form the embryonic gonad. The fascinating processes of germ cell formation and migration have been extensively reviewed12,13 and so we will not discuss them in detail. Similarly, the SGPs also migrate to form a single, cohesive tissue, and extend cellular processes to ensheath the germ cells. This involves adhesion molecules such as DE-cadherin,14 and is also regulated by the transcription factor Traffic Jam, which regulates SGP behavior but not initial SGP identity.15 These processes have also been recently reviewed.1 All of this has occurred by the mid-point in embryogenesis (stage 14, 12 h after fertilization), resulting in an embryonic gonad with germ cells along with differentially patterned SGPs, creating the foundation for sex-specific development of the testes and ovaries.
Establishing Sexual Identity in the Gonad
The somatic gonad
For sexually dimorphic development to occur, cells must establish a male or female identity that will govern their sex-specific development. The mechanism for initiating this identity in Drosophila somatic cells is well understood, although the mechanisms by which sexual identity leads to sex-specific control of development are still being elucidated. Sexual identity is controlled by the sex chromosome genotype (XX female, XY male), which in flies depends on the number of X chromosomes (reviewed in refs. 16, 17). Somatic cells with two X’s activate expression of the switch gene Sex lethal (Sxl), which encodes an RNA binding protein that influences both alternative splicing and translation of target RNAs (reviewed in ref. 17). For somatic sexual identity, SXL regulates alternative splicing of transformer (tra) mRNA, such that a functional TRA protein is produced only in females. TRA, together with the non-sex specific splicing factor Transformer 2, acts in the alternative splicing of two downstream targets, doublesex (dsx) and fruitless (fru), while these genes undergo default splicing in males. The result is the formation of different isoforms of DSX protein in males and females (DSXM and DSXF), while a functional FRU protein is only made in males. Though this pathway has been established for many years, few targets for the DSX or FRU transcription factors are known and the mechanism by which these genes act to translate sexual identity into sex-specific development is still largely unknown.
The earliest manifestations of sexual dimorphism in the gonad are observed at the time of gonad formation, when sex-specific gene expression is observed in SGPs,18,19 male SGPs signal to the germ cells through the JAK/STAT pathway,20 and the msSGPs undergo apoptosis specifically in females.6 At this time, dsx is expressed specifically in SGPs,8,18 while fru expression is not observed. Consistent with this, dsx controls all aspects of initial sexual dimorphism in gonad development so far described (discussed below). This is similar to the expression pattern observed for dsx homologs in other animal species (the dsx/mab3 related transcription factors, DMRTs). The DMRTs play a role in sex-specific gonad development in many animal species examined, including mammals, and a human syndrome of gonad sex reversal has been linked to deletions affecting DMRTs (reviewed in ref. 21). Thus, it appears that dsx plays an ancient role in the control of gonad sexual dimorphism.
The highly tissue-specific pattern of dsx expression in the gonad during embryogenesis illustrates that not all cells of the animal “know” their sex. In order for a cell to translate its sex chromosome constitution into information it can use to control sex-specific development, it needs to express a transcription factor, such as DSX or FRU, which is regulated by the sex chromosome genotype. While all cells may need to “count” their X chromosomes in order to properly conduct X chromosome dosage compensation, cells are on a “need to know basis” regarding their sexual identity; only those cells that need to undergo sex-specific development express DSX or FRU.8,18,22-26 Indeed, it is detrimental, even lethal, for an animal to express DSX where it is not supposed to be expressed.27,28
The germ cells
In contrast to our understanding of how sexual identity is initiated in the soma, we know little about how this process is regulated in the germline. Germline sex determination is regulated by two important inputs: autonomous cues derived from the sex chromosome genotype of the germ cells combined with non-autonomous signals from the surrounding soma (Fig. 2, reviewed in refs. 29, 30). Consequently, in Drosophila, the “sex” of the germline must match the “sex” of the soma in order for proper germline development to occur. Neither XY germ cells present in a female soma nor XX germ cells developing in a male soma can give rise to gametes, and these situations often result in a severe germline degeneration.
