Abstract
Klotho (KL) is a transmembrane protein that can be shed, and act as a circulating hormone and modulate several signaling pathways. There also exists a splice variant of Klotho mRNA, which encodes a putative secreted protein (Klotho-S, KL-S) in both human and mouse. The potential anti-senescence gene Klotho has been recently found to participate in the progression of several different human cancers. In the current study, we undertook to study the expression and activity of Klotho in lung cancer cell line A549. Klotho expression was studied by using RT-PCR and western blotting. Effects of Klotho on cell growth and motility were assessed using MTT and scratch motility assay, and the apoptosis was assessed by TUNEL. Wnt signaling pathway activity was measured by western blotting. We established that the Klotho was endogenous expressed in A549 cells, but the expression level is lower compared with normal lung tissues. The overexpression of KL or KL-S could inhibit the cell proliferation, motility, and induce apoptosis in a dose-dependent manner. Also, we report KL could inhibit activation of Wnt -TCF/β-catenin signaling pathway, and it is involved in KL-induced growth inhibition. These studies indicate Klotho works as a potential tumor suppressor in lung cancer, and suggest that the Klotho tumor suppressive activities could be mediated through its KL-S isoform. These results suggest the use of Klotho or KL-S as potential strategy for the development of novel therapeutic interventions for lung cancers.
Keywords: Wnt, apoptosis, gene therapy, klotho, lung cancer, proliferation
Introduction
Lung cancer is the leading cause of cancer-related death, patients with advanced stage of lung cancer, have a median survival time of only 10 months. Lung cancer can be separated into two major forms: non-small cell lung cancer (NSCLC) and small cell lung cancer, which account for 80 and 20% of all lung carcinomas, respectively.1 Deregulated Wnt signaling in cancer has been primarily in colon cancer, and more recently, studies focused on lung cancer, NSCLC in particular.2,3 Increased expression of Wnt proteins or decreased expression of Wnt regulators is likely involved in the multistep process of lung carcinogenesis.
The Klotho gene codes for a single pass transmembrane protein, which is a 1012-amino acid protein, abundantly expressed in various tissues. The extracellular domain of Klotho is composed of two internal repeats, KL1 and KL2, which share amino-acid sequence homology to β-glucosidase but lack glucosidase activity.4 KL1 could also be transcribed through an alternative splicing, named Klotho-S. Klotho is an inhibitor of ligand-dependent activation of the insulin and IGF-I pathways.5-7 The transmembrane form of Klotho was a co-factor essential for activation of FGF signaling by FGF23.8,9 Klotho bound to various Wnt family members. In a cell culture model, the Wnt-Klotho interaction resulted in the suppression of Wnt biological activity. Ectopic expression of Klotho antagonized the activity of endogenous and exogenous Wnt. So Klotho was shown to be a secreted antagonist of the Wnt signaling pathway. In cervical carcinoma, epigenetic silencing of Klotho may occur during the late phase of cervical tumorigenesis, and consequent functional loss of Klotho may contribute to aberrant activation of the canonical Wnt pathway.
In this study we report that in lung cancer, overexpression of Klotho or Klotho-S inhibit the cell proliferation, motility and induce apoptosis in a dose-dependent manner; Klotho could inhibit activation of Wnt-TCF/β-catenin signaling pathway and it is involved in Klotho- induced growth inhibition. These studies indicate Klotho works as a potential tumor suppressor in lung cancer, and suggest that the Klotho tumor suppressive activities could be mediated through its Klotho-S isoform.
