Abstract
In all animals, germline cells differentiate in intimate contact with somatic cells and interactions between germline and soma are particularly important for germline development and function. In the male gonad of Drosophila melanogaster, the developing germline cells are enclosed by somatic cyst cells. The cyst cells are derived from cyst stem cells (CySCs) of somatic origin and codifferentiate with the germline cells. The fast generation cycle and the genetic tractability of Drosophila has made the Drosophila testis an excellent model for studying both the roles of somatic cells in guiding germline development and the interdependence of two separate stem cell lineages. This review focuses on our current understanding of CySC specification, CySC self-renewing divisions, cyst cell differentiation, and soma-germline interactions. Many of the mechanisms guiding these processes in Drosophila testes are similarly essential for the development and function of tissues in other organisms, most importantly for gametogenesis in mammals.
Keywords: stem cells, cyst cells, germline, signaling, codifferentiation
Introduction
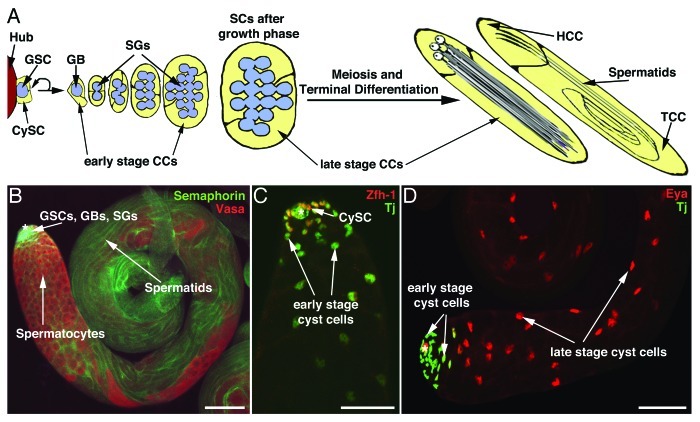
Adult stem cells have been identified from many tissues where they constantly produce highly specialized cells, such as germline, blood, and skin cells.1-5 A Drosophila testis contains two types of stem cells, germline stem cells (GSCs) and cyst stem cells (CySCs), which give rise to the germline cells (blue in Fig. 1A), and cyst cells (yellow in Fig. 1A), respectively.3,6 Electron-microscopy studies revealed that GSCs and CySCs are arranged in rosettes around a single group of terminally differentiated somatic cells, called the hub, at the apical tip of a wildtype testis.3 Each GSC is flanked by a pair of CySCs which extend cytoplasmic protrusions around the GSC and into the hub (illustrated in Fig. 1A). GSCs and CySCs divide asymmetrically to produce daughter cells (gonialblasts and cyst cells, respectively) that form developmental units, termed cysts. During cyst formation, two cyst cells grow cytoplasmic extensions around a single gonialblast to completely enclose it, thereby isolating it from direct contact with any other cell type. Once a cyst is formed, the two cyst cells and the enclosed gonialblast codifferentiate. The gonialblast undergoes four rounds of transit amplification divisions to produce 16 spermatogonia, which then become spermatocytes, and grow in size. After the growth phase, the spermatocytes undergo meiosis and differentiate into elongated spermatids. During all stages of germline differentiation, the two cyst cells continue to enclose the germline cells, grow tremendously in size, and codifferentiate with the germline. During terminal differentiation, morphological differences between the two cyst cells become apparent, as one cyst cell, the tail cyst cell, becomes much larger than the other cyst cell, the head cyst cell (TCC, HCC; Fig. 1A). The developing cysts become progressively displaced toward more basal regions of the testis. This results in a spatio-temporal arrangement of cysts along the apical to basal axis of the wildtype testis.3,7-9
Figure 1. The organization of somatic cells and germline cells in wildtype testes. (A) Graphic depicting the organization and development of the cysts along the apical (left) to basal (right) axes of the testis. CySC, cyst stem cell; GSC, germline stem cell; GB, gonialblast; SG, spermatogonia; SC, spermatocytes; CC, cyst cells; HCC, head cyst cells; TCC, tail cyst cells. (B–D) Immunofluorescent labeling of wildtype testes as indicated. (B) Visualization of cyst cells enclosing the germline cells in a whole testis. (C) The apical tip of a testis showing colocalization of Zfh-1 and Tj. (D) The apical region and the testis coil. Tj is expressed in early stage cyst cells near the tip, and Eya is expressed strongly in late stage cyst cells. *hub cells; scale bars, 50 µm.
The cyst cell lineage can be visualized easily by immunofluorescence microscopy. Several cell surface markers, such as Semaphorin (green in Fig. 1B), are highly expressed on the membranes of cyst cells (Zoller and Schulz, unpublished data) and allow for visualization of the cytoplasmic extensions enclosing the germline cells (red in Fig. 1B). Antibodies raised against the transcription factors Zinc finger homeodomain-1 (Zfh-1), Traffic jam (Tj), and Eyes absent (Eya) can be used to assess the developmental stage of the cyst cells. Zfh-1 (red in Fig. 1C) is expressed at high levels in CySCs, fading rapidly in early stage cyst cells (cyst cells associated with proliferating germline cells).10 Tj (green in Fig. 1C and D) is expressed at equal levels in CySCs and in early stage cyst cells.11 Eya (red in Fig. 1D) shows a very low level of expression in early stage cyst cells and a high level of expression in late stage cyst cells (cyst cells associated with post-mitotic germline cells).12 In addition to these antibodies, marker genes expressed from transposon insertion lines or driven by tissue-specific Gal4-transactivators provide useful tools for identifying and staging cyst cells throughout the Drosophila testis.13-15
The arrangement of the germline and the cyst cells in Drosophila testes is similar to the arrangement of germline and somatic cells in mammalian testes. Just as in a Drosophila testis, the germline cells in a mammalian testis are arranged in a spatio-temporal order along the axis, with the youngest stage germline cells located next to the basal membrane and the more differentiated germline stages located toward the lumen of the seminiferous tubules.16,17 Mammalian germline cells are enclosed in large somatic Sertoli cells, which act as a physical barrier as well as a source for nutrients and regulatory molecules. Physical isolation of the differentiating germline cell clusters from each other is achieved via localization of specialized cell junctions between the Sertoli cells and the germline cells.16,18-21 It appears that the enclosure of the developing germline cells, therefore, is dependent upon different mechanisms in Drosophila and mammals. While each cluster of germline cells in a Drosophila testis has a pair of microenevironment cyst cells generated by CySC divisions, each cluster of germline cells in a mouse testis sits in a compartment within the microenvironment Sertoli cell marked by the localization of junction proteins. The similarity in the tight association between germline and soma in Drosophila and mammalian testes, though, suggests that mechanisms and molecules setting up and maintaining the organization, and the communication pathways between germline and soma may be conserved.
The Origin and Identity of CySCs
In most organisms, the germline and somatic cells of the gonad are specified independently and coalesce during development to form the gonad.17 The Drosophila somatic cyst cell lineage originates from somatic gonadal precursor cells (SGPs) specified in parasegments 10–12 during embryogenesis. As the primordial germline cells migrate from the posterior end of the embryo toward the position of the future gonad in parasegment 10, they pass through parasegments 12 and 11 where they associate with the SGPs. Together, the germline and the SGPs from parasegments 12 and 11 migrate anteriorly and join the SGPs of parasegment 10. There, the germline cells and the SGPs coalesce to form the gonad and the SGPs become specified into the different somatic cell types of the testis.22-24 (For a comprehensive review of gonad formation, please visit the review by Mark Van Doren in this issue of Spermatogenesis).
