Abstract
Among most animals with internal fertilization, females store sperm in specific regions of their reproductive tract for later use. Sperm storage enables prolonged fertility, physical and temporal separation of mating from fertilization and, when females mate with multiple males, opportunities for differential use of the various males’ sperm. Thus, stored sperm move within the female reproductive tract as well as to several potential fates – fertilization, displacement by other sperm or ejection by the female. Drosophila melanogaster is a leading model system for elucidating both the mechanisms and evolutionary consequences of female sperm storage and differential male fertilization success. The prominence of Drosophila is due, in part, to the ability to examine processes influencing sperm movement and fate at several biological levels, from molecules to organ systems. In this review, we describe male and female factors, as well as their interactions, involved in female sperm storage and differential male fertilization success.
Keywords: cryptic female choice, cryptic male choice, female reproductive glands, genetic polymorphism, seminal fluid proteins, seminal receptacle, sperm competition, spermathecae
Introduction
For many animal species, mating and egg laying occur at different times and locations. This temporal and physical separation is mediated by the female’s ability to store sperm. Sperm storage includes the phases of sperm recruitment, maintenance, and release from particular regions of the female reproductive tract – frequently specialized sperm-storage organs (SSOs). Sperm storage allows the female to remain fertile for prolonged periods in the absence of potential mates, and to control the location and timing of oviposition.1-3 Females can influence sperm fate by moving and supporting stored sperm. Male products, in particular seminal fluid proteins (SFPs), also influence storage processes and sperm fate, in some cases at the expense of the female.4 When females mate with more than one male, male-male and male-female interactions within the female reproductive tract, particularly the SSOs, have profound consequences for sperm fate and, ultimately, male and female fitness. These consequences manifest most clearly as sperm precedence — the differential fertilization success of rival males. Drosophila melanogaster females mate frequently and store the sperm of multiple males in their SSOs. The wealth of well-developed genetic and genomic tools in Drosophila can therefore be applied to study the mechanisms and evolutionary consequences of female sperm storage. Here, we will review advances in our understanding of female sperm storage and sperm precedence in Drosophila. We will focus on molecular and cellular mechanisms underlying the functions of the SSOs, the effects of SFPs on sperm storage and use, and male and female factors affecting the precedence of one male’s sperm over another’s. For additional, comprehensive reviews of female remating, SFPs, and additional aspects of male-female interactions, see refs.5-12
Female Sperm Storage
Sperm-storage organs
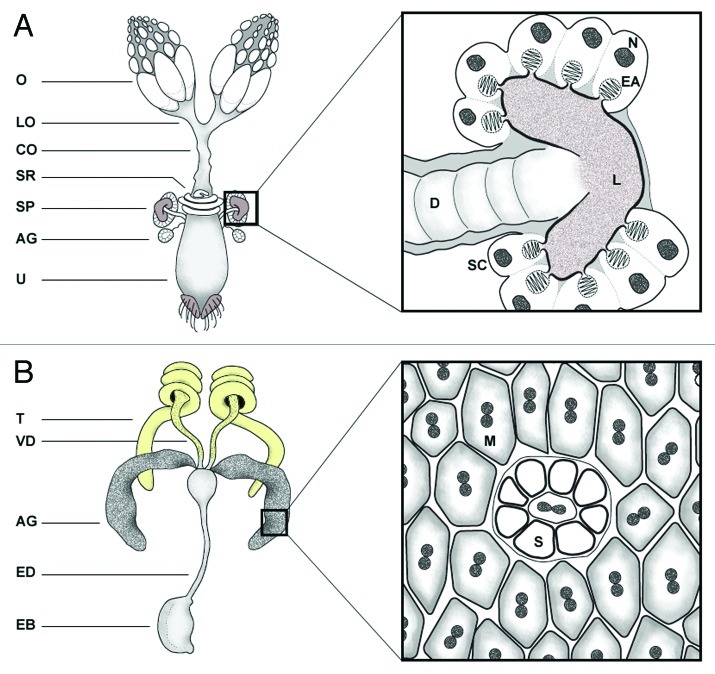
The D. melanogaster female possesses two types of SSOs located at the anterior of the uterus: a tubular seminal receptacle and the paired, mushroom-shaped spermathecae (Fig. 1). The seminal receptacle is a long, slender, closed-ended tube that narrows at the proximal end, whereas each of the spermathecae is composed of a duct that leads to the lumen of a cuticular capsule lined by secretory cells.7,13-16 Near the junctions between the spermathecal ducts and the uterus are two narrow ducts that lead to the female accessory glands (also known as parovaria), which have some known functions in immunity and fertilization in other insect species17-19 yet are poorly characterized in Drosophila. The spermathecae also function as glandular structures.14,19 Indeed, some Drosophila species do not store sperm in their spermathecae yet retain ducts and cells of presumably secretory function.20 The sperm stored in the seminal receptacle, rather than the spermathecae, constitute the primary source of sperm used for fertilization.21 The morphological and physiological differences between the SSOs suggest the spermathecae and seminal receptacle function independently in sperm storage, yet there is also evidence of communication between the two organs (see “Female influences on sperm recruitment, viability and usage,” below).
Figure 1. Overview of D. melanogaster female and male reproductive structures and glandular tissues. (A) The female reproductive system is shown in ventral view, with anterior to the top. It contains a pair of ovaries (O), from which mature eggs pass to the lateral oviducts (LO), which join to form the common oviduct (CO). Eggs are activated152 in the common oviduct before passing to the uterus (U), where fertilization takes place. The entrance to the egg, or micropyle, is adjacent to the openings of the ducts to the spermathecae (SP) and seminal receptacle (SR). Aside from their role as SSOs, the spermathecae function as glandular structures. Each spermathecal duct (D), which is surrounded by a thin layer of muscle and epithelial tissue, leads from the anterior-dorsal uterus to the lumen (L) of a cuticular capsule where sperm are stored. Surrounding the capsule is a ring of polarized secretory cells (SC), with nuclei (N) distal to the capsule, that release the contents of the end apparatus (EA), a large membrane-rich secretory organelle, into the lumen.28 Small accessory glands (AG) also connect through ducts to the anterior-dorsal uterus. (B) The male reproductive system is shown with anterior to the top. It contains a pair of testes (T), which connect through vasa deferentia (VD) to the anterior ejaculatory duct. A pair of lobed accessory glands (AG) also connect to the anterior ejaculatory duct. The male accessory glands are composed of a single layer of two distinct, binucleated, secretory cell types: the ‘main’ cells (M) and ‘secondary’ cells (S).153 The spherical secondary cells are located primarily at the distal tip of each gland, interspersed among the predominant hexagonal main cells. Each lobe is surrounded by a sheath of muscle that presumably squeezes the secretions of the cells into the ejaculatory duct (ED) and bulb (EB) to mix with sperm and other SFPs.153 Sperm are released from the vasa deferentia into the ejaculatory duct. Contractions in the ejaculatory duct propel the sperm and SFPs through the bulb and into the female at the time of ejaculation.16
Female reproductive physiology after a single mating
Sperm storage occurs in three major phases: recruitment to SSOs, maintenance of viability while in storage, and release from the SSOs. As early as 5 min from the start of mating, SFPs enter the posterior of the uterus followed by sperm.22-24 The secretions of the female reproductive tract are predicted to interact with the components of the ejaculate to form a sperm sac25 and allow for the hyperactivation26,27 and possibly modification of sperm.28 The activation of Pkd2, also known as Amo, a homolog of the TRPP2 calcium-permeable nonselective cation channel encoded by the Polycystic Kidney Disease 2 gene (PKD2) in humans, is required for sperm hyperactivation, reflected in higher tail-beat frequency of sperm, which swim predominantly tail first once inside the female reproductive tract.26,27 Shortly after sperm are transferred, and continuing after mating ends, a series of uterine conformational changes move the sperm from the posterior of the uterus to the anterior end and adjacent to the SSO entrances29,30 According to different estimates, approximately 1,500 to 4,000 sperm are passed to females during a ~20 min mating, yet only 25–35% of sperm enter the SSOs.16,21,31 The majority of stored sperm, ~75%, are rapidly stored within the seminal receptacle, with the remainder accumulating at a slower rate in the two spermathecae.21,31 Although the majority of sperm is stored within ~1 h after mating, total accumulation and movement among SSOs does not end until several hours later.30,31 The process of accumulation and movement corresponds temporally with changes in gene expression and tissue composition in the uterus, SSOs and oviduct.32-35 After this time, and before the first egg is ovulated, unstored uterine sperm are expelled.21,25,36 From a single mating, females can lay between 300 and 400 eggs and remain fertile for nearly two weeks.3,31,37-39
Female Influences on Sperm Recruitment, Viability and Usage
Female input is required for sperm recruitment to the SSOs
The female influences all phases of sperm storage through interactions that occur at the tissue and molecular levels. As described above, conformational changes in the female reproductive tract correspond with sperm movement toward and into the SSOs. Furthermore, as the spermathecae and seminal receptacle have distinct morphologies, tissue compositions and evolutionary histories, it is not surprising that they appear to have distinct mechanisms of sperm storage. Storage dynamics (rates and quantities of sperm accumulating in the SSOs) differ between the seminal receptacle and spermathecae. Also, an intact female central nervous system is necessary for recruitment of sperm to the SSOs, but apparently more so for the spermathecae than the seminal receptacle. Disrupting the signaling between the female’s central nervous system and the SSOs, either by masculinizing the central nervous system or by separating the female abdomen from the thorax, resulted in significantly reduced sperm storage, particularly in the spermathecae when compared with the seminal receptacle.40
Female-expressed proteins influence the recruitment of sperm to the SSOs (Table 1). Glucose dehydrogenase (Gld) is upregulated upon mating in the proximal and distal portions of the spermathecal ducts, yet absent in the seminal receptacle.41-44 Although females mutant for Gld store a wildtype number of sperm in the seminal receptacle, they store fewer sperm in the spermathecae and often show an asymmetry in storage, with one spermathecal capsule containing many sperm and the other containing none.41
Table 1. Reproductive Proteins with Demonstrated Roles in Sperm Storage and Sperm Precedence.