Results
130 kDa Klotho protein was endogenous expressed in A549 cells
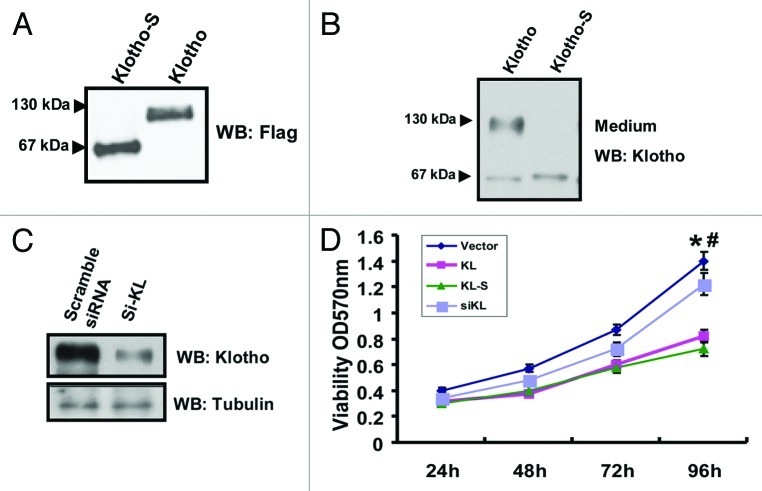
While disruption of the klotho gene causes pulmonary emphysema in mice, the expression pattern of Klotho in normal lung tissues or in lung cancers are still unknown. We characterized the klotho transcripts in normal lung tissues and A549 cells by RT-PCR, the result revealed high Klotho expression level in normal lung tissues, while low expression level in A549 cells (Fig. 1A). The RT-PCR fragments cannot distinguish the transmembrane form or secreted form (KL-S). We therefore investigated which Klotho form was primarily expressed in A549 cells. Western blot analysis of Klotho protein expression in A549 showed that the 130 kDa Klotho was endogenous expressed primarily (Fig. 1B). Klotho expression was also analyzed using quantitative RT-PCR in 5 lung cancer samples and adjacent normal lung tissue. Reduced klotho expression in the tumors compared with adjacent normal tissues was found in 4 (out of 5) of the samples (Fig. 1C).
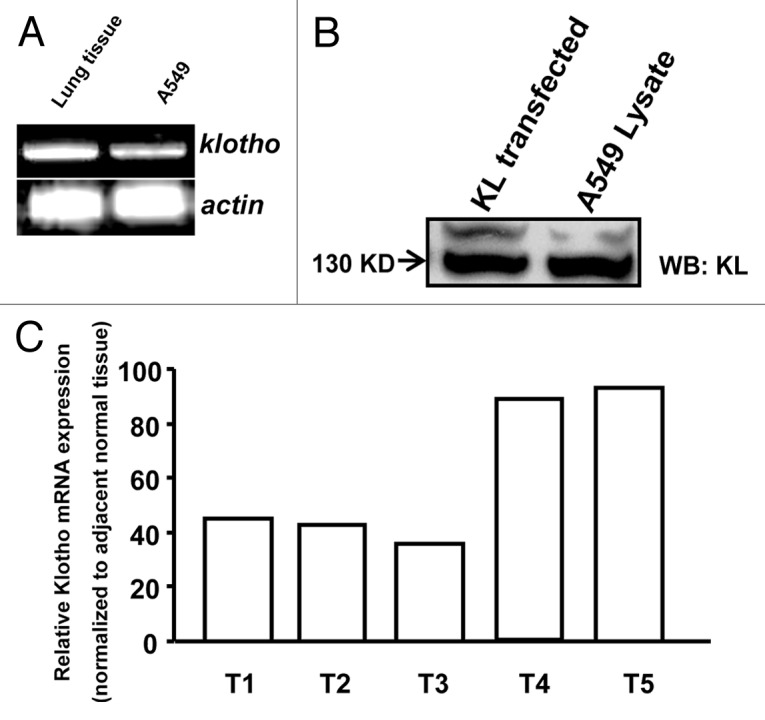
Figure 1. Endogenous expression of Klotho in A549 cells. (A) The KL transcripts detected by RT-PCR. (B) Western blot analysis of Klotho protein, HEK293 lysate transfected with Klotho as positive control. (C) Klotho mRNA levels were determined by quantitative RT-PCR in lung cancer clinical samples and adjacent normal tissue.