A common origin of cyst cells and hub cells from SGPs was shown by lineage tracing experiments. When gonadal precursor cells were labeled in parasegment 11, label-positive progeny were subsequently found among both the hub cells and the cyst cells of adult testes.25 Consistent with the common origin of the two somatic cell types, the cyst cells and the hub cells both express Zfh-1, Tj, and marker genes from enhancer trap lines.10,11,13
The specification of the SGPs as CySCs vs. hub cells was suggested to depend on signaling via the Notch (N), the Epidermal Growth Factor (EGF) and the Sevenless (Sev) signaling pathways. N signaling between the SGPs was sufficient to specify hub fate in SGPs throughout the embyonic gonad. Throughout the gonad, except for the most anterior region, N is opposed by EGF and Sev signaling from the germline to the SGPs. This results in specification of SGPs as hub cells at the anterior tip of the embryonic gonad, and in the specification of SGPs as CySCs in the remainder of the embryonic gonad.26-28
Both specification and maintenance of CySC vs. hub cell fate in Drosophila testes requires the antagonistic roles of lines (lin) and brother of odd with entrails limited (bowl).25 The bowl gene encodes a zinc finger transcription factor and lin encodes a cytoplasmic protein with catalytic activity. During Drosophila epidermal cell differentiation, Lin binds directly to Bowl to reduce Bowl abundance, possibly by targeting Bowl for degradation. Another protein, Drumstick (Drm), competes with Lin for binding to Bowl and, thus, promotes Bowl accumulation.29 Transcription of Bowl was upregulated in testes with excess stem cells based on microarray analysis.30 This observation led to the investigation of a requirement for Bowl and it’s antagonist Lin in testes. While bowl mutant embryonic gonads had fewer hub cells, lines mutant embryonic gonads had an increased number of hub cells in comparison to wildtype gonads. Likewise, when CySCs in adult testes were depleted of lines, their progeny accumulated in aggregates that expressed a variety of hub-specific marker genes, including a hedgehog-reporter and Cactus. Similar to hub cells, these hub-like aggregates appeared to recruit CySCs. The hub-like aggregates failed to recruit functional GSCs based on the expression of several GSC specific markers genes, implying that they are not identical to hub cells.25 How exactly Bowl specifies CySC vs. hub cell fate has not been revealed and no target genes of Bowl have been identified in testes.
Substantiating the common origin of cyst cells and hub cells, CySCs are able to convert into hub cells: they can associate with the hub cells and express hub cell markers. In testes devoid of germline cells, CySCs obtain hub cell identity at a high frequency, suggesting that germline cells play a central role in maintaining the CySC population.6 The ability of CySCs to convert into hub cells was also observed in wildtype testes, yet reports on the frequency of this conversion are conflicting.25,31 Voog31 reported a high frequency of conversion (one converted CySC in 46% of 74 testes examined), while others reported that CySCs very rarely generated daughter cells that adopted hub cell fate. Dinardo25 did not find any converted CySCs out of 40 testes, even though they used the same tools for lineage tracing as exploited by Voog.31 It remains to be resolved whether different genetic backgrounds or growth conditions may be the reason for the wide range in conversion frequency.
Lineage tracing experiments confirmed beyond any doubt that CySCs are indeed functional stem cells, as they both self-renew and produce differentiating daughter cells. In a key experiment, a Flippase recombinase was transiently expressed to induce a recombination event that randomly and permanently marked single cells and their progeny by reconstitution of a β-galactosidase (lacZ) marker gene. LacZ-positive cells persisting next to the hub had to be either GSCs or CySCs. GSCs produce many clusters of LacZ-positive, proliferating germline cells. CySCs, in contrast, produce daughter cyst cells that do not divide further, but together with a second cyst cell, enclose the developing germline. As expected, LacZ-positive CySCs were observed next to the hub and these stem cells produced a series of single, LacZ-positive cells that associated with developing germline cells.6 With these experiments, it was established that the CySCs in Drosophila testes are indeed stem cells, as they contains self-renew and produce differentiating daughter cells. Subsequent research shifted toward studying the specification of both stem cell populations the GSCs and the CySCs.
CySC Self-renewal Depends on Signals from the Hub
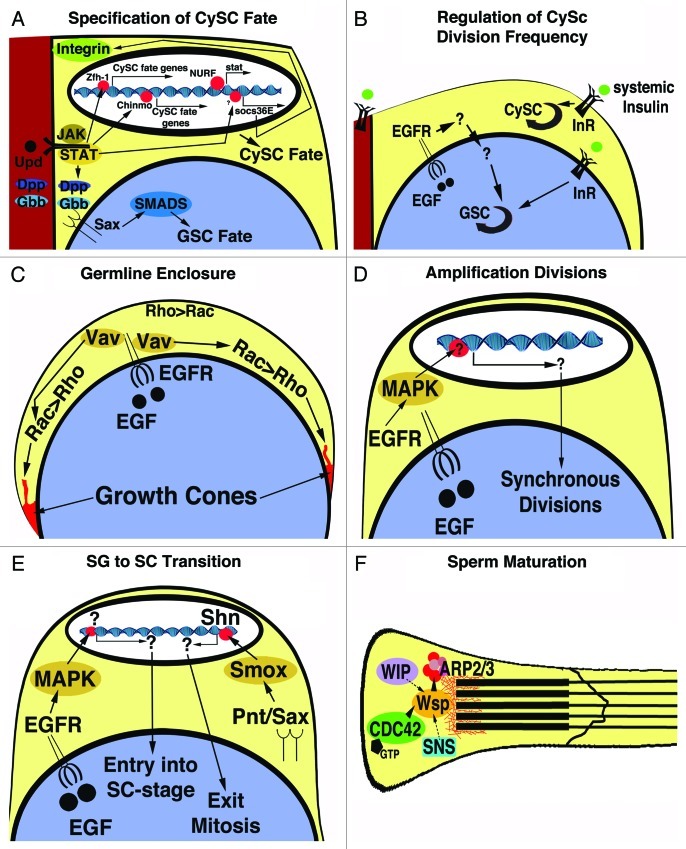
A series of elegant genetic experiments demonstrated how the cell fate decisions of CySCs to become either new CySCs or cyst cells is regulated (illustrated in Fig. 2A). CySC fate is dependent on signaling from the hub cells via the Janus kinase and Signal Transducer and Activator of Transcription (JAK/STAT) pathway.32,33 The highly conserved Jak/STAT signaling pathway is essential in a number of developmental processes in Drosophila and in mammals, and has been associated with a variety of cancers.34-39 The JAK/STAT signaling pathway was originally discovered as a cytokine-induced signaling pathway required by the myelid and lymphoid cell lineages and is now known to regulate many stem cell populations, including stem cells in the Drosophila ovary and intestine, and murine embryonic stem cells.40-43 The core JAK/STAT signaling pathway is relatively simple. Binding of the cytokine to its receptor induces conformational changes that lead to activation of associated JAKs. Activated JAKs phosphorylate the cytokine receptors and the tyrosine-phosphorylated motifs in the cytokine receptors serve as docking sites for the SH2 domains of STATs. Once bound to the receptor, the STATs become activated by tyrosine phosphorylation. Phosphorylated STATs dissociate from the receptors, dimerize, and translocate into the nucleus to regulate transcription of target genes.44
Figure 2. Regulation of cyst development.62 Graphics depicting specific steps of germline and cyst cell interactions. Molecules and Interactions as indicated. See main text for details. (A) Signaling events at the tip of the testes regulating CySC and GSC fate. (B) Signaling events regulating CySC and GSC division frequency (black round arrows). (C) The regulation of germline enclosure by EGF signaling. (D) The regulation of germline proliferation by EGF signaling. (E) Signaling events regulating the transition of the spermatogonia into the spermatocyte stage. (E) Sperm maturation depends on the cap proteins in the head cyst cells.
Drosophila males carrying a temperature sensitive allele of the JAK, hopscotch (hop), or stat were raised at a permissive temperature and then shifted to a restrictive temperature to induce a phenotypic change in the gonad. In testes from shifted animals, CySCs and GSCs were progressively lost. This suggested that GSCs and CySCs failed to self-renew and instead differentiated into cyst cells. Conversely, hyperactivation of JAK/STAT signaling had the opposite effect. Overexpression of the ligand Unpaired (Upd), that is normally only expressed in the hub, throughout the germline resulted in the excessive accumulation of CySCs and GSCs, as evident by their expression of stem cell specific marker genes.32,33 These studies demonstrated that JAK/STAT signaling induces CySC and GSC fate in Drosophila testes. (For a comprehensive review of GSC specification and function, please visit the review by Erika Matunis in this issue of Spermatogenesis).