| Protein | Origins | Protein class | Effect | Reference |
|---|---|---|---|---|
| Sex peptide (Acp70A)1 |
AGa |
Prohormone |
Decreases female receptivity, oogenesis, sperm release |
70
,
71
,
148
,
149
|
| Glucose Dehydrogenase (Gld) |
SPb, EDc, EBd |
Enzyme |
Sperm storage and release |
41
-
44
,
63
|
| Acp36DE |
AG |
Prohormone |
Sperm storage |
30
,
65
,
66
,
135
|
| Esterase-6 |
ED |
Enzyme |
Sperm storage and release from storage |
31
,
75
|
| Acp29AB |
AG |
Lectin |
Sperm retention in storage |
76
|
| CG9997 |
AG |
Serine Protease |
Sperm retention in the seminal receptacle |
11
,
63
|
| CG1757 |
AG |
Crisp |
Sperm retention in the seminal receptacle |
11
,
63
|
| CG1652 |
AG |
C-type Lectin |
Sperm retention in the seminal receptacle |
11
,
63
|
| CG1656 |
AG |
C-type Lectin |
Sperm retention in the seminal receptacle |
11
,
63
|
| Acp62F |
AG |
Protease Inhibitor |
Sperm precedence |
68
,
69
,
136
|
| CG11864 |
AG |
Metalloprotease |
Sperm storage and ovulation |
62
,
64
|
| Seminase |
AG |
Trypsin-type Serine Protease |
Sperm storage and ovulation |
62
|
| CG6168 |
AG |
Protease |
Female latency to remate and sperm precedence |
59
|
| Sdic |
Se |
Sperm Dynein Intermediate Chain |
Sperm precedence, possibly motility |
74
|
| Wasted | S | Unknown | Sperm retention in SSOs, sperm chromatin decondensation, efficiency of egg entry | 73 |
a) AG, Accessory Glands; b) SP, Spermathecae; c) ED, Ejaculatory Duct; d) EB, Ejaculatory Bulb; e) S, Spermatozoa. 1. The female sex peptide receptor (SPR) has a hypothesized role in sperm storage and sperm precedence, although it has not been definitively demonstrated.
Although Table 1 only lists a single female-expressed gene with a demonstrated role in sperm storage (Gld), reflecting the paucity of molecular knowledge of female contributions to the process, there is evidence that there are more genes to be discovered. In particular, gene products made in the spermathecal secretory cells appear necessary for recruitment of sperm into storage in the spermathecae. The identification of regulatory regions of genes expressed exclusively in the spermathecal secretory cells has made it possible to develop genetic tools that allow the manipulation of these cells in a precise spatiotemporal manner. Genetic ablation of the spermathecal secretory cells using these regulatory sequences to drive an apoptosis-promoting protein in otherwise normal females results in decreased sperm recruitment to the spermathecae, but not the seminal receptacle.45
Maintenance of sperm in the SSOs
Although sperm are recruited normally to the seminal receptacles of females lacking the spermathecal secretory cells, sperm begin to lose motility within 1 d of mating.45 This result implies that products of these cells act at a distance in the reproductive tract, and is consistent with prior results showing a loss of sperm viability in mutants impaired for the development of the spermathecae and female accessory glands.13,28 It is also consistent with the view that the glandular function of the spermathecae is as important as their function as storage receptacles.20,28 Notably, ablation of the spermathecal secretory cells after mating does not affect sperm maintenance in either the spermathecae or seminal receptacle.45 The cells’ secretions are therefore required before or around the time of mating and may potentially interact with SFPs or female targets within the reproductive tract.
The particular spermathecae-secreted gene products that are required for sperm storage are not known, although several notable functional categories are found in the lists of genes expressed highly in the spermathecae and, more broadly, in response to mating in the reproductive tract. These categories include serine proteases, which might participate in signaling cascades or process SFPs to active or inactive forms; proteins involved in carbohydrate and lipid metabolism, which might participate in sperm maintenance or maturation; and antimicrobial peptides, which might protect sperm or females from infectious agents transferred during copulation.28,32,34,35,42,46-51
Female control over sperm release from the SSOs
Some of the same types of factors that influence recruitment of sperm also appear to influence sperm release. In particular, the nervous system plays an important role. Nerve terminals with putative octopaminergic-tyraminergic function innervate the proximal portion of the seminal receptacle and extensively along the spermathecae. In both SSO types, nerve terminals are associated with muscle fibers.52 Female control of sperm release is coordinated by the neuromodulators tyramine and its derivative octopamine, which act via these SSO-associated octopaminergic-tyraminergic neurons.52 The effects of removing tyramine or octopamine differ between the SSOs; in the absence of both tyramine and octopamine, sperm release from the seminal receptacle and spermathecae is reduced, whereas in the absence of octopamine alone, only sperm release from the seminal receptacle is reduced.52 Octopamine and tyramine also influence female fertility in that they are necessary for egg laying.53-55 The nature of the coordination between ovulation and sperm release from storage sites remains unknown. Although sperm release from storage corresponds with ovulation, it does not directly depend on it.56 Gld also plays a role in sperm release. Gld-mutant females use sperm at a slower rate and retain fertility longer than wildtype controls.41
Sperm usage might depend on the post-mating maturation of the female reproductive tract. Mating induces substantial changes in the physiology and structure of the reproductive tract. For example, as the rate of ovulation increases dramatically during the ~6 h after mating, the common oviduct exhibits changes in gene expression, undergoes tissue remodeling, and becomes highly innervated.34 During this period, fertilization efficiency is lower when compared with the overall fertile period of females.57 It is yet to be determined whether or not this lower efficiency is due to the inability of the maturing tissues of the female reproductive tract to support a high fertilization rate.34 The mechanism by which sperm leave or are released from the SSOs remains unknown, let alone how this mechanism changes as the reproductive tract matures.