Overexpression of Klotho could inhibit the cell proliferation and promote apoptosis of A549
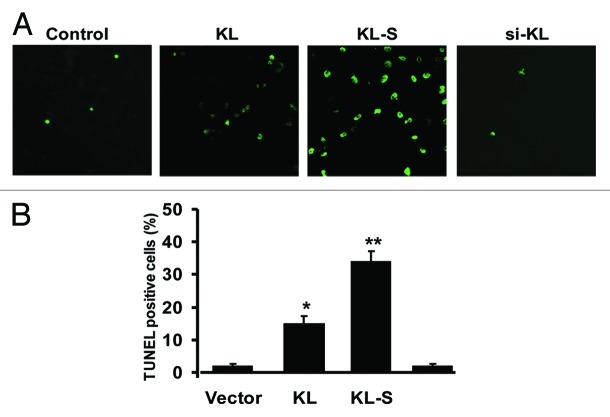
Cell proliferation and apoptosis can regulate the fate of tumor at any given time. Therefore, to determine whether the expression level of Klotho is involved in the proliferation or apoptosis of A549, we constructed expression plasmids of Klotho. Klotho may be shed and act as a circulating hormone, and it has been shown that either soluble klotho (KL-S) or conditioned medium taken from klotho overexpression is active.4,5,11,12 Therefore, to determine which isoform of KL function primarily in A549 cells, we constructed the soluble Klotho isoform plasmids, and tested which of them affect the cell more. Both of them were expressed at similar levels, and both of them could be detected in the cell medium (Fig. 2A and B). We also designed small interfering RNAs (siRNA) to see whether lower Klotho expression could increase the cell growth. Si-Klotho repressed KL expression in transfected A549 cells compared with control siRNA (Fig. 2C). All the constructs were transfected into A549 cells respectively, then cells were seeded on 96-well plates, and viability was assayed by time-lapse MTT assay. The results revealed that both KL-transfected and KL-S transfected group showed a lower growth curve, compared with scramble siRNA transfected group. But the KL-siRNA transfected group has similar growth curve as scramble siRNA group. It indicates that the overexpression of Klotho or Klotho-S could repress the proliferation of A549 cells, whereas Klotho knock-down could not affect the cell proliferation (Fig. 2D). Then we performed TUNEL assay to measure apoptosis. The result revealed that both Klotho-transfected and Klotho-S transfected group have more TUNEL positive cells, when compared with scramble siRNA transfected group. But the KL-siRNA transfected group has no effect on the apoptosis ratio. The result indicates that the overexpression of Klotho or Klotho-S could enhance the apoptosis of A549 (Fig. 3). These data suggest both Klotho and Klotho-S could inhibit the proliferation of A549 and promote the apoptosis, but Klotho knockdown seems to have no obvious effect.
Figure 2. Klotho regulates the cell proliferation of A549. (A) A549 cells were transfected with Klotho or Klotho-S, and western blot was performed to verify similar expression levels of proteins in cell lysates. (B) Klotho in cell culture supernatants were immunodetected by Klotho antibodies. (C) Extracts from A549 cells transfected with si-Klotho were subjected to western blot and probed with antibodies to Klotho or β-tubulin (loading control). (D) MTT assay for A549 cells transfected with indicated vectors. Data are shown as the mean ± SEM from three independent experiments. *p < 0.05, KL group vs. control group; # < 0.05, KL-S vs. control group.
Figure 3. Induction of A549 cells apoptosis by Klotho expression. (A) Representative images of TUNEL assay. A549 cells were transfected with indicated constructs. (B) Data of TUNEL positive cells were summarized. Data are shown as the mean ± SEM from three independent experiments. *p < 0.05, one-way ANOVA.