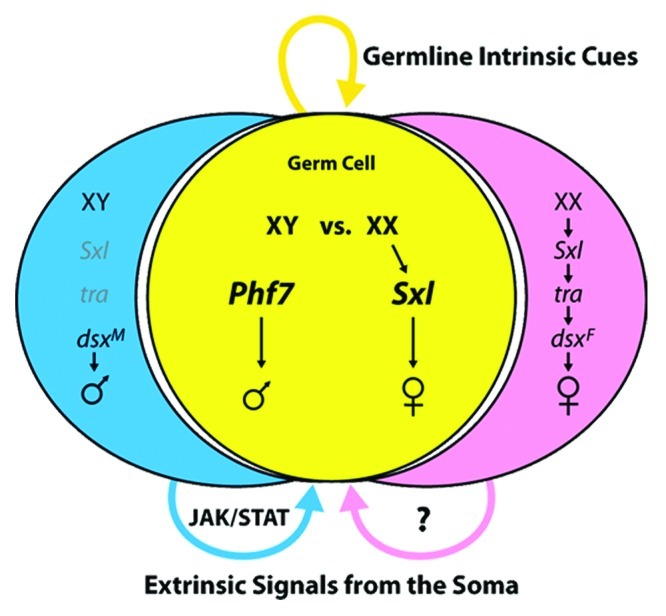
Figure 2. A simplified view of sex determination in the somatic gonad and germline.
Sex determination in the germline is manifested early in germ cell development,31 at the time that the germ cells first become generally competent for zygotic transcription.32 Female-specific activation of Sxl in the germ cells is observed at this time, prior to their migration and contact with SGPs.31 Soon after, the germ cells reach the SGPs and exhibit male-specific activation of the JAK/STAT pathway.20 Once the embryonic gonad forms, sex-specific expression of a number of genes is observed in the germline, indicating that their sexual identity has been robustly specified by this time.19,33
As in the soma, autonomous control of germline sex determination depends on Sxl, but the regulation of Sxl and its mechanism of action are quite different in the germline (reviewed in refs. 17, 29, 30). Germ cells with two X chromosomes autonomously activate expression of Sxl, independent of their somatic environment. The X chromosome genes that activate Sxl in the soma are not required for its activation in the germline,19,34,35 and how Sxl is regulated in the germline remains unknown. The key targets for regulation by SXL in the soma are also not required in the germline.36,37 Until recently, the role of Sxl as a sexual “switch” gene in the germline was still in question. While expression of Sxl in a male soma is sufficient to create a female identity in those cells, forced expression of Sxl in male germ cells has surprisingly little effect.31,38 However, recent work demonstrates that Sxl expression is sufficient to feminize XY germ cells and allow them to form eggs, provided they are transplanted into a female somatic environment.31 Thus, Sxl is capable of providing the autonomous female sexual identity that would normally be derived from the presence of two X chromosomes. However, such germ cells must receive proper signals from a female soma in order to develop properly as female germ cells.
Signals from both the male and female soma are required for germline sex determination. An important signal from the male somatic gonad acts through the JAK/STAT pathway to promote and maintain male germline identity.20 Germ cells receive this signal as they contact male SGPs during their migration, and both XY and XX germ cells are competent to respond to this signal. Initially all germ cells exhibit activation of the JAK/STAT pathway before this signal becomes restricted to the germline stem cells as they form.20,39 Evidence also supports a role for an important signal from the female soma to control germline sexual identity,19.33 but the identity of this signal is currently unknown.
Recent work has identified a new factor, Plant Homeodomain Finger protein 7 (PHF7) that plays an important role in regulating male germline identity, about which little was known.40 Phf7 is expressed specifically in male germ cells in the embryonic gonad and in the adult testis. The expression of Phf7 is largely dependent on signals from the soma, indicating it is a key target by which somatic signals influence germline sex determination. Loss of Phf7 disrupts proper male identity in the germ cells, and ectopic expression of Phf7 is toxic to female germ cells unless they are present in a male soma. Phf7 is even able to induce XX germ cells to make sperm when present in a testis. Phf7 encodes a chromatin-associated protein that specifically recognizes epigenetic marks on histone H3, indicating that global epigenetic regulation of the germline genome is important for proper germline sex determination. Phf7 also has clear homologs in other animals, including mouse and humans, that are also specifically expressed in the male germline, and human Phf7 can rescue Phf7 mutants in Drosophila. Thus, Phf7 represents an evolutionarily conserved epigenetic “reader” that may play a role in germline sex determination in diverse species.40
Testis Development
The embryonic gonad has a number of distinct cell types, including germ cells, anterior SGPs, posterior SGPs and msSGPs. Further, these cells have a clear sexual identity, as indicated by the expression of a number of sex-specific molecular markers. In the next stage of gonad development, this information is then combined to allow sexually dimorphic formation of the ovaries and testes. Here we will focus on testis development. One interesting difference between testis and ovary development is that the testis is largely complete by the end of embryogenesis or the early larval stages (24–30 h after fertilization), while morphogenesis of the ovary occurs later during the larval-pupal transition (5 d after fertilization).