Male Influences on Sperm Recruitment, Viability and Usage
Seminal fluid proteins
SFPs produced in the male accessory glands and ejaculatory bulb mediate physiological and behavioral changes that affect sperm storage and usage. Several studies have identified key SFPs that influence sperm dynamics and significantly impact female reproductive physiology and behavior6,58-61 also reviewed in ref.6,8,58 SFPs act to promote the male’s reproductive interests, which theory predicts should in some cases match the female’s reproductive interests and in other cases run counter to them. Either way, the female sperm storage tissues and their secretions likely interact with male proteins to influence patterns of sperm usage. One apparent case of male-female cooperation is a proteolytic cascade inside the female that tightly regulates the cleavage and activation of prohormones that secure sperm in the SSOs then increase ovulation.62,63 CG11864, a predicted metalloprotease, undergoes self-cleavage after activation by the trypsin-type serine protease Seminase.62 While being passed to the female during mating, CG11864 in turn cleaves the SFPs Acp36DE and ovulin, which promote sperm storage and ovulation, respectively (see below).64
Several SFPs facilitate the recruitment of sperm to the SSOs. A male lacking accessory glands transfers a normal number of sperm to his mate, yet the recruitment of sperm into the SSOs is reduced by 90%.39 The 122 kDa glycoprotein Acp36DE is necessary for maximizing the total number of sperm stored in these organs.65,66 In the female, the active, cleaved version of Acp36DE stimulates conformational changes in the uterus that position sperm adjacent to the SSO entrances.30
Sperm viability
It is unclear how SFPs promote sperm viability while stored in the female SSOs, as the majority of these proteins are no longer detected within the female shortly after mating.30,67 However, indirect evidence of effects on sperm maintenance come from studies of multiply mated females. For example, Acp62F, which localizes to the SSOs within one hour post-mating68 is required for sustained success of the first male’s sperm (see “Male influences on sperm precedence: sperm competition” below).69
Sperm release from the SSOs
The most extensively studied SFP, sex peptide, binds to sperm and is slowly cleaved off during storage.70 Sex peptide is required to promote efficient sperm release from storage71 and to maintain the sexual refractory period during which females reject subsequent courting males.70,72 Several SFPs promote the binding of sex peptide to sperm in a precise spatiotemporal manner during transfer to the female.11,62,67,71 Physical properties of the sperm themselves may also impact sperm release as sperm of males mutant for genes encoding sperm components — such as wasted and the Sdic (Sperm dynein intermediate chain) gene family — exhibit abnormal release from storage in single or double matings, respectively.73,74
As with female influences, there appears to be overlap between male factors that promote storage in the SSOs and those that promote release from the SSOs. The SFPs Esterase-6 and Gld (see also “Female influences on sperm recruitment, viability and usage” above), promote both sperm storage and release from the SSOs.41,75 However, other SFPs appear only to influence release. The predicted lectin Acp29AB localizes to the spermathecae (and possibly also the seminal receptacle) and promotes sperm retention in both SSOs.76 The SFPs CG9997 (a serine protease), CG17575 (a cysteine-rich secretory protein), and CG1652/CG1656 (C-type lectins) promote sperm retention in the seminal receptacle, but not the spermathecae.11
A mating plug influences remating but not sperm storage
SFPs contribute to a temporary mating plug that forms posterior to the sperm mass in the uterus. In at least one other Drosophila species, D. hibisci, it appears that this plug acts to secure sperm in the SSOs,77 but in D. melanogaster there is no evidence that the plug affects sperm storage or release.78 Instead, the plug appears to play a role in delaying remating. The refractory period to remating in females takes several hours to develop79 and, at least in laboratory settings, females can remate until this effect takes place78 The mating plug reduces remating rates in the first 4 h after mating.78 PEBII and PEB-me, two SFPs made in the ejaculatory bulb, are required for the formation of this short-term barrier to reinsemination.23,24,78,80 The mating plug persists in the female reproductive tract until it is ejected by the female.21,36
Female Multiple Mating
Remating prevalence and sperm precedence
Despite the continued fertility afforded by storing sperm, as well as the refractory effects of SFPs and the mating plug, females in the laboratory mate with another male before their sperm stores are entirely depleted, typically within ~5d after mating.81 Mature females appear to have control over whether or not to accept a mate,82 although newly eclosed females may not.83 Female remating tendency shows natural variation, and polymorphisms in two genes, Obp56a, encoding an odorant binding protein, and CG9897, encoding a serine protease, have been associated with this variation.84 Female receptivity to subsequent courting males is also influenced by male size,85 quantities of stored sperm,86-88 and the previous receipt of SFPs8,9,89,90 whose effects are prolonged by the presence of sperm91 (reviewed in ref.5). Female multiple mating in their natural environment is also well-documented.92,93 A large proportion (46% - 100%) of captured females retain sperm from more than one male92-94 and as many as six males in their storage organs.94,95 Although remating frequencies may be slightly elevated in established laboratory populations relative to field-caught females,96 female multiple mating is an integral part of the Drosophila melanogaster life history.
Because the sperm from more than one male co-occur within the female’s reproductive tract, an opportunity exists for non-random, differential fertilization success among the different males’ sperm. In D. melanogaster, the last mating male fertilizes, on average, ~80% of the subsequent offspring.38,97-99 This last male sperm precedence is an important determinant of male reproductive fitness (measured as offspring production).100 In principle, sperm precedence can be partitioned into distinct male and female components: competition between/among males, called sperm competition,101-103 and female choice between/among male ejaculates, called female sperm preference or cryptic female choice (cryptic because it is internal and not easily observable).2,104 However, because the outcome of sperm precedence depends on both male and female genotypes (reviewed below), it is difficult to disentangle sperm competition and cryptic female choice both experimentally and conceptually. Evidence from a range of animal systems indicates that sperm competition and cryptic female choice are powerful evolutionary processes shaping male and female reproductive behavior, morphology, physiology and biochemistry,2,4,102,103 as well as maintaining polymorphisms within populations.105,106 Males also allocate their ejaculate among mates in response to social environment or female condition, known as cryptic male choice.107-110
Sperm precedence assays offer distinct benefits over single-pair mating assays when exploring causes and consequences of sperm fate. As described above, observing reproductive outcomes of sperm competitive (male) and preference (female) scenarios more accurately reflects the reproductive biology of this species. Also, the examination of sperm fate in competitive scenarios is often a more sensitive assay for sperm performance than is the examination of reproductive outcomes from single-pair matings, as shown for Sdic mutants.74 Despite the challenge of identifying and characterizing specific mechanisms by which cryptic processes occur, the last ten years have been particularly fruitful in the following areas: a) describing sperm localization and displacement dynamics within the female reproductive tract, b) identifying male genes involved in differential fertilization success, c) dissecting cryptic male responses to the social environment and female condition, and d) elucidating genetic interactions underlying sperm competition and cryptic female choice.