Additional studies revealed that two proteins act downstream of JAK/STAT to regulate CySC fate in Drosophila testes. These are Zfh-1 and Chronologically inappropriate morphogenesis (Chinmo), a protein that may act as a transcriptional regulator or play a role in protein degradation.10,45,46 Analogous to hop and stat mutants, zfh-1 or chinmo deficient CySCs failed to self-renew. Analogous to Upd overexpression in the germline, overexpression of Zfh-1 or Chinmo in the cyst cells resulted in an accumulation of CySCs and GSCs.10,46 These findings strongly suggest that the activities of Zfh-1 and Chinmo depend on JAK/STAT signaling and link the JAK/STAT signaling event to transcriptional regulation of target genes. However, Zfh-1 and Chinmo appear to act in an independent manner, based on their expression patterns and genetic interaction. While Zfh-1 is predominantly expressed in CySCs, Chinmo appears to be expressed at similar levels in CySCs and in early stage cyst cells. Zfh-1 and Chinmo expression was unaffected in testes mutant for the other gene and overexpression of zfh-1 did not restore CySCs in chinmo mutants.46
In addition to the core signaling event from the hub cells to the CySCs, the levels of JAK/STAT signaling is further regulated cell autonomously within the CySCs.47,48 Suppressors Of Cytokine Signaling (SOCS) are highly conserved transcriptional targets of JAK/STAT signaling and antagonize the JAK/STAT pathway via several distinct mechanisms.49-51 In Drosophila testes, Socs36E is expressed in the hub cells and the CySCs. Yet surprisingly, testes from animals carrying a viable allele of socs36E showed a defect in the germline. The testes from these socs36E mutant animals progressively lost their GSCs from the position next to the hub. Notably, the CySCs in socs36E mutant testes had abnormally broad contact regions with the hub and expressed increased levels of βPS-Integrin at the hub-CySC interface. This indicated that JAK/STAT signaling acts via socs36E to regulate the expression, stability, or localization of cell adhesion molecules.52 The authors further hypothesized that increased cell adhesion between CySCs and hub cells in testes from socs36E mutant animals displaces the GSCs away from their position next to the hub. Consistent with these ideas, overexpression of βPS-Integrin in CySCs phenocopied the loss of socs36E, and reduction of βPS-Integrin from socs36E mutant animals rescued the GSC loss in the testes from socs36E mutant animals.52 While socs36E downregulates JAK/STAT signaling, the Nucleosome-Remodeling Factor (NURF) appears to positively regulate JAK/STAT signaling within the CySCs.52,53 Mosaic analysis experiments revealed that CySCs were not maintained when the cells were mutant for subunits of the NURF complex, specifically nurf301, nurf38, and iswi, suggesting a role for the NURF complex in CySC self-renewal. Restoring STAT expression in nurf301 mutant CySCs restored the CySCs loss, showing that STAT acts downstream of the NURF complex, and suggesting that stat may be a transcriptional target of the NURF complex.53 Following up on the importance of JAK/STAT signaling for stem cells, microarray studies were performed that identified transcripts up- or downregulated in testes with excess stem cells that are now being investigated for their roles in gametogenesis.25,30
CySCs Specify GSC Fate
JAK/STAT signaling is required for the self-renewal of GSCs and CySCs yet specification of GSCs also requires crosstalk between the two stem cell populations.32,33,47 When animals were created that expressed stat in the CySCs but lacked stat specifically in GSCs, surprisingly, the GSCs were maintained. These GSCs had lost contact with the hub yet remained associated with the CySCs and appeared functional. These observations suggested that stat activation in the CySCs is sufficient to mediate self-renewal of the GSCs, and that a second pathway from the CySCs to the GSCs has to induce, or reiterate GSC fate. This other pathway is the Transforming Growth Factor-β (TGFβ) signaling pathway.47
TGFβ is homologous to the Bone Morphogenetic Protein (BMP) and the signaling pathways activated by TGFβ and BMP play important roles in development and stem cell function across species.4,54-56 BMP signaling has also been implicated in regulating mammalian spermatogenesis, yet studying the role of the BMP pathway in mammals is more difficult due to the high number of BMP family members that may act redundantly.57-60 The core BMP signaling pathway consists of the active receptor and the SMAD transcription factors. The active TGFβ family receptor is a transient complex of two receptors with serine-threonine activity and a ligand. The active receptor phosphorylates receptor-regulated Smad type proteins which complex with non-receptor-regulated Smad proteins, enter the nucleus, and regulate transcription of target genes.55,56
The Drosophila TGFβ ligands Decapentaplegic (Dpp) and Glass bottom boat (Gbb) are expressed in CySCs and hub cells of wildype testes, and pathway activation has been observed in GSCs of wildtype testes.61-64 Leatherman47 reported that the TGFβ pathway was activated in GSCs from testes with germline-depleted stat. The GSCs in these testes had high levels of phosphorylated SMAD in their nuclei, and expression of TGFβ antagonists in the stat-depleted testes led to a loss of GSCs. This strongly suggested that CySCs signal to neighboring germline cells via the TGFβ pathway to induce GSC fate.47 It remains to be investigated whether the Dpp and Gbb signals from the hub are also sufficient to induce GSC fate and whether Dpp and Gbb activity in CySCs is directly dependent on the JAK/STAT pathway.
Regulation of CySC Divisions
Stem cells have to adjust to the demand for differentiated cells in order to keep a tissue fully functional. For example, skin stem cells have to produce more skin cells during childhood when an individual grows than they have to produce during adulthood. Likewise, GSCs and CySCs have to adjust to the overall metabolism of the fly and to the demand for sperm. This novel aspect of stem cell biology is only recently being addressed and the Drosophila gonad has emerged once again as one of the pioneer systems to approach this unknown territory. A few studies have already addressed the mechanisms by which stem cells regulate their division frequency to produce more or less differentiating germline and cyst cells (illustrated in Fig. 2B). One factor regulating the division frequency of CySCs and GSCs is nutrient availability. When animals were starved, CySCs and GSCs divided at lower frequencies compared with CySCs and GSCs in testes from fed animals.65,66 This suggested that insulin signaling regulates the division frequencies of stem cells in the testis in a manner similar as has been proposed for insuling signaling to the GSCs of the ovary.67-69 Complicating matters, CySCs, in turn, influence the division frequency of the enclosed GSCs in an only partially understood feedback loop. In testes from animals mutant for EGF signaling, GSCs divided at frequencies two to three times higher than GSCs of control testes.70
The EGF signaling pathway is highly conserved and plays multiple roles in embryonic development, stem cell biology, and gametogenesis of several species.71-74 In Drosophila, the major ligand for the pathway, Spitz (Spi), and its receptor, the Epidermal Growth Factor Receptor (EGFR), are assumed to be ubiquitously expressed in many tissues and pathway activation depends on the cell type-specific activity of ligand processing proteases.72 In testes, the germline cells process Spi into the active, secreted form via activity of the protease Stet, while EGFR is stimulated on the CySCs and cyst cells.9,70,75 Once activated, the tyrosine kinase EGFR phosphorylates cascades of downstream signal transducers.76
Removal of either spi or stet from the germline cells, or removal of the EGFR from the soma resulted in increased division frequencies of GSC but did not affect the division frequencies of CySCs.70 This strongly suggested that EGF signaling normally downregulates GSC divisions. However, this effect is indirect as EGF is produced by the germline and received by the CySCs which presumably respond with an unknown return signal to the GSCs. Thus, the division frequency of stem cells depends on both systemic factors and interaction between the CySCs and GSCs.