Overexpression of Klotho could inhibit the cell motility of A549
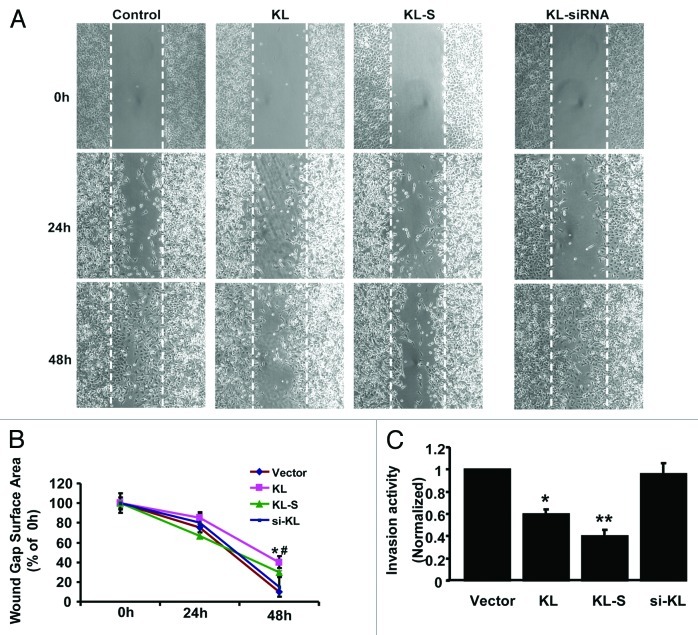
Cell motility is important for many physiological and pathological processes including organ development, cancer invasion, and metastasis. To examine whether the Klotho expression level could modulate A549 cell invasive capacity, we performed scratch wound closure assays that is considered to be an in vitro model for epithelial cell migration during repair and invasion. Linear scrape wounding of A549 cells was performed and cultures were allowed to undergo healing in the presence of serum for 24 and 48 h. We assessed the effect of Klotho, Klotho-S or KL-siRNA on wound healing, as compared with wounds in control cells population transfected with scramble siRNA. As quantitated by multiple measurements of the width of the wound space, as shown in Figure 4A, Klotho and Klotho-S inhibited wound healing, with approximately 60 and 70% respectively, of initial injured width at 48 h. In contrast, A549 cells transfected with KL-siRNA showed the same sharp as control group, nearly complete healing (Fig. 4A and B). To provide a more quantitative assay of invasion, we performed a matrigel invasion assay in this study. As shown in Figure 4C, both Klotho and Klotho-S, could suppress the invasion activity of A549 cells, while KL-siRNA seems to have no detectable effect. The results indicate that the ability of A549 lung cancer cells to migrate appears affected by Klotho or Klotho-S expression.
Figure 4. Klotho regulates the cell motility of A549. (A) Scratch wound closure assay for motility of A549 cells transfected with indicated constructs. The photographs of wound closure from each group were taken and assessed every 24 h for 2 d. (B) Quantitative assays were performed by calculating cell numbers. The ratio of migrating cells was calculated into line chart. Data are shown as the mean ± SEM from three independent experiments. *p < 0.05, KL group vs. control group; # < 0.05, KL-S vs. control group. (C) Klotho suppresses A549 invasion activity. A549 cells (2 × 104 cells) were seeded to the top chamber of a Matrigel invasion chamber in the absence of serum, and then subjected to invasion assay. Deteminations were made in triplicate. Data are shown as the mean ± SEM from three independent experiments. *p < 0.05, KL group vs. control group; ** < 0.01, KL-S vs. control group.
KL functions in a dose-dependent manner
As shown above, both Klotho and Klotho-S could inhibit the cell proliferation and motility, but knockdown Klotho seems to have no obvious effects in the proliferative or motile state of cells. That may be due to one reason, namely, Klotho functions in a dose-dependent manner, the endogenous Klotho in A549 is lower than its working- concentration. If it is true, it is not surprising that when the expression level is lower it will not affect cells anymore. To prove this, we performed MTT assay to investigate whether these is a dose-dependent inhibition of A549 proliferation by Klotho. As seen in Figure S1, when transfected with 0.5 ng Klotho, the cells still not shown proliferation inhibition; when the KL-S concentration was higher than 1 ng/group, Klotho began to suppress cell growth. The results confirm that Klotho functions in A549 cells in a dose-dependent manner.