Storage of multiple ejaculates
Female remating initiates a series of events ultimately resulting in the displacement of a portion of the previous male’s sperm and storage of the most recent mating male’s sperm.21,111-113 This phenomenon is typically examined as two components: the continued use of the previous mating male’s sperm (resistance to displacement), and the use of the most recent mating male’s sperm (ability to displace previously stored sperm). The development of transgenic males with either GFP- or RFP-labeled sperm has been valuable for elucidating interactions between competing ejaculates within the female reproductive tract in real time.21 Within minutes of a female’s remating, and before sperm transfer has begun, a small portion of sperm from the previous male leaves her SSOs and returns to the uterus.21 Sperm transfer occurs shortly thereafter (within 4–10 min of mating21,29,114) and previously stored sperm continue to leave the SSOs during storage of the mating male’s sperm. Although the accumulation of sperm in storage appears to be maximized by one hour after the start of mating (reviewed in ref.6), storage dynamics – including displacement of stored sperm by uterine sperm and mixing of the two males’ sperm due to their movement – continues for several hours.21,112 Eventually, unstored sperm are ejected21,36 in a process that precedes, and may be independent from, oviposition.21 Second-male sperm compose the majority of the dumped mass.21
While it is clear that sperm are physically displaced from female storage organs, observations of decreased first-male paternity without clear decreases in the storage of his sperm led to the hypothesis that sperm are also functionally displaced, by death or incapacitation.111,113 Currently, alternative explanations exist for the observed decline in fertility, such as female sperm dumping36 and differential fertilization efficiency.115 Vital staining of stored sperm reflects a small proportion of death over time in storage,36,116 although it is not attributable to remating.36,117 Additionally, observation of interactions between living sperm from two transgenic males failed to detect any effect of the presence of second-male ejaculate on the motility of first-male sperm.21 Support for the incapacitation hypothesis is further diminished by evidence that a second-mating male ejaculate does not distinguish self from other,118 and even supports sperm use and viability (the latter in vitro) from a previously mating male.91,117
Sperm remaining in the female reproductive tract 5 h after mating are called the fertilization set. A direct relationship between the relative proportion of each male’s stored sperm and short-term (72 h) relative fertilization success exists for the seminal receptacle, but not for the spermathecae.21,112 Within the spermathecae, displacement is more variable, as measured by the proportion of second-male sperm stored (there are more cases of relatively fewer second-male sperm).21 This, along with the direct observation that sperm of the two males are physically mixed within the seminal receptacle21,111 indicates that short-term sperm utilization in the seminal receptacle is a ‘fair raffle’ – a function of proportion – and that sperm within this fertilization (sub)set are unlikely to be preferentially selected by the female.
Female influences on sperm precedence, cryptic female choice
The female reproductive tract is the environment in which ejaculates from multiple males interact with each other and with female tissues and molecules.2,7 Therefore, females are predicted to have profound effects on the fates of stored sperm, although unequivocal demonstration of cryptic female choice has been challenging.119-122 These female effects appear to occur at the levels of behavior, morphology, physiology and biochemistry. Because a female’s first mating induces additional reproductive tract maturation at both molecular and morphological levels,32-34 a male mating with a previously-mated female encounters an environment that differs from that of a virgin female. This may have implications for the fate of a male’s sperm as the female continues to mate (and store sperm from) additional males. One experiment, deducing sperm displacement within 18 h post-mating based on offspring phenotypes, did not detect a difference between second-mated and third-mated females.97 However, another experiment, also deducing short-term sperm fate by observing offspring phenotypes, did detect virgin effects on sperm-use patterns. Although this result is consistent with mating-induced reproductive tract maturation, it might also be explained by female age effects.123 Together, these results highlight the need to further investigate how female mating history influences sperm fate over time.
Insight into the mechanisms of cryptic female choice – and the central role of sperm storage in the process - comes from multiple experimental approaches. At the behavioral level, females can dump stored sperm by a mechanism that is both sperm- and ejaculatory fluid-independent: second males transferring only seminal fluids (i.e., spermless) or transferring no ejaculate at all triggered the complete removal of sperm from the seminal receptacle in 32% of experimental females.36 The extent to which the amount expelled can be modulated, what causes a female to dump sperm, and the ultimate fate of the sperm expelled into the uterus remain unknown. Also unknown are the contributions of factors demonstrated to affect aspects of sperm storage in single matings, such as the female nervous system, neuromodulators, and uterine conformational changes (reviewed above), to cryptic female choice. However, examination of SSO morphology, particularly with regard to interactions with sperm morphology, supports the existence of cryptic female choice and suggests possible mechanisms by which it occurs. Females with supernumerary spermathecae favor the use of the last mating male’s sperm, but only within a few days after the second mating.124 It is not clear whether this difference is related to an altered geometry of the reproductive tract, an altered secretory capacity, or another factor. However, the difference does not appear to be directly attributable to an increased storage capacity because these ‘supernumerary’ females produce fewer offspring than wild-type females.124 Using lines independently selected for different seminal receptacle length or sperm length, Miller and Pitnick (2002, 2003) demonstrated that variation in seminal receptacle length influences sperm precedence though its interaction with variation in sperm tail length. Variation in seminal receptacle length alone was insufficient to affect sperm-precedence patterns (although females with longer seminal receptacles recruited more sperm).125,126
Male influences on sperm precedence, sperm competition
Because a male’s fertilization success is a critical component of his reproductive fitness,100 it is not surprising that males possess multiple mechanisms promoting their success in sperm competition. Disruption of sperm performance (e.g., viability, motility, ability to bind seminal fluid proteins, etc.) naturally or experimentally will likely alter sperm competitive ability, and therefore measures of sperm precedence. The ability to isolate components of the copulatory stimulus (copulation, male accessory gland products, and sperm) facilitates dissection of male effects on the ability to displace and resist displacement of stored sperm.91,127-129 Both direct examination of sperm presence and indirect inference from offspring phenotypes provide evidence that the second male’s sperm enters the SSOs and displaces the previous male’s sperm (21,112 and suggested by ref.38). Moreover, direct examination of sperm localization in the female reproductive tract has revealed that a component of the mating stimulus that is neither sperm nor male accessory gland product is sufficient to cause physical displacement of sperm.21,36 Finally, SFPs influence levels of sperm competition through their effects on sperm recruitment, retention and distribution among SSOs (described below, and reviewed in refs.8,9,130).
In D. melanogaster, genes harboring polymorphisms affecting sperm competition occur across the X, Y, second and third chromosomes.131-134 Relatively few of the X chromosome genes associated with sperm competitive ability code for SFPs. Characterization of these genes will provide novel insights into the mechanisms involved in sperm competition. For example, Sdic is necessary for sperm competitive ability; males lacking the Sdic family demonstrate a poor capacity to displace sperm relative to controls.74 Although these sperm-tail proteins have a hypothesized role in sperm motility, mutant motility appears superficially normal in vitro and mutant males have normal fertility in single-mating situations.74 This result highlights the sensitivity of sperm-precedence assays to detect subtle aspects of sperm performance on reproductive outcomes. At least seven SFPs have been identified (described in the following paragraph) that, when inhibited, influence sperm precedence through their effects on sperm storage dynamics or as yet-to-be identified mechanisms (Table 1; reviewed in refs.8,9,130). Their varied effects reinforce the idea that multiple processes affect the outcome of sperm competition.
The ability to manipulate SFP levels has revealed their diverse roles in sperm fate. Some SFPs appear to affect sperm competitive ability by influencing the first male’s representation in the fertilization set, as deduced from offspring phenotypes. For example, Acp36DE-deficient males experience lower fertilization success because fewer of their sperm accumulate in storage, resulting in a lower contribution to the subsequent fertilization set.65,135 Another SFP, Acp29AB, is required to retain sperm in female storage sites.76 As a likely result of this, males who do not transfer Acp29AB to their mates have a lower capacity to resist sperm displacement relative to controls.76 Still other SFPs support sperm utilization from storage sites. Sex peptide, CG9997, CG17575, and CG1652/CG1656 interact with each other to support normal sperm release from the seminal receptacle.11,63 As a result of their effects enabling normal sperm depletion, decreased levels of each of these SFPs by RNA interference increase a male’s resistance to sperm displacement.30,71 Finally, some SFPs’ effects on sperm competition occur by as-yet unidentified mechanisms. Acp62F, a protease inhibitor that is toxic upon ectopic expression,68,136 is associated with increased displacement; Acp62F mutants exhibit elevated second-male sperm precedence.69 While Ap62F mutants in single-mating scenarios do not alter their mates’ egg-laying rates or sperm storage dynamics,69 polymorphisms in this gene are associated with female fecundity and the ability to displace resident sperm.132 This last example also illustrates how examining the effects of natural genetic variation on sperm precedence provides an important complement to studies of mutants and is crucial to understanding how evolutionary selection shapes reproductive function (see ‘The role of natural genetic variation’, below).