Germline stem cells show a characteristic orientation of their mitotic spindles during cell division in both mouse and Drosophila testes. In Drosophila testes, the spindles of the GSCs are oriented perpendicular to the hub.77 In rat testes, mitotic spindles of spermatogonia were mainly oriented with angles ranging from 60 to 90° perpendicular in relation to the basement membrane of the seminiferous tubules.78 CySCs in Drosophila testes also undergo strict asymmetric divisions yet they employ a different cellular mechanism for orienting their plane of division than GSCs do.77,79 In GSCs, the mitotic spindle is oriented perpendicular to the hub throughout mitosis.77 CySC spindles do not show a consistent orientation. Instead, one of the spindle poles is repositioned to the hub-CySC interface specifically during anaphase.79 Spindle positioning in both CySC and GSC divisions requires Centrosomin, as loss of Centrosomin disrupts the spindle orientation in both GSCs and CySCs. While GSCs also require activity of Adenomatous Polyposis Coli (APC), this factor is dispensable for CySC spindles.77,79 Instead, CySCs require Moesin, a linker between the membrane and the cytoskeleton.79,80 Moesin knockdown via RNA-Interference in CySCs resulted in reduced spindle repositioning during anaphase. In contrast, Moesin knockdown in GSCs had no effect on spindle positioning. In the course of their experiments, the authors noticed that both overexpression and loss of Moesin increased the number of GSCs, CySCs, and cyst cells. Though the mechanism for the dose effect of Moesin is not understood, these results suggested that the correct expression level of Moesin is required for maintaining the correct number of stem cells in wildtype testes.79
The above discussed studies showed that a combination of multiple mechanisms, including Upd signaling from the hub, the cell autonomous activity of lines and bowl, and attachment of the CySCs to the hub via cell adhesion molecules assure the maintenance and self-renewal of CySCs, as well as proper interactions between CySCs and GSCs in Drosophila testes. In the following section, we will focus on discussing cyst cell differentiation and the roles the cyst cells play in guiding germline proliferation and germline differentiation.
Cyst Formation is Regulated by EGF Signaling
The cysts in Drosophila testes are one of the few examples in animal development where one cell type completely encloses another. Other examples are the enclosure of nerve cells by the myelin sheath and the formation of biofilm during the mating of yeast.81-83 Research in Drosophila testes has revealed insights as to how a specific arrangement between two different cell types is established. Genetic experiments showed that signaling from the germline cells via EGF to the cyst cells is required to induce and organize the growth of the cyst cells around the germline cells (illustrated in Fig. 2C). A conditional allele of spi displayed germline enclosure defects of increasing severity with increasing temperature. Removing one copy of the small monomeric GTPase, rac1, from spi mutant animals raised at an intermediate temperature drastically enhanced the germline enclosure defects resembling testes from spi mutant animals raised at higher temperatures. Likewise, reducing the expression of the docking protein and guanidyl exchange factor, Vav, or Rac1 specifically from the cyst cells of spi mutant resulted in a similarly strong enhancement of the germline enclosure defects. Together with binding studies of EGFR and Vav, the above genetic data suggested that Vav and Rac1 act in a signaling branch downstream of the EGFR. Conversely, reducing the expression of the small monomeric GTPase Rho1 from the cyst cells had the opposite effect on testes from spi mutant animals, restoring germline enclosure even when the animals were raised at high temperatures. This indicated that Rho1 acts in a pathway opposing EGFR for germline enclosure.84 In cultured mammalian cells, Rac and Rho play antagonistic roles in regulating cell shape changes and growth via different effects on the actin cytoskeleton.85-88 Based on electron microscopy, expression of dominant negative Rac1- and Rho1-constructs in the cyst cells of otherwise wildtype testes specifically affected the structure of the cyst cell membranes. In wildtype testes, the cyst cell membranes were wavy. Loss of Rac1 from the cyst cells resulted in smoother membranes than those seen in cyst cells of wildtype testes, while loss of Rho1 resulted in the appearance of filopodia in the cyst cell membranes. These findings supported the idea that EGF signaling from the germline cells produces a differential of Rac- and Rho-activities across the cyst cells that, perhaps by organizing the actin cytoskeleton, leads to the directional growth of the cyst cells around the germline cells.84 In mammalian testes, the displacement of the differentiating germline cells toward the lumen of the seminiferous tubules requires reorganization of the germline-Sertoli cell contacts.89 The Sertoli cells in mouse testes contain a most elaborate cytoskeleton that is essential for their structure and function and likely to play an active role in the constant reorganization of the germline-Sertoli cell contacts.19,90 Intestingly, the mammalian EGFR homolog was reported to localize to Sertoli cells opening the possibility that some of the roles for EGF signaling in somatic cell shape or function are conserved between Drosophila and mammals.90-92
Further studies in Drosophila led to the identification of several other conserved proteins that are required for the physical interactions between germline and cyst cells. For example, the large musculo-aponeurotic fibrosarcoma (Maf) factor homolog Tj acts cell autonomously in cyst cells to mediate cyst cell association with the germline by activating the transcription of cell adhesion molecules.11 Similarly, the conserved motor protein Dynein Light Chain 1 (DDLC1) is required for the presence of several cell adhesion molecules on the cyst cell membranes and the presence of Eya in the cyst cell nuclei.93 However, it remains to be addressed whether this is due to a role of DDLC1 in regulating gene expression, protein localization, or protein stability. Likewise, it would be interesting to learn if loss of ddlc1 has an effect on EGF signaling and whether it plays a parallel role in gametogenesis of other organisms.
Cyst Cells Regulate Germ Cell Proliferation
In most tissues maintained by stem cells, the stem cell daughters undergo several rounds of transit amplifying divisions to increase the numbers of precursors for terminally differentiated cells.1,4,5,94 The proliferation of cells needs to be tightly regulated to avoid loss or tumorous growth of a tissue and the mechanisms restricting cell proliferation are of wide interest in development, stem cell biology, and cancer biology. The Drosophila testis is an excellent model tissue in which to study tumorigenesis.95,96 In Drosophila testes, proliferation of the stem cell daughters relies on both germline intrinsic and extrinsic factors.6,9,97 The requirement for germline-soma interactions to restrict cell proliferation is most evident in testes that lack either germline cells or germline enclosure by cyst cells. In testes without a germline (agametic testes), the cyst cells lost the ability to differentiate into late stage cyst cells and proliferated as early stage cyst cells instead. The somatic cells appeared to be properly specified and initially behaved normally. However, after five days, the number of early stage cyst cells increased and the number of late stage cyst cells decreased. In addition, the cyst cells underwent mitotic divisions, a trait normally restricted to CySCs. This suggests that germline cells normally restrict the ability of cyst cells to reenter the CySC proliferation program.6 Interestingly, cyst cells associated with gonialblasts normally fail to elongate their centrioles and to recruit pericentriolar material, possibly due to downregulation of SAS-6, a main regulator of centriole architecture.98 It remains to be investigated whether downregulation of SAS-6 is the cause for the withdrawal of cyst cells from the cell cycle and whether cyst cells in agametic testes continue to express SAS-6.