Klotho functions as a secreted Wnt antagonist in A549 cell line
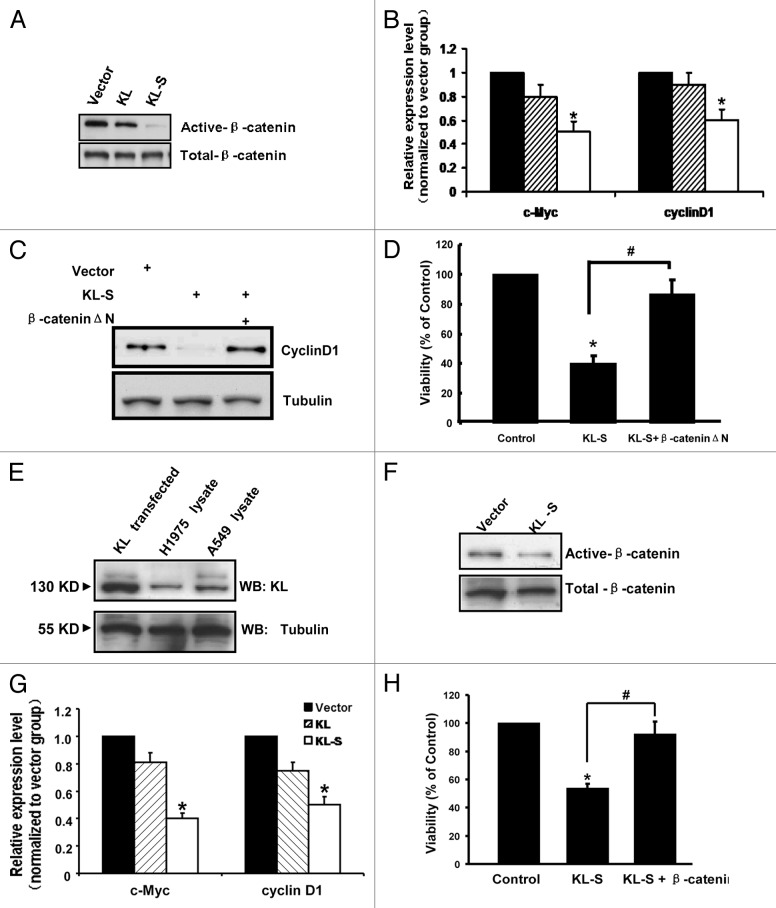
We have previously reported the inhibition of IGF-1/insulin pathways by Klotho.10 To identify additional molecular mechanisms, we detected whether Klotho-S overexpression could regulate Wnt signaling pathway. According to previous reports, the Wnt pathway plays an important role in the development of lung cancer,1,2 and Klotho inhibits activation of this pathway.13 So we examined the ability of Klotho to inhibit activation of the Wnt pathway in A549 cells. For these studies, cells were transfected with pCDNA3.1-Klotho-Flag or an empty vector and analyzed using western blotting for the expression and active form of β-catenin 48 h after transfection. Ectopic expression of Klotho-S protein resulted in a dramatic reduction in the active form of β-catenin (ABC), which is dephosphorylated on S37 or T41 residues; without changing the total β-catenin levels (Fig. 5A). The expression of representative Wnt pathway target genes, including c-Myc and CyclinD1, was deceased in Klotho-S overexpressed group (Fig. 5B). A549 cells transfected with KL-siRNA did not change the active form of β-catenin (ABC) or the total β-catenin levels (Fig. S2). CyclinD1 is not only target of β-catenin transcriptional activity, but also regulated by other pathways affected by Klotho, such as IGF-1.14,15 Therefore, we transfected a stabilized, transcriptionally active form of β-catenin lacking the NH2-terminal 90 amino acids (ΔN90beta-catenin)16,17 together with Klotho-S, to investigate whether the downregulation of cyclinD1 caused by Klotho-S could be rescued. An increase in cyclinD1 mRNA levels in response to co-expression ofΔN90beta-catenin and KL-S was observed (Fig. 5C). We next asked whether ΔN90beta-catenin was able to rescue the growth inhibition of A549 cells by Klotho-S. Using MTT assay, the ΔN90beta-catenin was found to be functional on mitigating the Klotho-S caused viability reduction (Fig. 5D). These findings indicate that the canonical Wnt pathway is inhibited by Klotho-S in A549 cell line, and this inhibition is involved in Klotho-S caused the growth inhibition of cancer cells. Conversely, the downregulation of Klotho in lung cancer may be the cause led to abnormal Wnt pathway activation.
Figure 5. Inhibition of the Wnt/β-catenin pathway by Klotho-S in A549 and H1975 cells. (A) Western blot analysis of total β-catenin, and active β-catenin (A, B and C) in A549 cells transfected with either the Klotho/Klotho-S expression vector or the empty vector. (B) RT-PCR analysis of TCF/β-catenin target genes: c-MYC and cyclinD1 in A549 cells transfected with indicated constructs. (C) Western blot analysis of CyclinD1 in A549 cells transfected with either Klotho-S or co-transfected with Klotho-S andβ-cateninΔN. (D) MTT assay for A549 cells transfected with either Klotho-S or co-transfected with Klotho-S andβ-cateninΔN. (E), Western blot analysis of Klotho protein, HEK293 lysate transfected with Klotho as positive control. (F) Western blot analysis of total β-catenin, and active β-catenin (A, B and C) in H1975 cells transfected with either the Klotho-S expression vector or the empty vector. (G) RT-PCR analysis of c-MYC and cyclinD1 in H1975 cells transfected with indicated constructs. (H) MTT assay for H1975 cells transfected with either Klotho-S or co-transfected with Klotho-S and β-cateninΔN. Data are shown as the mean ± SEM from three independent experiments. *p < 0.05, one-way ANOVA.