Drosophila melanogaster males respond strategically to perceived level of sperm competition in ways that increase their fertility. One means of achieving this is by altering their copulatory behavior in response to the physical presence of other males. While the initiation of copulation may be under female control,82 its duration appears to be largely, although not completely (described below), under male control.137,138 After sperm transfer occurs, additional time in copula increases the female’s latency to remating114 and, possibly, fecundity,138 (but see ref.114) thereby increasing male resistance to sperm displacement. In the presence of rivals, males shorten both their latency to mating and copulation duration, resulting in decreased displacement of a previous male’s sperm. However, when exposed to other males prior to mating, males had longer copulation durations resulting in higher short-term (24 h) fecundity, displacement of rival sperm, as well as resistance to sperm displacement by other males.89 The latter was likely attributable to the induction of longer female remating latencies. Strategic responses extend to quantitative and qualitative shifts in SFP allocation. In the presence of a rival, males increase the quantity of sex peptide and ovulin transferred to the female108 resulting in increased male fertility by stimulating female short-term fecundity and delaying both cryptic female choice and sperm competition by decreasing female remating rates. Consistent with predictions that males benefit by manipulating female latency to remating, but not fecundity (because fecundity does not increase with repeated matings138,139) second-mating males transfer decreased levels of ovulin, but similar levels of sex peptide as males mating with virgin females.110
Male influences of sperm precedence, cryptic male choice
Males also respond strategically to female condition. The number of sperm a mating male transfers to the female corresponds with the extent of displacement from storage of the previous-mating male’s sperm.21 Perhaps because of this, males copulate for longer durations with81,138 and transfer more sperm to109 previously mated females than virgins. Males appear to be responding, in part, to female cuticular hydrocarbon composition. Male copulation durations with virgin females carrying the odor of a mated female did not differ from durations with mated females – both of which were significantly longer than copulation durations with virgin females.138 In single-pair scenarios, males transfer more sperm to younger females than older females.109 This might help explain why second-male sperm precedence decreases with older females (effect first detected 17 d post-eclosion),140 which is unlikely to be due to decreasing sperm viability with female age.116 Instead, males may make a decreased investment in older females, resulting in insufficient storage of last-mating male sperm or compromised displacement of first-male sperm.
Female-male interactions influencing sperm precedence
Reproduction involves both cooperation and conflict. While successful reproduction requires males and females to coordinate the introduction of mature and activated gametes, characteristics increasing male reproductive success in a competitive environment do not necessarily improve female reproductive success and may even be detrimental to it.9,10,129,139,141,142 Several lines of evidence illuminate how male-female interactions determining sperm utilization patterns are mediated at the level of behavior and morphology. While males appear to have a large influence on copulation duration, it is also affected by the genotype of the female with whom they mate via yet unidentified traits.137 The previously described effect of the interaction between variation in seminal receptacle length and variation in sperm length on sperm precedence126 highlights the complex interdependence of male and female traits that ultimately determines sperm fate.
The role of natural genetic variation
Observed variation in sperm precedence values and associated fertility components is due, in part, to genetic variation.106 Examination of the genetic architecture of sperm precedence (the identification of genes involved in sperm precedence, their relative effects on sperm precedence and their interactions with each other) has provided valuable insights into the nature of male and female influences on sperm precedence, as well as the maintenance of genetic variation in the presence of strong selective pressures,106 and has yielded novel genes with potential roles in sperm competition and cryptic female choice. These insights clarify the opportunities and limits for selection on male and female fertility phenotypes to result in evolutionary change.
Males from distinct genetic lines exhibit significant variability in their capacity both to displace sperm of other males and to resist displacement of their own sperm.38,99,111,112,131,134 These capacities are, to some extent, non-transitive; the outcome of sperm precedence depends on the particular combination of competing males. In some cases, these population-level differences in sperm displacement are not directly attributable to differences in quantities of sperm stored,112 suggesting that more than one mechanism is involved in the ability to displace sperm and resist displacement. Female genetic variation is also associated with the extent of sperm displacement84,134,143 and differences in remating latency.84,134 Moreover, identification and examination of the relationships among genetic polymorphisms associated with different levels of sperm precedence and associated fertility components reveal significant male-female interactions.134,144 These male-male and male-female interactions likely reflect the multiple interacting traits involved in both male and female fertility and might serve to maintain genetic variation and decrease potential inbreeding144,145 (also see ref.146). These interactions are also important, because they indicate that there is unlikely to be a single ‘best male’ genotype for sperm precedence,105 and that the fertilization environment (competing males and even female144) is critical for determining fertilization success.
Although it remains possible that an allele conferring fertilization success could go to fixation in a population, the high extant levels of relevant genetic variation suggest that such events are rare. Although selection on male and female traits involved in sperm precedence may be intense, it may not be consistent; non-transitivity of sperm precedence may maintain polymorphisms for genes with important effects on sperm precedence in both males106 and females.134,144 Antagonistic pleiotropy is another phenomenon maintaining genetic variation, as evidenced by an allele of the gene encoding CG17331, a predicted endopeptidase, that both increases a male’s ability to displace resident sperm and decreases a female’s latency to remate.131 Pleiotropy is well-documented for male reproductive genes131,132,147 and may reflect common mechanisms among various components of fertilization success. For example, sex peptide acts in the female reproductive tract and central nervous system to affect female fecundity,148 latency to remate,79 cost of mating,149 sleep and locomotion.150 Epistatic effects are also well documented131,132 and possibly reflect interactions among reproductive proteins, such as the interactions among sex peptide, CG9997, CG17575, and CG1652/CG1656 needed for appropriate retention of stored sperm.11,63
Because SFPs have well-documented effects on sperm storage dynamics, female fecundity, female longevity, and sperm precedence,8,9,129,130 they are attractive candidates for augmenting male reproductive success. Some of the variation in both components of sperm competition (the ability to displace and resistance to displacement) and closely associated female responses such as fecundity, remating rate and sperm storage corresponds to variation in specific reproductive proteins.99,131,132,134,147 This relationship reflects ample available variation for natural and sexual section to occur. Some of these relationships further characterize genes with already-identified functions, such as sex peptide and Esterase-6.132 However, associations between polymorphisms in uncharacterized male reproductive genes with components of sperm competition suggest functions for these genes that can be tested. For example, polymorphisms in CG6168, encoding a predicted protease,59 are associated with female latency to remate, an ability to displace sperm as well as the ability to resist displacement.132 Based on these phenotypes, future experiments could examine whether CG6168 mutants appropriately retain stored sperm or processed SFPs. Female receptors for male SFPs likely mediate the magnitude of the female’s response although additional genes are likely involved.147 Quantitative trait locus mapping identified three regions of the female genome corresponding with each of three female traits affecting sperm precedence: ability to displace sperm, remating latency, and fertility.151 Although these accounted for significant levels of variation in female phenotypes assayed, large (> 85%) unexplained variation suggests additional factors influence expression of these characteristics.151 Narrowing the focus to examination of polymorphisms involved in a single male-female protein interaction, sex peptide and the corresponding female sex peptide receptor, revealed two significant allelic interactions affecting the male’s ability to resist displacement.134 These results suggest the value of additional assays examining the functional interaction(s) between sex peptide and sex peptide receptor as well as their effects on sperm precedence.
Future Directions for Female Sperm Storage and Utilization
The past 10 y have produced important insights into the timing and mechanisms of sperm movement within the female reproductive tract from a single male as well as overlapping ejaculates from multiple males. In particular, there is a better understanding of the SFPs involved in various aspects of sperm storage and how they interact with each other and the female to influence sperm movement and fate. In general, understanding female contributions to sperm storage and usage have lagged behind elucidation of male contributions. However, expression profiles of the SSOs, increased knowledge of the secretory tissues of the female reproductive tract, and documented interactions between SFPs and the female reproductive tract set the stage for continued exploration of female responses to the presence of sperm and SFPs. This holds promise for exploring male-female reproductive interactions from the level of protein-protein interactions to coevolutionary dynamics. For example, understanding the functions of the numerous spermathecae-expressed proteases will likely shed light on cooperative and antagonistic aspects of reproduction.