The male-specific SGPs
One of the first sexual dimorphisms to be identified in the developing gonad was the presence of the msSGPs, which are found in the posterior of the embryonic gonad in males but not females (ref. 6, Figure 1). In addition to expression of molecular markers characteristic of SGPs, the msSGPs express the transcription factor SOX100B [6]. It is remarkable that SOX100B is the fly homolog of mammalian Sox9, a protein that is essential for sex determination in the gonads of mouse and human (reviewed in ref. 41). Sox100B mutants exhibit severe defects in testis development but show no defects in the ovary.42 Thus, this transcription factor is critical for gonad sexual dimorphism in diverse species. The msSGPs ultimately give rise to the terminal epithelium of the testis,42 which is critical for the final differentiation of spermatids, and likely also plays a role in connecting the testis to other portions of the reproductive tract.
While msSGPs are only found in the male gonad, these cells are initially present in both sexes prior to gonad formation but undergo sex-specific apoptosis in females.6 In males, these cells actively migrate to join the SGPs and germ cells in forming the gonad.43 If programmed cell death in the msSGPs is blocked in females, these cells survive and join the gonad, indicating that sex-specific apoptosis is the key step controlling their sexually dimorphic development.6 Interestingly, even though these cells express DSX,8,18 they do not decide for themselves whether to behave as male or female, but receive their instructions from the other SGPs. Experiments with sexual mosaics indicate that the behavior of the msSGPs depends on the sex of the SGPs, rather than on the sex of the msSGPs. Further, in the absence of SGPs, the msSGPs die in both sexes, indicating that a sex-specific survival cue from male SGPs is what determines the fate of the msSGPs.18
The behavior of the msSGPs highlights a general principle of how sexual dimorphism is created in the gonad; many cell types do not decide for themselves what sex they should be, but rather receive signals from other cell types, namely the SGPs, about what sexual path they should follow. This is true for the msSGPs and is also true for the pigment cell precursors and the germ cells (Fig. 1). This is contrary to the commonly-held belief that, in Drosophila, “all cells decide for themselves” what sex they should be.44 Thus, the creation of sexual dimorphism in the fly gonad is similar to gonad development in mammalian systems, where non-autonomous sex determination is known to be common.
The pigment cell precursors
An additional male-specific cell type arises in the testis during the last stage of embryogenesis (stage 17, ≈20 h after fertilization). These cells were originally discovered because they also express SOX100B and were thought to be derived from the msSGPs, but were found to be of independent origin.18 They surround the outside of the embryonic gonad and give rise to the pigment cells that ensheath the adult testis and parts of the reproductive tract. While the function of these cells is not clear, their relationship to the fat body suggests they may influence testis function by means such as hormonal signaling.
Interestingly, the pigment cell precursors provide another example of non-autonomous sex determination in the developing gonad. These cells are not part of the embryonic gonad at the time of its formation, but are specified from the surrounding fat body via male-specific signaling from the SGPs.18 SGPs express Wnt2 in a male-specific manner under control of the somatic sex determination pathway, and Wnt2 is necessary and sufficient for formation of pigment cell precursors.18,45 The sex of the fat body itself does not influence this process; both male and female fat body are competent to produce pigment cell precursors when provided with the Wnt2 signal.18,45 Further, male-specific pigment cell precursor formation is under control of dsx,18 which is expressed in SGPs but not the pigment cell precursors themselves.8 Instead, dsx regulates Wnt2 expression in the SGPs.18 The sex-specific recruitment of neighboring cell types to become part of the developing gonad is a common feature of gonad development in different animals, such as in the recruitment of mesonephric cells into the mouse testis, a process which is controlled by the sex of the gonad rather than the sex of the mesonephros (reviewed in ref. 46).