Conversely, germline proliferation is restricted by surrounding cyst cells. In spi or stet mutant animals, germline cells and cyst cells were present but the cyst cells did not enclose the germline. In these testes, the germline cells proliferated at early stages and failed to differentiate.9,84 Though previous experiments had suggested that EGF signaling produces a feedback loop from the cyst cells to the enclosed germline cells to restrict germline proliferation, these experiments did not address germline enclosure.75,99 Therefore, it remained unclear whether the germline proliferation defects in the egfr mutants were due to defects in enclosure or whether EGF also restricts germline proliferation past the enclosure event. Recent studies have shed more light on the specific roles of EGF in restricting germline proliferation and cyst maturation (Hudson and Schulz, unpublished data). By using many alleles and conditions for spi, stet, raf, and egfr, as well as overexpression studies, we and others demonstrated that EGF signaling has a range of effects on cyst cells and germline cells. EGF signaling from the germline to the soma regulates the division frequency of GSCs, the encloure of the gonialblast by cyst cells, the progression of the spermatogonia through amplification divisions, and EGF signaling also promotes the entry of the germline cells into the spermatocyte stage (illustrated in Fig. 2B–E).9,70,75,84,99(Hudson and Schulz, unpublished data) This suggests that the cyst cells send different sets of return signals to the enclosed germline cells at different stages of spermatogenesis. The EGFR also appears to be required cyst cell-intrinsically for their viability, as some EGFR alelles are cell lethal.75
In order for the spermatogonia to enter the spermatocyte stage, they first have to exit from the mitotic amplifciation divisions. The exit of the spermatogonia from mitosis specifically at the 16-cell stage depends on TGFβ signaling (illustrated in Fig. 2E). Loss of the TGFβ receptors Punt (Pnt) or Saxophone (Sax), the signal transducer Smad on X (Smox), or the downstream transcription factor Schnurri (Shn) from cyst cells resulted in the accumulation of large clusters of spermatogonia of multiples of 16. For example, these cysts contained 32, 64, or 128- spermatogonia.100,101 In addition to JAK/STAT, EGF and TGFβ signaling, a number of candidate genes have been identified that affected germline proliferation but their mode of action and whether their homologs my play a role in tissue homeostasis in other organisms or cancer is yet to be determined.62
Germline proliferation was suggested to depend on motor-based processes within the cyst cells, based on the phenotypes of mutations in a cytoplasmic dynein, motor proteins and a GTPase.93 Animals carrying a hypomorphic allele of ddlc1 displayed a testes phenotype in which the germline cells overproliferated and accumulated as single cells and 2-cell spermatogonia. In an effort to investigate the mechanism of DDLC1 action, the potential binding partners of DDLC1 were knocked down via RNA-Interference and the effect on germline and cyst cells investigated.93,102 The binding partners for DDLC1 are Dhc64C, which encodes a microtubule motor protein, and Myosin V (didum), which encodes an actin binding protein.103 The mammalian homolog of Myosin V, Myosin 5b, displayed motor protein function during membrane recycling, suggesting a similar role as a motor protein in Drosophila.93,104 Knockdown of either didum or dhc64C in the cyst cells resulted in a similar germline overproliferation phenotype as knockdown of ddlc1 and removing one copy of didum or dhc64C from animals carrying a hypomorphic allele of ddlc1 enhanced the germline proliferation phenotype. This suggested that the three proteins act in a pathway to restrict germline proliferation. However, the effect on cell adhesion molecules observed in the ddlc1 mutants was not recapitulated upon reduction of didum or dhc64C, suggesting that ddlc1 acts in two distinct pathways, one involved in cell adhesion, and the other in restricting germline proliferation. Finally, cyst cell-reduction of the GTPase, Rab11, which was shown to play a role in Myosin V-dependent secretion in developing photoreceptors, also resulted in germline overproliferation.93,105 The authors hypothesized that Rab11 acts in the same pathway as DDLC1, Myosin V, and Dhc64C, and that they play a role in the exocytosis of a yet-to-be-identified cyst cell derived signal regulating germline proliferation.93 Interestingly, another Dynein, Dhc1, has been associated with EGF secretion in the developing eye and cytoplasmic dynein has been identified in rat Sertoli cells suggesting conserved roles for Dyneins in regulating intracellular processes during development and germline differentiation.106,107
Cyst Cells Regulate Germline Survival
In addition to providing regulatory cues for the development of the germline cells, cyst cells are also required for germline survival, yet for most of the genes mentioned below, their exact mechanisms of action and how they relate to each other is unknown. Germline survival specifically at the spermatocyte stage depends on the transcription factors Eya and Sine oculis (So), suggesting a checkpoint after mitotic amplification divisions. Eya and So are both expressed in cyst cells associated with spermatocytes and clonal analysis revealed that they act in the cyst cells to prevent the death of spermatocytes prior to their maturation. Mutations in eya and so show synergistic genetic interactions, suggesting that they function in different pathways.12
Several other factors are required for germline survival prior to the spermatocyte stage. The zero population growth (zpg) gene encodes a gap junction protein which is expressed in the germline. Mutations in zpg were associated with underproliferating spermatogonia that eventually died, which suggested that germline cells and surrounding cyst cells exchange small molecules for promoting their viability.108 Likewise, mutations in the tumor suppressor gene discs large (dlg), which encodes a septate junction protein, resulted in the death of spermatogonia as well as their enclosing cyst cells, suggesting that germline viability depends on direct connections between the germline and the cyst cells.109 slow motion (slowmo), a mitochondrial gene of unknown function, is expressed in cyst cells and affected survival of germline cells at all stages of development.110
When the role of cyst cells in GSC self-renewal and GSC daughter differentiation was first explored, two outstanding studies also suggested a role for EGF signaling in CySC viability or maintenance.75,99 This was not recapitulated in testes from animals carrying a temperature sensitive allele of spi, animals mutant for the protease stet, or animals with CySC-depleted EGFR.62,70,84 Kiger75 reported a failure to detect egfr mutant CySCs or cyst cells in clonal analysis experiments. This failure to detect EGFR mutant somatic cells is not surprising as the mutant alleles used in the clonal analysis experiments had been reported to be cell lethal.75,103 Maybe, the EGFR produced from the cell-lethal alleles folds in a manner interfering with basic cellular functions and, therefore, induces cell death. As the alleles have no effect on the germline, it suggests that the folding defects would depend on ligand binding. We tested the temperature sensitive allele of the EGFR used by Kiger.75 We observed that, after the shift to the restrictive temperature, the animals themselves died within a few days supporting the idea that this alleles is cell-lethal (Hudson and Schulz, unpublished data).
The other study reported that the somatic cells next to the hub in adult animals depleted of the Mitogen activated protein (Map)-Kinase, Raf, expressed the late cyst cell marker Eya, suggesting that the CySCs may have died or differentiated into late stages.99 In these animals, Raf activity was systemically reduced, leaving open in which cell type Raf acts to maintain CySCs. As Raf acts downstream of several signaling pathways it is plausible that the observed defects do not reflect loss of EGF signaling from the germline.111,112 Consistent with this, we never detected somatic cells next to the hub with high levels of Eya expression in testes maternally and zygotically depleted for EGF signaling (Hudson and Schulz, unpublished data). The strongest argument to refute the idea that EGF signaling from the germline to the cyst cells directly prevents CySC death or differentiation is the observation that somatic cells next to the hub in agametic testes did not undergo cell death or express Eya.6
Cyst Cells Regulate Late Stages of Germline Differentiation
Somatic and germline cells remain intimately associated throughout spermatogenesis in many species, suggesting that interactions between the two cell types also regulate late stages of spermatogenesis.1,16,113 An excellent example for this was observed in advanced stages of Drosophila spermatogenesis. As spermatids elongate, the cyst cells undergo a series of morphological changes whereby they become structurally distinct from one another. Tail cyst cells grow dramatically in size to accommodate the elongating sperm tails, while the head cyst cells grow to a much lesser extent. Following elongation of the cysts, the head cyst cells associate with the terminal epithelium of the testes, upon which the entire cysts coil to form compact structures.8 As the spermatids mature, the head cyst cells grow a cap around the sperm heads (illustrated in Fig. 2F). This cap is rich in F-actin and cell adhesion molecules, and organized by the Arp2/3 complex. A requirement for the cap was shown by phenotypic analysis of testes from animals carrying mutant alleles and expressing RNA-interference-constructs for genes encoding cap proteins specifically in the cyst cells. These studies included knockdown of Wsp, the Arp2/3 complex proteins Sop2 and Arp3, Wasp-interacting protein (WIP), the cell adhesion protein Sticks and stones (SNS), and the small monomeric GTPase Cdc42. Any of these mutants resulted in abnormal caps and were associated with an arrest in development of the germline cells prior to cyst coiling. Furthermore, it was shown that release of mature sperm from the cap depends on the activity of the dynamin Shibire. Interestingly, spermatogenesis was also arrested when the mammalian WASp homolgue was disrupted in mouse Sertoli cells, and the nature of the molecular mechanisms and the degree of parallels have become a future target of this line of research.114,115
Though the cyst cells are clearly required for proper germline proliferation and differentiation in tissue, it appears that germline cells have some cell-intrinsic properties for differentiation. An interesting cell culture study of Drosophila male germline cells suggested that the entry into terminal differentiation may be independent from surrounding cyst cells. When the authors dissociated 16-cell spermatogonia from the encapsulating cyst cells, the dissociated spermatogonia transitioned into spermatid-like elongated cells. Unfortunately, it was not addressed whether these germline cells had undergone meiosis, whether they had normal DNA-content or chromating structure, or whether the mitochondria assembled properly to produce the long flagellum of the sperm tail. The authors nevertheless concluded that cyst cells are dispensible for spermatocytes to undergo spermiogenesis.116 Similarly, in some mutant situations, germline cells can enter the spermatocyte stage independent from surrounding cyst cells. For example, germline cells from animals double-mutant for stet and the nucleoporin98–96 locus were not enclosed by cyst cells but developed into spermatocyte-like cells.117
Although the intrinsic properties of germline cells may give them some independence from the soma, the constant and coordinated production of mobile sperm from GSCs remains under cyst cell control. All of the findings discussed in this review (see Table 1 for overview of discussed proteins) clearly prove this point and have changed our view of the cyst cell lineage in Drosophila testes. While cyst cells were originally thought of as nurturing cells in Drosophila testes, the research discussed in this review shows that the CySC lineage in Drosophila testes plays an active role in gametogenesis and put them into the spotlight. In Drosophila testes, germline and cyst cells undergo coordinated interactions that regulate their codifferentiation and assure the production of functional sperm. A similar interdependence of stem cell lineages was discovered in mammals and similar mechanisms of interactions are being discussed.118-121 For example, melanocyte stem cells are closely associated with epithelial stem cells in the hair follicle of the skin, and here as well signaling between the two lineage is an important key in coordinating the differentiation of the two stem cell lineages to make pigmented hair.122
Table 1. Alphabetical listing of the genes discussed in this review that play roles in or for the CySC lineage.
