Abstract
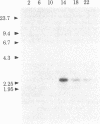
The accumulation pattern of a number of mRNAs during the cell cycle of Chlamydomonas was examined by two-dimensional gel analysis of in vitro translation products and by RNA blot hybridization analysis. Two-dimensional gel analysis revealed that 10-15% of the 300 most abundant translation products are differentially synthesized from RNA obtained at various cell cycle stages. RNAs that direct the synthesis of alpha- and beta-tubulins and that hybridize to cloned alpha- and beta-tubulin probes accumulate coordinately during the predivision period of the cell cycle, reaching peak levels before or during division. Other RNAs represented by selected cloned cDNA probes show a number of different cell cycle patterns of accumulation. The accumulation patterns of these RNAs are not directly influenced by ongoing illumination conditions, even though alternating light-dark illumination cycles are used to synchronize Chlamydomonas cells. The results suggest that there may be a complex program of gene expression correlated with cell cycle progression in Chlamydomonas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci. 1974 Dec;16(3):529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Lee A. S. Coupling of histone and DNA synthesis in the somatic cell cycle. Science. 1982 Jan 1;215(4528):79–81. doi: 10.1126/science.7053561. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Cell division from a genetic perspective. J Cell Biol. 1978 Jun;77(3):627–637. doi: 10.1083/jcb.77.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Pirkl E., Zentgraf H., Schaller H. Conversion of bacteriophage fd into an efficient single-stranded DNA vector system. Mol Gen Genet. 1980 Jan;177(2):231–242. doi: 10.1007/BF00267434. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Posakony J. W., Hill K. R. The cell cycle program of polypeptide labeling in Chlamydomonas reinhardtii. J Cell Biol. 1977 Feb;72(2):223–241. doi: 10.1083/jcb.72.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Laffler T. G., Chang M. T., Dove W. F. Periodic synthesis of microtubular proteins in the cell cycle of Physarum. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5000–5004. doi: 10.1073/pnas.78.8.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Nordstrom S. A., Moulder J. E., Rosenbaum J. L. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978 Jul;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Minami S. A., Collis P. S., Young E. E., Weeks D. P. Tubulin induction in C. reinhardii: requirement for tubulin mRNA synthesis. Cell. 1981 Apr;24(1):89–95. doi: 10.1016/0092-8674(81)90504-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Chiang K. S., Kates J. R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. I. Isotopic transfer experiments with a strain producing eight zoospores. J Mol Biol. 1967 Apr 14;25(1):47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction and synthesis of tubulin during the cell cycle and life cycle of Chlamydomonas reinhardi. Dev Biol. 1979 Apr;69(2):400–407. doi: 10.1016/0012-1606(79)90300-2. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell. 1976 Sep;9(1):15–27. doi: 10.1016/0092-8674(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P., Gealt M. A. Control of induction of tubulin synthesis in Chlamydomonas reinhardi. Nature. 1977 Aug 18;268(5621):667–668. doi: 10.1038/268667a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]