The testis stem cell niche
One of the more interesting aspects of testis development is the formation of the germline stem cells (GSCs) and the somatic environment, or “niche,” that regulates them. GSCs are critical for the production of large numbers of gametes throughout an extended period of adult life. There is clear sexual dimorphism in gonad stem cell development. In Drosophila, both the testis and the ovary have GSCs, as well as somatic stem cells that produce the differentiated somatic cells that nurture the germline during gametogenesis. However, there are many differences between the male and female GSCs, somatic stem cells, and the niches that control these stem cells. In other species, such as mouse and human, there is a clear GSC population only in the testis, and the ovary has a more limited capacity to produce gametes. How sexual dimorphism in both the soma and the germline lead to differences in the gonad stem cell systems is thus an important question for understanding sex-specific development and reproductive health.
The testis stem cell system is located at the proximal end of the testis, and is organized around an important cluster of somatic cells that make up the “hub” (ref. 47, Figure 1). The GSCs are arranged around the hub, along with the Cyst Stem Cells (CySCs), the somatic stem cells that produce the cyst cells that surround the germline during spermatogenesis (see reviews on GSCs and CySCs by Matunis and Schultz, this issue). The hub acts as a key organizing center by adhering to the stem cells, allowing them to remain in the niche and regulating their pattern of cell division. The hub also signals to the stem cells through multiple signaling pathways, including JAK/STAT, TGF-β and other pathways (Matunis and Schultz, this issue). In addition to producing the cyst cells, the CySCs also form an important part of the niche that regulates the GSCs.48
The hub forms during the last stage of embryogenesis (Stage 17, ≈20–24 h after fertilization) from a subset of SGPs in the anterior region of the embryonic gonad.49 Initially, SGPs with a combination of anterior and male identities express unique molecular markers such as escargot. Subsequently, a subset of these anterior male cells coalesce to form a tightly associated cluster of cells that express many of the molecular markers also expressed in the adult hub. This includes expression of several cell-cell adhesion molecules (DE-cadherin, DN-cadherin, Fasciclin 3) that are likely to mediate the sorting of these cells away from other SGPs and into the tight cluster of the embryonic hub.49 A subset of germ cells associate with the embryonic hub as it forms and take on GSC identity.39 Although all germ cells in the male gonad initially exhibit activation of the JAK/STAT pathway,20 this response becomes restricted to those germ cells associated with the hub.39 Further, the hub-proximal germ cells exhibit the oriented divisions characteristic of GSCs and produce progeny that enter differentiation.39 Thus, by the embryo/larval transition, a fully functional stem cell niche has formed in the testis.
Since differentiating spermatogonial cysts are observed soon after formation of the hub and GSCs, it is likely that the CySCs also form at this time. However, no specific markers are available that distinguish these cells at this early time point. Lineage analysis indicates that the CySCs are derived from the same pool of anterior SGPs that form the hub (PS10 and PS11 SGPs).50 It is possible that posterior SGPs (PS12) associate with those germ cells that are not selected to become GSCs to directly form spermatogonial cysts (“one shot spermatogenesis”).39 However, it is also possible that posterior SGPs utilize their unique identity to contribute to some other cell type in the adult testis that remains to be identified.