The study of natural genetic variation also holds increasing importance. The revolution in next-generation DNA sequencing has been a boon to population-genetic studies in all fields. The study of sperm fate will benefit from the increased power and resolution that such studies can now achieve. This kind of study will be made even more meaningful by better knowledge of natural mating behavior and more efforts to study sperm precedence in experimental settings that more accurately reflect the environment in which they have evolved, especially settings involving more than two males or, even better, populations. After all, it is because of its rich and varied sexual life, as well as the extensive toolkit for its experimental manipulation, that Drosophila melanogaster serves as such an excellent model for sperm storage and usage in animals.
Acknowledgments
We are grateful to Julie Brill and Mariana Wolfner for the invitation to contribute this review. S.L.S. was supported by a US-Israel Binational Science Foundation grant to MLS and Yael Heifetz (Hebrew University). M.C.B.Q. was supported by a Research, Scholarship and Creativity grant from Gustavus Adolphus College. We appreciate the thoughtful and constructive feedback provided by Anthony Fiumera, Yael Heifetz and four anonymous reviewers. Finally, we apologize to colleagues whose work was not included in this review due to space limitations.
Glossary
Abbreviations:
- Gld
glucose dehydrogenase
- SFP
seminal fluid protein
- SSO
female sperm storage organ
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest and have no financial interests to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/21655
References
- 1.Birkhead TR, Møller AP. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biological Journal of the Linneaen Society. 1993;50:295–311. doi: 10.1111/j.1095-8312.1993.tb00933.x. [DOI] [Google Scholar]
- 2.Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton, NJ: Princeton University Press, 1996. [Google Scholar]
- 3.Neubaum DM, Wolfner MF. Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Curr Top Dev Biol. 1999;41:67–97. doi: 10.1016/S0070-2153(08)60270-7. [DOI] [PubMed] [Google Scholar]
- 4.Arnqvist G, Rowe L. Sexual Conflict. Princeton: Princeton University Press, 2005. [Google Scholar]
- 5.Singh SR, Singh BN, Hoenigsberg HF. Female remating, sperm competition and sexual selection in Drosophila. Genet Mol Res. 2002;1:178–215. [PubMed] [Google Scholar]
- 6.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/S0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 7.Heifetz Y, Rivlin PK. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology. 2010;73:723–39. doi: 10.1016/j.theriogenology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, Chow CY, et al. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet. 2009;68:23–56. doi: 10.1016/S0065-2660(09)68002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered. 2009;100:399–410. doi: 10.1093/jhered/esp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007;3:1–11. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitnick S, Wolfner M, Suarez S. Sperm-female interactions. In: Birkhead TR, Hosken DJ, Pitnick S, eds. Sperm Biology: An Evolutionary Perspective. London: Academic press, 2009:247-304. [Google Scholar]
- 13.Anderson RC. A Study of the Factors Affecting Fertility of Lozenge Females of Drosophila Melanogaster. Genetics. 1945;30:280–96. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filosi M, Perotti ME. Fine structure of the spermatheca of Drosophila melanogaster Meig. J Submicrosc Cytol. 1975;7:259–70. [Google Scholar]
- 15.Blaney WM. Some observations on the sperm tail of Drosophila melanogaster. Drosoph Inf Serv. 1970;45:125. [Google Scholar]
- 16.Fowler GL. Some aspects of the reproductive biology of Drosophila: sperm transfer, sperm storage, and sperm utilization. Adv Genet. 1973;17:293–360. doi: 10.1016/S0065-2660(08)60173-X. [DOI] [Google Scholar]
- 17.Leopold RA, Degrugillier ME. Sperm penetration of housefly eggs: evidence for involvement of a female accessory secretion. Science. 1973;181:555–7. doi: 10.1126/science.181.4099.555. [DOI] [PubMed] [Google Scholar]
- 18.Marchini D, Bernini L. L M, Giordano PC, R D. The female reproductive accessory glands of the medfly Ceratitis capitata: Antibacterial activity of the secretion fluid. Insect Biochem. 1991;21:573–696. doi: 10.1016/0020-1790(91)90029-E. [DOI] [PubMed] [Google Scholar]
- 19.Gillott C. Arthropoda - Insecta. In: Adiyodi KG, Adiyodi RG, eds. Reproductive Biology of Invertebrates Accessory Sex Glands: John Wiley & Sons, 1988:356-71. [Google Scholar]
- 20.Pitnick S, Markow T, Spicer GS. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–22. doi: 10.2307/2640442. [DOI] [PubMed] [Google Scholar]
- 21.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–7. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 22.Bairati A. Structure and ultrastructure of the male reproductive system in Drosophila melanogaster Meig. 2. The genital duct and accessory glands. Ital J Zool (Modena) 1968;2:105–82. [Google Scholar]
- 23.Ludwig MZ, Uspensky II, Ivanov AI, Kopantseva MR, Dianov CM, Tamarina NA, et al. Genetic control and expression of the major ejaculatory bulb protein (PEB-me) in Drosophila melanogaster. Biochem Genet. 1991;29:215–39. doi: 10.1007/BF00590103. [DOI] [PubMed] [Google Scholar]
- 24.Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol. 2001;31:543–51. doi: 10.1016/S0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Pimentel H, Tolbert LP, Heed WB. Ultrastructural examination of the insemination reaction in Drosophila. Cell Tissue Res. 1994;275:467–79. doi: 10.1007/BF00318816. [DOI] [PubMed] [Google Scholar]
- 26.Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, et al. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One. 2011;6:e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Lu X. Drosophila sperm motility in the reproductive tract. Biol Reprod. 2011;84:1005–15. doi: 10.1095/biolreprod.110.088773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–21. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- 29.Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol. 2007;53:319–31. doi: 10.1016/j.jinsphys.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci U S A. 2009;106:15796–800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert DG. Ejaculate esterase 6 and initial sperm use by female Drosophila melanogaster. J Insect Physiol. 1981;27:641–50. doi: 10.1016/0022-1910(81)90112-8. [DOI] [Google Scholar]
- 32.Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:10358–63. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapelnikov A, Rivlin PK, Hoy RR, Heifetz Y. Tissue remodeling: a mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 2008;8:114. doi: 10.1186/1471-213X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapelnikov A, Zelinger E, Gottlieb Y, Rhrissorrakrai K, Gunsalus KC, Heifetz Y. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc Natl Acad Sci U S A. 2008;105:13912–7. doi: 10.1073/pnas.0710997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 2009;18:465–75. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 36.Snook RR, Hosken DJ. Sperm death and dumping in Drosophila. Nature. 2004;428:939–41. doi: 10.1038/nature02455. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman BP, Demerec M. Utilization of sperm by the female Drosophila melanogaster. Am Nat. 1942;76:445–69. doi: 10.1086/281068. [DOI] [Google Scholar]
- 38.Lefevre G, Jr., Jonsson UB. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics. 1962;47:1719–36. doi: 10.1093/genetics/47.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tram U, Wolfner MF. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics. 1999;153:837–44. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur BI, Hauschteck-Jungen E, Nothiger R, Ward PI. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. P Roy Soc Lond B Bio. 1998;265:1749–53. doi: 10.1098/rspb.1998.0498. [DOI] [Google Scholar]
- 41.Iida K, Cavener DR. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J Exp Biol. 2004;207:675–81. doi: 10.1242/jeb.00816. [DOI] [PubMed] [Google Scholar]
- 42.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–14. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Schiff NM, Feng Y, Quine JA, Krasney PA, Cavener DR. Evolution of the expression of the Gld gene in the reproductive tract of Drosophila. Mol Biol Evol. 1992;9:1029–49. doi: 10.1093/oxfordjournals.molbev.a040777. [DOI] [PubMed] [Google Scholar]
- 44.Cavener DR. The response of enzyme polymorphisms to developmental rate selection in Drosophila melanogaster. Genetics. 1983;105:105–13. doi: 10.1093/genetics/105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9:e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawniczak MK, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–10. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- 47.Prokupek A, Hoffmann F, Eyun SI, Moriyama E, Zhou M, Harshman L. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution. 2008;62:2936–47. doi: 10.1111/j.1558-5646.2008.00493.x. [DOI] [PubMed] [Google Scholar]