The KL-S caused downregulation of Wnt signaling activity exist in H1975 cell line also
Though we have found the klotho expression level is decreased in many lung cancer samples, still we do not know whether Klotho was involved in abnormal activation of Wnt signaling pathway in other lung cancer cell lines. To address this question, we detected the role of Klotho upon Wnt activity in H1975 cell lines, which was found had detectable Wnt activity. As shown in Figure 5E, there were endogenous expressions of 130 KD Klotho also. What is more, when KL-S was transfected into H1975, the amount of active form of β-catenin was decreased by ~50% compared with empty vector transfection, While the total β-catenin levels was not affected by KL-S transfection (Fig. 5F). Moreover, the expression of c-Myc and CyclinD1, was deceased in Klotho-S overexpressed group (Fig. 5G). The result was further confirmed by the MTT assay. Using MTT assay, we found the KL-S expression could cause viability reduction in H1975 cells, while the ΔN90beta-catenin was found to be functional on rescue the Klotho-S caused viability reduction (Fig. 5H). The results indicate Klotho is expressed in H1975 lung cancer cell line and its expression level is inversely correlates with the activity of Wnt signaling pathway. Moreover, the Klotho-S overexpression plays role in cell viability in H1975 cells, while theΔN90beta-catenin can block Klotho-S function.
Klotho regulates Wnt3a expression in A549 and H1975 cell lines
As we observed a decrease in Wnt activity in both A549 and H1975 cell lines by Klotho or Klotho-S overexpression (Fig. 5A and F). We examined whether it is caused by downregulation of wnt ligands. It has been shown that Wnt3a was expressed in A549 cell lines, and Wnt3a stimulation led to a strong increase of A549 cell proliferation.18 We also found H1975 has Wnt3a expression by RT-PCR and western blotting. To determine if Klotho-S could affect the expression level of Wnt3a, we used real-time PCR analysis to quantitate levels of Wnt3a gene expression. Levels of Wnt3α mRNA were reduced in both A549 and H1975 cells transfected with KLotho-S, when compared with vector transfection group (Fig. 6A). It has been reported that, Klotho could decrease Wnt5a internalization in melanoma cells.19 To determine whether Klotho-S plays a role in Wnt3a internalizeation in lung cancer cells, we looked at the Wnt3a expression in the culture medium. We treated the cells with Klotho-S conditional medium, and observed an increase expression of Wnt3a in the medium, while in the cell lysate, total Wnt3a amount was reduced (Fig. 6B). The results indicate Klotho-S may inhibit the internalization of Wnt3a, and the decrease of functional wnt3a will cause a downregulation of Wnt signaling pathway. Further, as the target gene of Wnt signaling, the transcript level of Wnt3a is reduced.
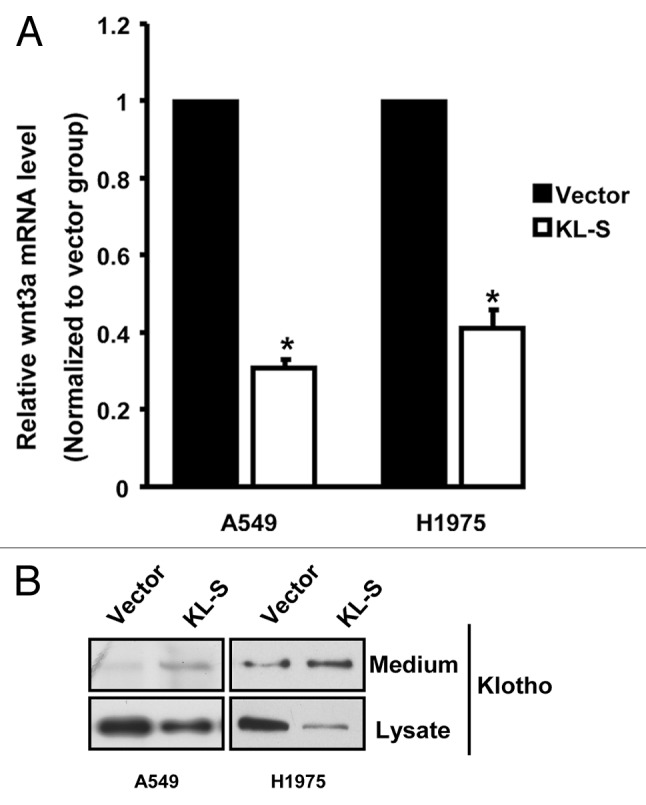
Figure 6. Klotho regulates Wnt3a expression in A549 and H1975 cell lines. (A) RT-PCR analysis of human Wnt3a gene in A549 and H1975 cells transfected with indicated constructs. (B) Western blot analysis of Wnt3a protein in A549 andH1975 cells transfected with either the Klotho-S expression vector or the empty vector.