If the same pool of anterior SGPs (PS10 and PS11) give rise to hub cells and CySCs,49,50 how then is the hub vs. CySC decision made such that the correct number of hub cells form? Recent work indicates that the transcription factor Bowl is important for this decision.50 Fewer hub cells are formed in bowl mutants, while removing an inhibitor of bowl, known as lines, increases hub cell number. Thus, bowl appears to act as a “pro-hub” factor in the hub vs. CySC decision. Interestingly, when bowl is activated by loss of lines in adult CySCs, they take on some hub cell character, indicating that the hub cells and CySCs remain closely related and their distinction must be maintained even in the adult testis.50
It is also clear that cell-cell signaling is important for determining which SGPs will form hub cells. Signaling between somatic cells via the Notch pathway regulates hub cell number, with Notch mutants showing a strong decrease in hub cells.51,52 Notch is expressed broadly in SGPs, and evidence for Notch activity is seen in SGPs in both anterior and posterior regions of the gonad.51,52 Thus, it is likely that anterior SGPs that have activated Notch will form hub cells, while those that do not form CySCs. Since Notch is known to upregulate bowl in other contexts, one possibility is that Notch acts at least in part through bowl to specify hub cell identity.50 However, there is disagreement about the nature and location of the ligands for the Notch pathway that control hub formation. Kitadake et al. observe that Serrate is expressed in SGPs and is the major ligand affecting hub formation.51 However, Okebe and DiNardo observe that the Delta ligand plays the major role, with a lesser role for Serrate, but that neither is expressed in the gonad.52 Instead, they postulate that Delta expression in the neighboring midgut signals to the SGPs. Further work is needed to resolve which somatic cells are important for activating Notch signaling to control hub cell specification.
Signaling from the germ cells to the SGPs through receptor tyrosine kinases (RTKs) is also involved in hub cell specification.51,53 Posterior SGPs express the Sevenless (SEV) RTK, while all SGPs express the Epidermal Growth Factor receptor (EGFR). Loss of either or both of these RTKs increases the number of SGPs that take on hub identity, but there is still a clear bias toward formation of hub cells from anterior SGPs, indicating that the anterior/posterior patterning of the SGPs is the major determinant for which SGPs can form hub cells. Ligands for each of these receptors (BOSS and Spitz) are expressed in germ cells, suggesting that germ cells limit the production of an important component of their own stem cell niche, the hub. One intriguing idea is that this allows the system to compensate for situations where fewer germ cells reach the gonad.51 When germ cells are plentiful, they restrict the number of cells that take on hub identity and allow for sufficient CySC production. However, when few germ cells are present, hub cell number is increased to ensure that some germ cells contact hub cells and become germline stem cells. Indeed, when germ cell number is reduced experimentally, this mechanism appears to be important for increasing the likelihood of germline stem cell production and formation of a functional testis.51
One problem with this model is that, if the same pool of cells generates both hub cells and CySCs, when the number of hub cells is increased, CySC number would be decreased. As CySCs and cyst cells are also essential for gametogenesis, it would be counterproductive to increase hub cells at the expense of CySCs unless some other mechanism was in place to ensure CySC production. It is important to note, however, that manipulation of either the Notch or RTK pathways only alters hub cell number by a modest amount. For example, double mutants of both sev and Egfr increase hub cell number from about 9 to about 16 cells from a total of approximately 40 SGPs.51 Thus, these pathways can alter the likelihood that an SGP will choose hub cell identity over CySC identity, but they are unlikely to convert the entire pool of anterior SGPs to hub cells, for the obvious reason that loss of CySC would be disastrous for testis function.
Later steps in testis development
Since the testis contains a functioning stem cell system and begins producing differentiating spermatogonial cysts by the early larval period, little needs to change regarding basic testis function between the larval and adult testes (a period of nine days of development). However, some mutations only affect the testis at the larval/pupal transition (e.g., Sox100B),42 indicating that there may be additional changes or checkpoints that occur at this important developmental transition. This is the time when ovary morphogenesis normally begins in females, and the developmental “timer” that controls ovary development may also trigger changes to the testis.
One important event that happens during the early pupal period is attachment of the testis to the rest of the reproductive tract, which develops from the genital imaginal disc (reviewed in ref. 16). The genital disc derivatives attach to the posterior of the testis where the msSGPs form the terminal epithelium (Fig. 1). In addition, the genital disc is the source of muscle cells that migrate to form a uniform layer around the testis underneath the pigment cell layer.45 Attachment of the genital disc derivatives and the migration of the muscle cells causes the testis to elongate from the spherical shape of the larval testis to the extended testis “tube” of the adult.
