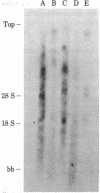
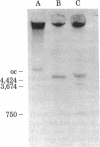
Abstract
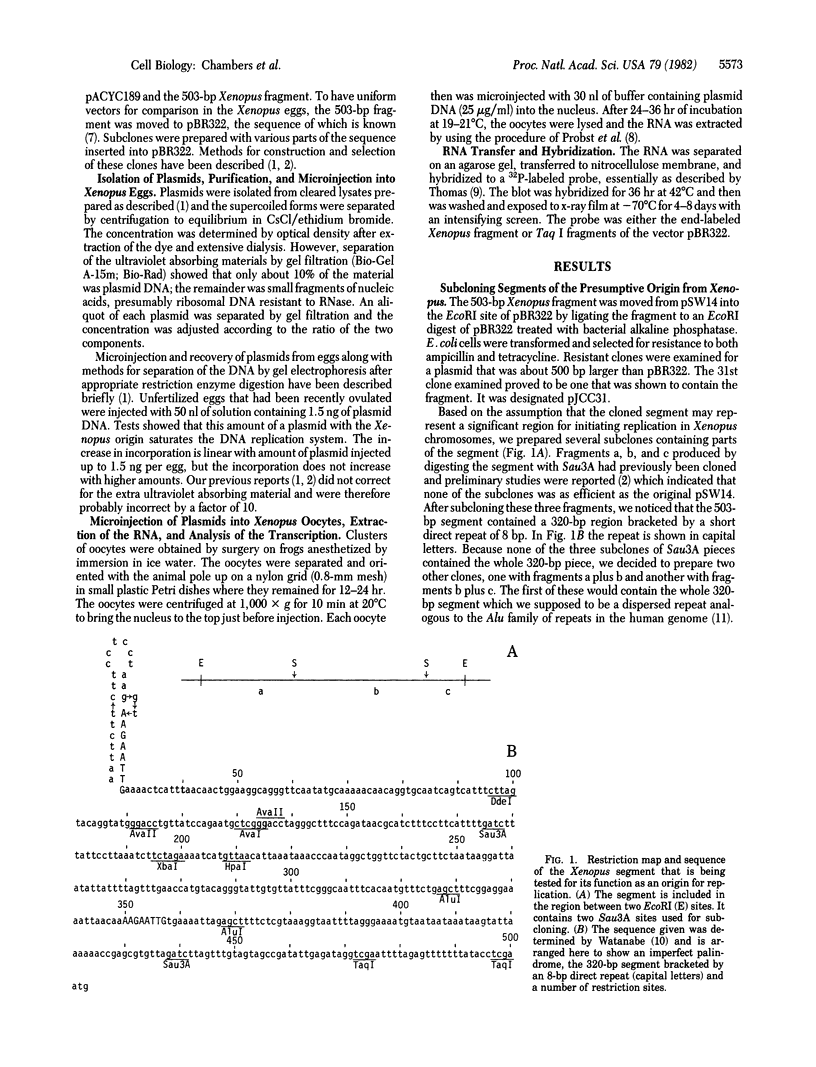
A previously cloned 503-base pair (bp) EcoRI segment of genomic DNA from Xenopus laevis selected for enhancement of replication of its vector plasmid was moved to the EcoRI site of pBR322. This plasmid designated pJCC31 and five other clones, which were made by cleaving the 503-bp segment in relation to a dispersed repeated sequence and subcloning, were compared with pBR322 for replication by microinjection into Xenopus eggs. The replication measured by incorporation of a 32P-labeled nucleotide as well as semiconservative segregation and dilution of N6-methyladenine at the EcoRI sites showed pJCC31 to be about 15 times as efficient as pBR322. The next most efficient subclone, pJCC31-2, contains an insert with a complete 320-bp dispersed repeated sequence bracketed by an 8-bp direct repeat. This observation, along with our previous report that repeated sequences of the Alu family in the human genome enhanced replication of the vector plasmid nearly as much as that of the presumptive Xenopus origin, leads to the hypothesis that members of a subset of the short dispersed repeated sequences in vertebrates function as origins for chromosomal replication. Preliminary studies also show that the presumptive Xenopus origin contains a RNA polymerase promoter that increases the transcription of the plasmid when it is microinjected into Xenopus oocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Callan H. G. DNA replication in the chromosomes of eukaryotes. Cold Spring Harb Symp Quant Biol. 1974;38:195–203. doi: 10.1101/sqb.1974.038.01.023. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Haynes S. R., Jelinek W. R. Low molecular weight RNAs transcribed in vitro by RNA polymerase III from Alu-type dispersed repeats in Chinese hamster DNA are also found in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6130–6134. doi: 10.1073/pnas.78.10.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Sudo T., Yoshida M., Kubota H., Ueyama H. In vitro replication of recombinant plasmids carrying chromosomal segments of Xenopus laevis. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3697–3701. doi: 10.1073/pnas.79.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Taylor J. H. Cloning of an origin of DNA replication of Xenopus laevis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5292–5296. doi: 10.1073/pnas.77.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]