| Gene name/Abbreviation | Molecular nature | Discussed function | References |
|---|---|---|---|
| Arp2/3 complex (Sop2, Arp3) |
Cytoskeletal regulator |
Sperm maturation |
114 |
| Brother of odd with entrails limited (bowl) |
Transcription factor |
CySC/hub cell specification |
25 |
| Cdc42 |
GTPase |
Sperm maturation |
114 |
| Centrosomin (cnn) |
Protein binding |
Stem cell fate |
79 |
| Chronologically inappropriate morphogenesis (chinmo) |
Transcription factor or Protein degradation |
CySC fate |
46 |
| Decapentaplegic (dpp) |
Protein binding/Ligand |
GSC fate |
61–64 |
| Discs large (dlg) |
Protein binding |
Cyst survival |
109 |
| dhc64C |
Microtubule motor activity |
GL proliferation |
93 |
| Dynein Light Chain 1 (ddlc1) |
Cytoplasmic Dynein |
GL-Soma adhesion |
93 |
| |
|
GL proliferation |
93 |
| Epidermal growth factor receptor (egfr) |
Receptor |
CySC specification |
27, 28 |
| |
|
CySC division frequency |
70 |
| |
|
GL proliferation |
75, 99 |
| |
|
Cell viability |
75 |
| Eyes absent (eya) |
Transcription factor |
Spermatocyte survival |
12 |
| F-actin |
Cytoskeletal component |
Sperm maturation |
113, 114 |
| Glass bottom boat (gbb) |
Protein binding/Ligand |
GSC fate |
61–64 |
| Hopscotch (hop) = JAK |
Protein tyrosine kinase |
CySC fate |
32, 33 |
| Insulin |
Protein binding/Ligand |
GCS + CySC division frequency |
65, 66 |
| Insulin Receptor (InR) |
Receptor |
GSC + CySC division frequency |
65, 66 |
| Ku80 |
Unknown |
Unknown |
123 |
| Lines (lin) |
Catalytic activity |
CySC specification |
25 |
| Moesin |
Protein binding |
Stem cell fate |
79 |
| Mothers against dpp (mad) |
Signal transducer |
GSC fate |
47 |
| Myosin V (didum) |
Actin binding |
GL proliferation |
93 |
| NOA |
Fatty acid elongase |
Unknown |
124 |
| Notch (N) |
Receptor |
Hub specification from SGPs |
27, 28 |
| NURF complex (nurf301, nurf38, iswi) |
Chromatin remodeling |
CySC fate |
53 |
| Protein Phosphatase (ppy) |
Phosphatase |
Unknown |
122 |
| Punt (pnt) |
Receptor |
GL proliferation |
100 |
| Rab11 |
Endosome transport |
GL proliferation |
93 |
| Rac1 |
GTPase |
GL enclosure |
84 |
| Rho1 |
GTPase |
GL enclosure |
84 |
| Saxophone (sax) |
Receptor |
GL proliferation |
101 |
| SAS-6 |
N/A |
Cyst cell cectriole architecture |
98 |
| Schnurri (shn) |
Transcription factor |
GL proliferation |
100 |
| Sevenless (sev) |
Receptor |
CySC specification from SGPs |
26 |
| Shibire (shi) |
Actin binding |
Sperm release |
113 |
| Sine oculis (so) |
Transcription factor |
GL survival |
12 |
| Slow motion (slomo) |
N/A |
Cyst survival |
110 |
| Smad on X |
Signal transducer |
GL mitosis |
102 |
| Signal transducer and activator of transcription (stat) |
Signal transducer |
CySC fate |
32, 33 |
| Suppressor of cytokine signaling 36E (socs36E) |
N/A |
CySC fate |
52 |
| Spitz (spi) |
Protein binding/ligand |
CySC division frequency |
70 |
| |
|
Germline enclosure |
84 |
| |
|
GL proliferation |
75, 99 |
| Stet |
Protease |
CySC division frequency |
70 |
| |
|
Germline enclosure |
9 |
| |
|
GL proliferation |
75, 99 |
| Sticks and Stones (sns) |
Protein binding |
Sperm maturation |
114 |
| Traffic jam (tj) |
Transcription factor |
GL-soma adhesion |
11 |
| Unpaired (upd) |
Protein binding/Ligand |
CySC fate |
32, 33 |
| Zero population growth (zpg) |
Gap junction protein |
Cyst survival |
108 |
| Vav |
Guanidyl exchange factor |
GL enclosure |
84 |
| Wsp |
Protein binding |
Sperm maturation |
114 |
| Wasp-interacting protein (wip) |
Actin filament binding |
Sperm maturation |
114 |
| Zinc finger homeodomain -1 (zfh-1) | Transcription factor | CySC fate | 10 |
CySC, cyst stem cells; GSC, germline stem cells; GL, germline; SGP, somatic gonadal precursors. References that present the research on these genes in Drosophila testes are listed.
Outlook
The interdependence of germline and cyst cells has been demonstrated for both the early and the final stages of spermatogenesis in Drosophila. Cyst cells regulate spermatogonial divisions, germline cell survival, and sperm maturation via several conserved molecules and pathways. Though research on Drosophila cyst cells has dramatically advanced our knowledge in this respect, many questions remain to be adressed. For example, we are only beginning to understand how the divisions of CySCs and GSCs are regulated to assure that two cyst cells are generated for each gonialblast. To start to address these questions, the molecules and pathways regulating stem cell division frequencies need to be identified and studied in detail, followed by the identification of the means by which the two stem cell populations coordinate their activities.
What are the molecular natures of the signals presented by the cyst cells to the enclosed germline cells? Despite the importance of these signals for germline differentiation, not a single one has been identified. A possible explanation is that redundant pathways regulate the behavior of the enclosed germline. Consistent with this idea, attenuation of signaling pathways in sensitized backgrounds can have a strong effect on germline development while not causing an effect in an otherwise wildtype background (Zoller and Schulz, unpublished data; Ng and Schulz, unpublished data).
Molecules and pathways regulating early and late stages of germline and cyst cell development have been described. However, no reports are available that describe interactions between germline and cyst cells during germline meiosis or the initiation of terminal differentiation. Several factors, including Protein Phosphatase Y, the NOA fatty acid elongase, and Ku80, a protein involved in DNA damage repair, are expressed in late stage cyst cells and the study of these genes may shed light on these questions.123-125 However, we still need to create a pool of late stage cyst cell-specific markers and transcriptional profiles of differentiating cyst cells in order to study germline and cyst cell differentiation. These tools will ultimately aid us in identifying potential interactions and show whether cyst cells, like germline cells, pass critical cell-intrinsic checkpoints during their differentiation program.