The Role of dsx in Controlling Gonad Sexual Dimorphism
As mentioned above, dsx is expressed in the SGPs beginning before gonad formation, and is required for all aspects of somatic gonad sexual dimorphism so far discovered. dsx expression is controlled by genes such as eya that regulate SGP specification.8 Thus, an important part of SGP identity is expression of dsx so that these cells can use information about their sexual identity to allow for sex-specific development.
dsx is regulated by alternative splicing to produce functional proteins, DSXM and DSXF, in the respective sexes. These DSX isoforms have the same DNA binding domain, but their different C-termini allow them to have different effects on gene expression (reviewed in ref. 16). Since dsx is required for proper specification of both male and female identity, we would expect that SGPs mutant for dsx would develop with an intermediate phenotype, neither fully male nor fully female. Indeed, sex-specific gene expression in the SGPs of dsx mutants does appear to be intermediate between the male and female levels (ref. 18, Camara et al., submitted). Surprisingly, however, initial development of the sex-specific cell types described above appears fully male-like in dsx mutants; both XY and XX dsx mutant embryonic gonads have msSGPs, pigment cell precursors and hubs.18.49 This is perhaps understandable for cell types like the msSGPs and pigment cell precursors whose sexual development depends on signals from the SGPs. An intermediate level of a male-specific signal, such as Wnt2, may be sufficient to induce these cells to develop along the male path.
The observation that both XY and XX dsx mutants have hubs is less easy to understand. Data indicate that the sex determination pathway acts in an autonomous manner to specify hub development. In gonads with a mixture of male and female SGPs, the female SGPs do not contribute to the hub.49 Why then would an intermediate sexual identity give rise to male development? Even more curious, when dsx mutant gonads are examined as adults, half of both XY and XX gonads contain hubs while the other half have components of the female GSC niche, the terminal filaments and cap cells (Camara et al., submitted). The hubs and terminal filaments/cap cells are thought to be derived from the same pool of anterior SGPs49,54 and, indeed, it appears that the terminal filaments and cap cells that form later in dsx mutants come from the same cells that initially formed hubs (Camara et al., submitted). Thus, dsx likely acts on the same set of cells to determine whether they form components of the male or female niche. However, dsx is not a “micro-manager” of sexual development that instructs cells and tissues how to form male or female niche structures. Instead, these structures seem to form independent of dsx function, but do so stochastically in the absence of dsx regardless of the sex chromosome genotype. Thus, dsx appears to act only to ensure that the male pathway is activated in XY animals and the female pathway is activated in XX animals.
Future Directions
The above review demonstrates that we are clearly making progress in understanding testis development and how sexual dimorphism is regulated in the gonad. However, considering that sex determination has been studied in Drosophila for decades (reviewed in ref. 55), a surprising number of important unanswered questions remain. For the soma, we understand how the sex chromosome genotype influences the sex-specific function of the key downstream transcription factors DSX and FRU. However, few targets for regulation by DSX are known in any tissue, and none have been identified yet in the gonad. Any role that FRU may play in gonad development is also unknown.
Further, for sex determination in the germline, even basic questions such as how Sxl is activated and what its targets are remain to be answered. Similarly, much remains to be discovered about how sex determination in the soma influences germline sex determination through important signals, such as the unidentified signal from the female soma, and factors activated in the germ cells by these signals, such as Phf7. How the sex of the soma is integrated with the sex of the germ cells, and why the sex of the soma and germline need to match for proper germline development to occur, also remain to be uncovered. The timing of gonad development is also very different in males and females, and it will be interesting to discover how this timing is regulated in a sex-specific manner.
Finally, intriguing parallels have been found between how sexual dimorphism is regulated in the gonads in diverse species, such as flies and mammals. The logic for creating sexual dimorphism was thought to be very different between species, with cells in Drosophila primarily deciding “for themselves” what sex to be in an autonomous manner, while mammals exhibit much more non-autonomous sex determination through local and systemic (hormonal) signals. We now know that this logic of sex determination is much more similar between species, with a great deal of local cell-cell signaling regulating gonad sexual dimorphism in both flies and mammals. Also of interest is the role that dsx homologs (DMRTs) play in the development of gonad sexual dimorphism in a wide range of different animal species.21 In the future, it will be of great interest to explore how diverse species utilize similar mechanisms, and exhibit interesting evolutionary differences, in sex-specific development of the gonads.
Acknowledgments
The authors wish to express their regrets the scope of this review did not allow for inclusion of all the interesting work that has been done on testis development, including important background work that has been laid down through many years. We apologize to those authors whose work was not specifically included in this review. We also wish to thank our many colleagues who study sexually dimorphic development in flies and other systems for the many fruitful interactions and discussions that have occurred in this engaging and open community. Current work cited from the M.V.D. lab is supported by NIGMS (GM084356) and NICHD (HD66244).