- 48.Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development. 2004;131:2007–21. doi: 10.1242/dev.01077. [DOI] [PubMed] [Google Scholar]
- 49.Swanson WJ, Wong A, Wolfner MF, Aquadro CF. Evolutionary expressed sequence tag analysis of Drosophila female reproductive tracts identifies genes subjected to positive selection. Genetics. 2004;168:1457–65. doi: 10.1534/genetics.104.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–31. doi: 10.1016/S0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- 51.Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, et al. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;17:1217–27. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avila FW, Bloch Qazi MC, Rubinstein CD, Wolfner MF. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc Natl Acad Sci U S A. 2012;109:4562–7. doi: 10.1073/pnas.1117689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monastirioti M, Linn CE, Jr., White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–11. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–55. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 55.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Bloch Qazi MC, Wolfner MF. Emergence of sperm from female storage sites has egg-influenced and egg-independent phases in Drosophila melanogaster. Biol Lett. 2006;2:128–30. doi: 10.1098/rsbl.2005.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman T, Herndon LA, Heifetz Y, Partridge L, Wolfner MF. The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc Biol Sci. 2001;268:1647–54. doi: 10.1098/rspb.2001.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–84. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 59.Mueller JL, Ripoll DR, Aquadro CF, Wolfner MF. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc Natl Acad Sci U S A. 2004;101:13542–7. doi: 10.1073/pnas.0405579101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller JL, Ravi Ram K, McGraw LA, Bloch Qazi MC, Siggia ED, Clark AG, et al. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics. 2005;171:131–43. doi: 10.1534/genetics.105.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaFlamme BA, Ram KR, Wolfner MF. The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 2012;8:e1002435. doi: 10.1371/journal.pgen.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ram KR, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:15384–9. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravi Ram K, Sirot LK, Wolfner MF. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:18674–9. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–57. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206:3521–8. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- 67.Ravi Ram K, Ji S, Wolfner MF. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol. 2005;35:1059–71. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Lung O, Tram U, Finnerty CM, Eipper-Mains MA, Kalb JM, Wolfner MF. The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics. 2002;160:211–24. doi: 10.1093/genetics/160.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller JL, Linklater JR, Ravi Ram K, Chapman T, Wolfner MF. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics. 2008;178:1605–14. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–13. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 71.Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubli E. The sex-peptide. Bioessays. 1992;14:779–84. doi: 10.1002/bies.950141111. [DOI] [PubMed] [Google Scholar]
- 73.Ohsako T, Yamamoto MT. Sperm of the wasted mutant are wasted when females utilize the stored sperm in Drosophila melanogaster. Genes Genet Syst. 2011;86:97–108. doi: 10.1266/ggs.86.97. [DOI] [PubMed] [Google Scholar]
- 74.Yeh SD, Do T, Chan C, Cordova A, Carranza F, Yamamoto EA, et al. Functional evidence that a recently evolved Drosophila sperm-specific gene boosts sperm competition. Proc Natl Acad Sci U S A. 2012;109:2043–8. doi: 10.1073/pnas.1121327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbert DG, Richmond RC. Esterase 6 in Drosophila melanogaster: reproductive function of active and null males at low temperature. Proc Natl Acad Sci U S A. 1982;79:2962–6. doi: 10.1073/pnas.79.9.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong A, Albright SN, Giebel JD, Ram KR, Ji S, Fiumera AC, et al. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008;180:921–31. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polak M, Starmer WT, Barker JSF. A mating plug and male mate choice in Drosophila hibisci Bock. Anim Behav. 1998;56:919–26. doi: 10.1006/anbe.1998.0850. [DOI] [PubMed] [Google Scholar]
- 78.Bretman A, Lawniczak MK, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. J Insect Physiol. 2010;56:107–13. doi: 10.1016/j.jinsphys.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 79.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A. 2003;100:9923–8. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Markow TA, Ankney P. Insemination reaction in Drosophila: A copulatory plug in species showing male contribution to offspring. Evolution. 1988;42:1097–100. doi: 10.2307/2408926. [DOI] [PubMed] [Google Scholar]
- 81.Singh SR, Singh BN. Female remating in Drosophila: Comparison of duration of copulation between first and second matings in six species. Curr Sci India. 2004;86:465–70. [Google Scholar]
- 82.Markow TA, Hanson SJ. Multivariate analysis of Drosophila courtship. Proc Natl Acad Sci U S A. 1981;78:430–4. doi: 10.1073/pnas.78.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markow TA. Forced matings in natural populations of Drosophila. Am Nat. 2000;156:100–3. doi: 10.1086/303368. [DOI] [PubMed] [Google Scholar]
- 84.Giardina TJ, Beavis A, Clark AG, Fiumera AC. Female influence on pre- and post-copulatory sexual selection and its genetic basis in Drosophila melanogaster. Mol Ecol. 2011;20:4098–108. doi: 10.1111/j.1365-294X.2011.05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitnick S. Male size influences mate fecundity and remating interval in Drosophila melanogaster. Anim Behav. 1991;41:735–45. doi: 10.1016/S0003-3472(05)80340-9. [DOI] [Google Scholar]
- 86.Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–3. doi: 10.1038/194252a0. [DOI] [Google Scholar]
- 87.Manning A. The control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–50. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 88.Gromko MH, Newport MA, Kortier MG. Sperm dependence of female receptivity to remating in Drosophila melanogaster. Evolution. 1984;38:1273–82. doi: 10.2307/2408634. [DOI] [PubMed] [Google Scholar]
- 89.Bretman A, Fricke C, Chapman T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc Biol Sci. 2009;276:1705–11. doi: 10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fricke C, Wigby S, Hobbs R, Chapman T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J Evol Biol. 2009;22:275–86. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 91.Xue L, Noll M. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc Natl Acad Sci U S A. 2000;97:3272–5. doi: 10.1073/pnas.97.7.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ochando MD, Reyes A, Ayala FJ. Multiple paternity in two natural populations (orchard and vineyard) of Drosophila. Proc Natl Acad Sci U S A. 1996;93:11769–73. doi: 10.1073/pnas.93.21.11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marks RW, Seager RD, Barr LG. Local ecology and multiple mating in a natural population of Drosophila melanogaster. Am Nat. 1988;131:918–23. doi: 10.1086/284832. [DOI] [Google Scholar]
- 94.Harshman LG, Clark AG. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–41. doi: 10.2307/2411303. [DOI] [PubMed] [Google Scholar]
- 95.Imhof M, Harr B, Brem G, Schlötterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol Ecol. 1998;7:915–7. doi: 10.1046/j.1365-294x.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 96.Sgro CM, Partridge L. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. Am Nat. 2000;156:341–53. doi: 10.1086/303394. [DOI] [Google Scholar]
- 97.Morrow EH, Stewart AD, Rice WR. Patterns of sperm precedence are not affected by female mating history in Drosophila melanogaster. Evolution. 2005;59:2608–15. [PubMed] [Google Scholar]
- 98.Boorman E, Parker GA. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to femlae age and mating status. Ecol Entomol. 1976;1:145–55. doi: 10.1111/j.1365-2311.1976.tb01217.x. [DOI] [Google Scholar]
- 99.Clark AG, Aguadé M, Prout T, Harshman LG, Langley CH. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pischedda A, Rice WR. Partitioning sexual selection into its mating success and fertilization success components. Proc Natl Acad Sci U S A. 2012;109:2049–53. doi: 10.1073/pnas.1110841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parker G. Sperm competition and its evolutionary consequences in the insects. Biol Rev Camb Philos Soc. 1970;45:525–67. doi: 10.1111/j.1469-185X.1970.tb01176.x. [DOI] [Google Scholar]