Future research will shed light on these and other key questions. As discussed, many of the principles unraveled by studying gametogenesis in Drosophila testes may apply to gametogenesis in other species.
Acknowledgments
The authors thank Steve DiNardo, Erika Matunis, Bernard Mechler, Margaret Fuller, Leanne Jones, Yukiko Yamashita, Antony Mahowald, Erica Bach, Trudi Schupbach, Claude Desplan, Sally Tazuke, and members of the UGA Developmental Biology Group for many helpful discussions on germline and cyst cell development. We are indebted to Judy Willis, Wolfgang Lukowitz, Sarah Thomas, members of the Schulz laboratory, and unknown reviewers for suggestions on the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/21380
References
- 1.Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–57. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 2.Huckins C. The spermatogonial stem cell population in adult rats. 3. Evidence for a long-cycling population. Cell Tissue Kinet. 1971;4:335–49. doi: 10.1111/j.1365-2184.1971.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–90. doi: 10.1016/S0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 4.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf D. Concise review: hematopoietic stem cells and tissue stem cells: current concepts and unanswered questions. Stem Cells. 2007;25:2390–5. doi: 10.1634/stemcells.2007-0544. [DOI] [PubMed] [Google Scholar]
- 6.Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–47. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- 7.Tates AD. Cytodifferentiation during spermatogenesis in Drosophila melanogaster Rijksuniversiteit Leiden, 1972. [Google Scholar]
- 8.Fuller MT. Spermatogenesis in Drosophila. In: Bate M, Martinez Arias A, ed. The Development of Drosophila melanogaster. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press, 1993:71-148. [Google Scholar]
- 9.Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–34. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- 10.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 12.Fabrizio JJ, Boyle M, DiNardo S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol. 2003;258:117–28. doi: 10.1016/S0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 13.Gönczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005;171:571–81. doi: 10.1534/genetics.105.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White-Cooper H. Tissue, cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis. 2012;2:11–22. doi: 10.4161/spmg.19088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–6. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert SF. Developmental Biology. Sinauer Associates Inc., Publishers Sunderland, Massachusetts, USA, 2006. [Google Scholar]
- 18.de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998;13(Suppl 1):1–8. doi: 10.1093/humrep/13.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 19.Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis: a molecular and biochemical perspective. Cytokine Growth Factor Rev. 2007;18:299–311. doi: 10.1016/j.cytogfr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nel-Themaat L, Gonzalez G, Akiyama H, Behringer RR. Illuminating testis morphogenesis in the mouse. J Androl. 2010;31:5–10. doi: 10.2164/jandrol.109.008235. [DOI] [PubMed] [Google Scholar]
- 21.Nel-Themaat L, Jang CW, Stewart MD, Akiyama H, Viger RS, Behringer RR. Sertoli cell behaviors in developing testis cords and postnatal seminiferous tubules of the mouse. Biol Reprod. 2011;84:342–50. doi: 10.1095/biolreprod.110.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–25. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- 23.Riechmann V, Rehorn KP, Reuter R, Leptin M. The genetic control of the distinction between fat body and gonadal mesoderm in Drosophila. Development. 1998;125:713–23. doi: 10.1242/dev.125.4.713. [DOI] [PubMed] [Google Scholar]
- 24.Moore LA, Broihier HT, Van Doren M, Lunsford LB, Lehmann R. Identification of genes controlling germ cell migration and embryonic gonad formation in Drosophila. Development. 1998;125:667–78. doi: 10.1242/dev.125.4.667. [DOI] [PubMed] [Google Scholar]
- 25.Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–96. doi: 10.1242/dev.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitadate Y, Shigenobu S, Arita K, Kobayashi S. Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev Cell. 2007;13:151–9. doi: 10.1016/j.devcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Kitadate Y, Kobayashi S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc Natl Acad Sci U S A. 2010;107:14241–6. doi: 10.1073/pnas.1003462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okegbe T. A Notch above bowl: Specification of niche cells in Drosophila testis. University of Pennsylvania, Philadelphia, USA, 2011. [Google Scholar]
- 29.Hatini V, Green RB, Lengyel JA, Bray SJ, Dinardo S. The Drumstick/Lines/Bowl regulatory pathway links antagonistic Hedgehog and Wingless signaling inputs to epidermal cell differentiation. Genes Dev. 2005;19:709–18. doi: 10.1101/gad.1268005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terry NA, Tulina N, Matunis E, DiNardo S. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev Biol. 2006;294:246–57. doi: 10.1016/j.ydbio.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–6. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 33.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–9. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 34.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29. [PubMed] [Google Scholar]
- 35.Luo H, Dearolf CR. The JAK/STAT pathway and Drosophila development. Bioessays. 2001;23:1138–47. doi: 10.1002/bies.10016. [DOI] [PubMed] [Google Scholar]
- 36.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 37.Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–6. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 38.Mohri T, Iwakuri T, Nakayama H, Fujio Y. JAK-STAT signaling in cardiomyogenesis of cardiac stem cells. JAK-STAT. 2012;1:1–6. doi: 10.4161/jkst.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine RL. Janus kinase mutations. Semin Oncol. 2009;36(Suppl 1):S6–11. doi: 10.1053/j.seminoncol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Ihle JN. The Janus protein tyrosine kinases in hematopoietic cytokine signaling. Semin Immunol. 1995;7:247–54. doi: 10.1006/smim.1995.0029. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–9. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–10. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Gregory L, Came PJ, Brown S. Stem cell regulation by JAK/STAT signaling in Drosophila. Semin Cell Dev Biol. 2008;19:407–13. doi: 10.1016/j.semcdb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Schindler C, Darnell JE., Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Torrado R, Yamada D, Defossez PA. Born to bind: the BTB protein-protein interaction domain. Bioessays. 2006;28:1194–202. doi: 10.1002/bies.20500. [DOI] [PubMed] [Google Scholar]
- 46.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–68. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–10. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21:4812–21. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- 50.Rawlings JS, Rennebeck G, Harrison SM, Xi R, Harrison DA. Two Drosophila suppressors of cytokine signaling (SOCS) differentially regulate JAK and EGFR pathway activities. BMC Cell Biol. 2004;5:38. doi: 10.1186/1471-2121-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–16. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- 52.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–6. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherry CM, Matunis EL. Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell. 2010;6:557–67. doi: 10.1016/j.stem.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 55.Raftery LA, Sutherland DJ. TGF-beta family signal transduction in Drosophila development: from Mad to Smads. Dev Biol. 1999;210:251–68. doi: 10.1006/dbio.1999.9282. [DOI] [PubMed] [Google Scholar]
- 56.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–21. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 57.Zhao GQ, Liaw L, Hogan BL. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development. 1998;125:1103–12. doi: 10.1242/dev.125.6.1103. [DOI] [PubMed] [Google Scholar]
- 58.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 59.Puglisi R, Montanari M, Chiarella P, Stefanini M, Boitani C. Regulatory role of BMP2 and BMP7 in spermatogonia and Sertoli cell proliferation in the immature mouse. Eur J Endocrinol. 2004;151:511–20. doi: 10.1530/eje.0.1510511. [DOI] [PubMed] [Google Scholar]
- 60.Itman C, Loveland KL. SMAD expression in the testis: an insight into BMP regulation of spermatogenesis. Dev Dyn. 2008;237:97–111. doi: 10.1002/dvdy.21401. [DOI] [PubMed] [Google Scholar]
- 61.Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–72. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 62.Schulz C, Kiger AA, Tazuke SI, Yamashita YM, Pantalena-Filho LC, Jones DL, et al. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–23. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bunt SM, Hime GR. Ectopic activation of Dpp signalling in the male Drosophila germline inhibits germ cell differentiation. Genesis. 2004;39:84–93. doi: 10.1002/gene.20030. [DOI] [PubMed] [Google Scholar]