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/21780
References
- 1.Jemc JC. Somatic gonadal cells: the supporting cast for the germline. Genesis. 2011;49:753–75. doi: 10.1002/dvg.20784. [DOI] [PubMed] [Google Scholar]
- 2.Brookman JJ, Toosy AT, Shashidhara LS, White RA. The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development. 1992;116:1185–92. doi: 10.1242/dev.116.4.1185. [DOI] [PubMed] [Google Scholar]
- 3.Riechmann V, Rehorn KP, Reuter R, Leptin M. The genetic control of the distinction between fat body and gonadal mesoderm in Drosophila. Development. 1998;125:713–23. doi: 10.1242/dev.125.4.713. [DOI] [PubMed] [Google Scholar]
- 4.Moore LA, Broihier HT, Van Doren M, Lehmann R. Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development. 1998;125:837–44. doi: 10.1242/dev.125.5.837. [DOI] [PubMed] [Google Scholar]
- 5.Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–82. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- 6.DeFalco TJ, Verney G, Jenkins AB, McCaffery JM, Russell S, Van Doren M. Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev Cell. 2003;5:205–16. doi: 10.1016/S1534-5807(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 7.Clark IB, Boyd J, Hamilton G, Finnegan DJ, Jarman AP. D-six4 plays a key role in patterning cell identities deriving from the Drosophila mesoderm. Dev Biol. 2006;294:220–31. doi: 10.1016/j.ydbio.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Hempel LU, Oliver B. Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol. 2007;7:113. doi: 10.1186/1471-213X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greig S, Akam M. The role of homeotic genes in the specification of the Drosophila gonad. Curr Biol. 1995;5:1057–62. doi: 10.1016/S0960-9822(95)00210-7. [DOI] [PubMed] [Google Scholar]
- 10.Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–25. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- 11.DeFalco T, Le Bras S, Van Doren M. Abdominal-B is essential for proper sexually dimorphic development of the Drosophila gonad. Mech Dev. 2004;121:1323–33. doi: 10.1016/j.mod.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–3. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins AB, McCaffery JM, Van Doren M. Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development. 2003;130:4417–26. doi: 10.1242/dev.00639. [DOI] [PubMed] [Google Scholar]
- 15.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 16.Camara N, Whitworth C, Van Doren M. The creation of sexual dimorphism in the Drosophila soma. Curr Top Dev Biol. 2008;83:65–107. doi: 10.1016/S0070-2153(08)00403-1. [DOI] [PubMed] [Google Scholar]
- 17.Salz HK, Erickson JW. Sex determination in Drosophila: The view from the top. Fly (Austin) 2010;4:60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFalco T, Camara N, Le Bras S, Van Doren M. Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev Cell. 2008;14:275–86. doi: 10.1016/j.devcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casper AL, Van Doren M. The establishment of sexual identity in the Drosophila germline. Development. 2009;136:3821–30. doi: 10.1242/dev.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–7. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13:163–74. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J Neurobiol. 2000;43:404–26. doi: 10.1002/1097-4695(20000615)43:4<404::AID-NEU8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol. 2000;2:500–6. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- 24.Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. J Neurogenet. 2002;16:229–48. doi: 10.1080/01677060216292. [DOI] [PubMed] [Google Scholar]
- 25.Sanders LE, Arbeitman MN. Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol. 2008;320:378–90. doi: 10.1016/j.ydbio.2008.05.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jursnich VA, Burtis KC. A positive role in differentiation for the male doublesex protein of Drosophila. Dev Biol. 1993;155:235–49. doi: 10.1006/dbio.1993.1021. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Barmina O, Sanders LE, Arbeitman MN, Kopp A. Evolution of sex-specific traits through changes in HOX-dependent doublesex expression. PLoS Biol. 2011;9:e1001131. doi: 10.1371/journal.pbio.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casper A, Van Doren M. The control of sexual identity in the Drosophila germline. Development. 2006;133:2783–91. doi: 10.1242/dev.02415. [DOI] [PubMed] [Google Scholar]