- 102.Birkhead TR, Moller AP. Sperm Competition and Sexual Selection. London: Academic Press, 1998. [Google Scholar]
- 103.Simmons LW. Sperm Competition and Its Evolutionary Consequences in the Insects. Princeton, NJ: Princeton University Press, 2001. [Google Scholar]
- 104.Knowlton N, Greenwell SR. Male sperm competition avoidance mechanisms: The influence of female interests. In: Smith RL, ed. Sperm Competition and the Evolution of Animal Mating Systems. London: Academic Press, 1984:61 - 84. [Google Scholar]
- 105.Clark AG, Dermitzakis ET, Civetta A. Nontransitivity of sperm precedence in Drosophila. Evolution. 2000;54:1030–5. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 106.Clark AG. Sperm competition and the maintenance of polymorphism. Heredity (Edinb) 2002;88:148–53. doi: 10.1038/sj.hdy.6800019. [DOI] [PubMed] [Google Scholar]
- 107.Dewsbury DA. Ejaculate cost and male choice. Am Nat. 1982;119:601–10. doi: 10.1086/283938. [DOI] [Google Scholar]
- 108.Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FC, Bretman A, et al. Seminal fluid protein allocation and male reproductive success. Curr Biol. 2009;19:751–7. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lupold S, Manier MK, Ala-Honkola O, Belote JM, Pitnick S. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav Ecol. 2011;22:184–91. doi: 10.1093/beheco/arq193. [DOI] [Google Scholar]
- 110.Sirot LK, Wolfner MF, Wigby S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2011;108:9922–6. doi: 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Price CSC, Dyer KA, Coyne JA. Sperm competition between Drosophila males involves both displacement and incapacitation. Nature. 1999;400:449–52. doi: 10.1038/22755. [DOI] [PubMed] [Google Scholar]
- 112.Civetta A. Direct visualization of sperm competition and sperm storage in Drosophila. Curr Biol. 1999;9:841–4. doi: 10.1016/S0960-9822(99)80370-4. [DOI] [PubMed] [Google Scholar]
- 113.Harshman LG, Prout T. Sperm displacement without sperm transfer in Drosophila melanogaster. Evolution. 1994;48:758–66. doi: 10.2307/2410484. [DOI] [PubMed] [Google Scholar]
- 114.Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution. 2000;54:534–42. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 115.Newport MA, Gromko MH. The effect of experimental design on female receptivity to remating and its impact on reproductive success in Drosophila melanogaster. Evolution. 1984;38:1261–72. doi: 10.2307/2408633. [DOI] [PubMed] [Google Scholar]
- 116.Radhakrishnan P, Fedorka KM. Influence of female age, sperm senescence and multiple mating on sperm viability in female Drosophila melanogaster. J Insect Physiol. 2011;57:778–83. doi: 10.1016/j.jinsphys.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Holman L. Drosophila melanogaster seminal fluid can protect the sperm of other males. Funct Ecol. 2009;23:180–6. doi: 10.1111/j.1365-2435.2008.01509.x. [DOI] [Google Scholar]
- 118.Gilchrist AS, Partridge L. Male identity and sperm displacement in Drosophila melanogaster. J Insect Physiol. 1995;41:1087–92. doi: 10.1016/0022-1910(95)00068-6. [DOI] [Google Scholar]
- 119.Birkhead TR. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–8. doi: 10.2307/2411251. [DOI] [PubMed] [Google Scholar]
- 120.Birkhead TR. Defining and demonstrating postcopulatory female choice--again. Evolution. 2000;54:1057–60. doi: 10.1111/j.0014-3820.2000.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 121.Eberhard WG. Criteria for demonstrating postcopulatory female choice. Evolution. 2000;54:1047–50. doi: 10.1111/j.0014-3820.2000.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 122.Kempenaers B, Foerster K, Questiau S, Robertson BC, Vermeirssen ELM. Distinguishing between female sperm choice versus male sperm competition: a comment on Birkhead. Evolution. 2000;54:1050–2. doi: 10.1111/j.0014-3820.2000.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 123.Bjork A, Starmer WT, Higginson DM, Rhodes CJ, Pitnick S. Complex interactions with females and rival males limit the evolution of sperm offence and defence. Proc Biol Sci. 2007;274:1779–88. doi: 10.1098/rspb.2007.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bangham J, Chapman T, Smith HK, Partridge L. Influence of female reproductive anatomy on the outcome of sperm competition in Drosophila melanogaster. Proc Biol Sci. 2003;270:523–30. doi: 10.1098/rspb.2002.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller GT, Pitnick S. Functional significance of seminal receptacle length in Drosophila melanogaster. J Evol Biol. 2003;16:114–26. doi: 10.1046/j.1420-9101.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- 126.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–3. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 127.Kalb JM, DiBenedetto AJ, Wolfner MF. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc Natl Acad Sci U S A. 1993;90:8093–7. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 129.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–4. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 130.Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity (Edinb) 2001;87:511–21. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 131.Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–57. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fiumera AC, Dumont BL, Clark AG. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics. 2007;176:1245–60. doi: 10.1534/genetics.106.064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chippindale AK, Rice WR. Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:5677–82. doi: 10.1073/pnas.101456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chow CY, Wolfner MF, Clark AG. The genetic basis for male x female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics. 2010;186:1355–65. doi: 10.1534/genetics.110.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc Biol Sci. 2000;267:1097–105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mueller JL, Page JL, Wolfner MF. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics. 2007;175:777–83. doi: 10.1534/genetics.106.065318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MacBean IT, Parsons PA. Directional selection for duration of copulation in Drosophila melanogaster. Genetics. 1967;56:233–9. doi: 10.1093/genetics/56.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Friberg U. Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim Behav. 2006;72:1259–68. doi: 10.1016/j.anbehav.2006.03.021. [DOI] [Google Scholar]
- 139.Bateman AJ. Intra-sexual selection in Drosophila. Heredity (Edinb) 1948;2:349–68. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 140.Mack PD, Priest NK, Promislow DE. Female age and sperm competition: last-male precedence declines as female age increases. Proc Biol Sci. 2003;270:159–65. doi: 10.1098/rspb.2002.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–4. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 142.Holland B, Rice WR. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc Natl Acad Sci U S A. 1999;96:5083–8. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clark AG, Begun DJ. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149:1487–93. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clark AG, Begun DJ, Prout T. Female x male interactions in Drosophila sperm competition. Science. 1999;283:217–20. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- 145.Mack PD, Hammock BA, Promislow DEL. Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt. Evolution. 2002;56:1789–95. doi: 10.1111/j.0014-3820.2002.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 146.Ala-Honkola O, Manier MK, Lüpold S, Pitnick S. No evidence for postcopulatory inbreeding avoidance in Drosophila melanogaster. Evolution. 2011;65:2699–705. doi: 10.1111/j.1558-5646.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- 147.Greenspan L, Clark AG. Associations between Variation in X Chromosome Male Reproductive Genes and Sperm Competitive Ability in Drosophila melanogaster Int J Evol Biol 2011; 2011:214280. [DOI] [PMC free article] [PubMed]
- 148.Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–8. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 149.Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–21. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 150.Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lawniczak MK, Begun DJ. A QTL analysis of female variation contributing to refractoriness and sperm competition in Drosophila melanogaster. Genet Res. 2005;86:107–14. doi: 10.1017/S0016672305007755. [DOI] [PubMed] [Google Scholar]
- 152.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev Biol. 2001;234:416–24. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 153.Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech Dev. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-J. [DOI] [PubMed] [Google Scholar]