- 64.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–75. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 65.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–5. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, McLeod CJ, Jones DL. Regulation of adult stem cell behavior by nutrient signaling. Cell Cycle. 2011;10:2628–34. doi: 10.4161/cc.10.16.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 68.Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–12. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137:2117–26. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parrott BB, Hudson A, Brady R, Schulz C. Control of germline stem cell division frequency--a novel, developmentally regulated role for epidermal growth factor signaling. PLoS One. 2012;7:e36460. doi: 10.1371/journal.pone.0036460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiley LM, Adamson ED, Tsark EC. Epidermal growth factor receptor function in early mammalian development. Bioessays. 1995;17:839–46. doi: 10.1002/bies.950171005. [DOI] [PubMed] [Google Scholar]
- 72.Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284:140–9. doi: 10.1016/S0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 73.Moghal N, Sternberg PW. The epidermal growth factor system in Caenorhabditis elegans. Exp Cell Res. 2003;284:150–9. doi: 10.1016/S0014-4827(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 74.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–4. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 76.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 78.Lagos-Cabré R, Moreno RD. Mitotic, but not meiotic, oriented cell divisions in rat spermatogenesis. Reproduction. 2008;135:471–8. doi: 10.1530/REP-07-0389. [DOI] [PubMed] [Google Scholar]
- 79.Cheng J, Tiyaboonchai A, Yamashita YM, Hunt AJ. Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development. 2011;138:831–7. doi: 10.1242/dev.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110:3011–8. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 81.Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci. 2002;59:1851–71. doi: 10.1007/PL00012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahni N, Yi S, Daniels KJ, Huang G, Srikantha T, Soll DR. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: insights into the evolution of new signal transduction pathways. PLoS Biol. 2010;8:e1000363. doi: 10.1371/journal.pbio.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alby K, Bennett RJ. Interspecies pheromone signaling promotes biofilm formation and same-sex mating in Candida albicans. Proc Natl Acad Sci U S A. 2011;108:2510–5. doi: 10.1073/pnas.1017234108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 2007;17:1253–8. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 85.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaffe AB, Hall A. Cell biology. Smurfing at the leading edge. Science. 2003;302:1690–1. doi: 10.1126/science.1092874. [DOI] [PubMed] [Google Scholar]
- 87.Kurokawa K, Itoh RE, Yoshizaki H, Nakamura YO, Matsuda M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol Biol Cell. 2004;15:1003–10. doi: 10.1091/mbc.E03-08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 89.Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amlani S, Vogl AW. Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat Rec. 1988;220:143–60. doi: 10.1002/ar.1092200206. [DOI] [PubMed] [Google Scholar]
- 91.Vogl AW. Changes in the distribution of microtubules in rat Sertoli cells during spermatogenesis. Anat Rec. 1988;222:34–41. doi: 10.1002/ar.1092220107. [DOI] [PubMed] [Google Scholar]
- 92.Suárez-Quian CA, Niklinski W. Immunocytochemical localization of the epidermal growth factor receptor in mouse testis. Biol Reprod. 1990;43:1087–97. doi: 10.1095/biolreprod43.6.1087. [DOI] [PubMed] [Google Scholar]
- 93.Joti P, Ghosh-Roy A, Ray K. Dynein light chain 1 functions in somatic cyst cells regulate spermatogonial divisions in Drosophila. Sci Rep. 2011;1:173. doi: 10.1038/srep00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 95.Loveland KL, Hime G. TGFbeta superfamily members in spermatogenesis: setting the stage for fertility in mouse and Drosophila. Cell Tissue Res. 2005;322:141–6. doi: 10.1007/s00441-005-0008-0. [DOI] [PubMed] [Google Scholar]
- 96.Hime GR, Loveland KL, Abud HE. Drosophila spermatogenesis: insights into testicular cancer. Int J Androl. 2007;30:265–74, discussion 274. doi: 10.1111/j.1365-2605.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 97.Gönczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–71. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- 98.Riparbelli MG, Colozza G, Callaini G. Procentriole elongation and recruitment of pericentriolar material are downregulated in cyst cells as they enter quiescence. J Cell Sci. 2009;122:3613–8. doi: 10.1242/jcs.049957. [DOI] [PubMed] [Google Scholar]
- 99.Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–7. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 100.Matunis E, Tran J, Gönczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–91. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- 101.Li CY, Guo Z, Wang Z. TGFbeta receptor saxophone non-autonomously regulates germline proliferation in a Smox/dSmad2-dependent manner in Drosophila testis. Dev Biol. 2007;309:70–7. doi: 10.1016/j.ydbio.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 102.Makokha M, Hare M, Li M, Hays T, Barbar E. Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74. Biochemistry. 2002;41:4302–11. doi: 10.1021/bi011970h. [DOI] [PubMed] [Google Scholar]
- 103.FlyBase Consortium The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 2003;31:172–5. doi: 10.1093/nar/gkg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Z, Edwards JG, Riley N, Provance DW, Jr., Karcher R, Li XD, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–69. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neely MD, Erickson HP, Boekelheide K. HMW-2, the Sertoli cell cytoplasmic dynein from rat testis, is a dimer composed of nearly identical subunits. J Biol Chem. 1990;265:8691–8. [PubMed] [Google Scholar]
- 107.Iyadurai SJ, Robinson JT, Ma L, He Y, Mische S, Li MG, et al. Dynein and Star interact in EGFR signaling and ligand trafficking. J Cell Sci. 2008;121:2643–51. doi: 10.1242/jcs.027144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, et al. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–39. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- 109.Papagiannouli F, Mechler BM. discs large regulates somatic cyst cell survival and expansion in Drosophila testis. Cell Res. 2009;19:1139–49. doi: 10.1038/cr.2009.71. [DOI] [PubMed] [Google Scholar]
- 110.Reeve S, Carhan A, Dee CT, Moffat KG. Slowmo is required for Drosophila germline proliferation. Genesis. 2007;45:66–75. doi: 10.1002/dvg.20265. [DOI] [PubMed] [Google Scholar]
- 111.Wassarman DA, Therrien M, Rubin GM. The Ras signaling pathway in Drosophila. Curr Opin Genet Dev. 1995;5:44–50. doi: 10.1016/S0959-437X(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 112.Schnorr JD, Holdcraft R, Chevalier B, Berg CA. Ras1 interacts with multiple new signaling and cytoskeletal loci in Drosophila eggshell patterning and morphogenesis. Genetics. 2001;159:609–22. doi: 10.1093/genetics/159.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 114.Desai BS, Shirolikar S, Ray K. F-actin-based extensions of the head cyst cell adhere to the maturing spermatids to maintain them in a tight bundle and prevent their premature release in Drosophila testis. BMC Biol. 2009;7:19. doi: 10.1186/1741-7007-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Schejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development. 2011;138:2729–39. doi: 10.1242/dev.059865. [DOI] [PubMed] [Google Scholar]
- 116.Kawamoto T, Kawai K, Kodama T, Yokokura T, Niki Y. Autonomous differentiation of Drosophila spermatogonia in vitro. Dev Growth Differ. 2008;50:623–32. doi: 10.1111/j.1440-169X.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 117.Parrott BB, Chiang Y, Hudson A, Sarkar A, Guichet A, Schulz C. Nucleoporin98-96 function is required for transit amplification divisions in the germ line of Drosophila melanogaster. PLoS One. 2011;6:e25087. doi: 10.1371/journal.pone.0025087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7:358–63. doi: 10.1097/00062752-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 120.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–87. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 121.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–10. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 122.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–55. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Armstrong CG, Mann DJ, Berndt N, Cohen PT. Drosophila PPY, a novel male specific protein serine/threonine phosphatase localised in somatic cells of the testis. J Cell Sci. 1995;108:3367–75. doi: 10.1242/jcs.108.11.3367. [DOI] [PubMed] [Google Scholar]
- 124.Boutanaev AM, Mikhaylova LM, Nurminsky DI. Up-regulation of the Ku heterodimer in Drosophila testicular cyst cells. FEBS Lett. 2007;581:1707–15. doi: 10.1016/j.febslet.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jung A, Hollmann M, Schäfer MA. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm development. J Cell Sci. 2007;120:2924–34. doi: 10.1242/jcs.006551. [DOI] [PubMed] [Google Scholar]