- 30.Hempel LU, Kalamegham R, Smith JE, 3rd, Oliver B. Drosophila germline sex determination: integration of germline autonomous cues and somatic signals. Curr Top Dev Biol. 2008;83:109–50. doi: 10.1016/S0070-2153(08)00404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science. 2011;333:885–8. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
- 32.Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–6. doi: 10.1016/S0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- 33.Staab S, Heller A, Steinmann-Zwicky M. Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development. 1996;122:4065–71. doi: 10.1242/dev.122.12.4065. [DOI] [PubMed] [Google Scholar]
- 34.Steinmann-Zwicky M. Sex determination in Drosophila: sis-b, a major numerator element of the X:A ratio in the soma, does not contribute to the X:A ratio in the germ line. Development. 1993;117:763–7. doi: 10.1242/dev.117.2.763. [DOI] [PubMed] [Google Scholar]
- 35.Granadino B, Santamaria P, Sánchez L. Sex determination in the germ line of Drosophila melanogaster: activation of the gene Sex-lethal. Development. 1993;118:813–6. doi: 10.1242/dev.118.3.813. [DOI] [PubMed] [Google Scholar]
- 36.Marsh JL, Wieschaus E. Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature. 1978;272:249–51. doi: 10.1038/272249a0. [DOI] [PubMed] [Google Scholar]
- 37.Bachiller D, Sánchez L. Mutations affecting dosage compensation in Drosophila melanogaster: effects in the germline. Dev Biol. 1986;118:379–84. doi: 10.1016/0012-1606(86)90007-2. [DOI] [PubMed] [Google Scholar]
- 38.Hager JH, Cline TW. Induction of female Sex-lethal RNA splicing in male germ cells: implications for Drosophila germline sex determination. Development. 1997;124:5033–48. doi: 10.1242/dev.124.24.5033. [DOI] [PubMed] [Google Scholar]
- 39.Sheng XR, Posenau T, Gumulak-Smith JJ, Matunis E, Van Doren M, Wawersik M. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev Biol. 2009;334:335–44. doi: 10.1016/j.ydbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang SY, Baxter EM, Van Doren M. Phf7 controls male sex determination in the Drosophila germline. Dev Cell. 2012;22:1041–51. doi: 10.1016/j.devcel.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakob S, Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 2011;278:1002–9. doi: 10.1111/j.1742-4658.2011.08029.x. [DOI] [PubMed] [Google Scholar]
- 42.Nanda S, DeFalco TJ, Loh SH, Phochanukul N, Camara N, Van Doren M, et al. Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex Dev. 2009;3:26–37. doi: 10.1159/000200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark IB, Jarman AP, Finnegan DJ. Live imaging of Drosophila gonad formation reveals roles for Six4 in regulating germline and somatic cell migration. BMC Dev Biol. 2007;7:52. doi: 10.1186/1471-213X-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolpert LS, Jessell J, Lawrence T, Roberston P, Meyerowitz E. Principles of Development, 3rd ed. Oxford, 2006. Oxford University Press. [Google Scholar]
- 45.Kozopas KM, Samos CH, Nusse R. DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 1998;12:1155–65. doi: 10.1101/gad.12.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–21. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- 47.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–90. doi: 10.1016/S0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 48.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–96. doi: 10.1242/dev.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitadate Y, Kobayashi S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc Natl Acad Sci U S A. 2010;107:14241–6. doi: 10.1073/pnas.1003462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okegbe TC, DiNardo S. The endoderm specifies the mesodermal niche for the germline in Drosophila via Delta-Notch signaling. Development. 2011;138:1259–67. doi: 10.1242/dev.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitadate Y, Shigenobu S, Arita K, Kobayashi S. Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev Cell. 2007;13:151–9. doi: 10.1016/j.devcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Asaoka M, Lin H. Germline stem cells in the Drosophila ovary descend from pole cells in the anterior region of the embryonic gonad. Development. 2004;131:5079–89. doi: 10.1242/dev.01391. [DOI] [PubMed] [Google Scholar]
- 55.Bridges CB. The Genetics of Sex in Drosophila, in Sex and Internal Secretions, E. Allen, Editor. 1932, The Williams and WIlkins Co.: Baltimore. p. 55-93. [Google Scholar]