Discussion
Klotho is a new anti-aging gene. Genetic mutation of Klotho causes multiple premature aging-like phenotypes and strikingly shortens lifespan.6,8,13,20 Recently, Klotho was reported to function as a secreted Wnt antagonist and as a tumor suppressor.5,10,13,21-26 In the current study, our observations indicate Klotho as a tumor suppressor in A549 cells by inhibiting Wnt signaling pathway. Klotho inhibits activation of Wnt signaling pathway and slows down the growth of A549 cells. Furthermore, we found that the KL1 domain was as active as the full length protein. Taken together, our studies provide four new insights into the role of Klotho in A549 cells.
First, we established that the 130 kDa Klotho was endogenous expressed in A549 cells. We examined the mRNA levels of Klotho by RT-PCR and the protein levels by western blotting. Our results indicate that the expression level of Klotho was reduced in A549 cells and lung cancer samples compared with normal lung tissues. Recent studies show repression of Klotho by epigenetic mechanisms in human cervical carcinoma and pancreatic cancer.13,21 There might be the same mechanisms in lung cancer. If validated by further studies, the expression level of Klotho may serve as a novel diagnostic tool for the differentiation between normal and cancer cells.
Second, we report that the both full length Klotho and Klotho-S could inhibit the cell proliferation, motility, and induce apoptosis. The cleavage products of full length Klotho can be 68 and 64 kda protein representing KL1 and KL2, respectively. Moreover, Klotho gene can transcribe a secreted form of Klotho by alternative RNA splicing encoding by only the KL1 domain.11,27,28 Though 130 kDa Klotho was endogenous expressed in A549 cells, the secreted Klotho Klotho-S, could also be efficacious on inhibiting of A549 cells proliferation and motility. It has been reported that soluble Klotho and conditioned medium obtained from Klotho-overexpressing cells could effectively inhibit growth of breast and pancreatic cancer cells.5,21 The results are consistent with the studies in cervical carcinoma.13 What is more, only the full-length Klotho work as a coreceptor of FGF23, which can cause hypophosphatemia; while KL1 do not affect FGF23 signaling.9,21 Thus, secreted Klotho may be also functional in mediating growth-inhibitory activities as the full-length protein, but safer for avoiding to trigger FGF signaling.
Third, we showed that Klotho functions in a dose-dependent manner in A549 cells. We found that A549 has endogenous KL expression, but KL knocking down seems to have no obvious effect in the proliferative or motile state of cells. The results indicate that there is a threshold in cells. That’s to say, the endogenous KL level is lower than the threshold level of functional KL. Given this, we can control the cancer cells proliferation, invasion, and metastasis by epigenetic expressing Klotho. Also, the Klotho expression level detection may provide a new biomarker for a good prognosis in patients with lung cancer.
Fourth, we found that Klotho-S was associated with oncogenic inhibition of TCF/β-catenin in A549 cells. Many mechanisms may govern Klotho growth-inhibitory activities, some of which have been reported to mediate the growth inhibitory activities of Klotho in lung cancers, such as IGF-I pathway and bFGF pathway.29,30 Klotho was also shown to be a secreted antagonist of the Wnt signaling pathway by forming a complex with Wnt3.23 Studies in cervical carcinoma indicated functional loss of Klotho due to epigenetic silencing may contribute to aberrant activation of the canonical Wnt pathway in cervical carcinoma.13 The Wnt family of signaling proteins is essential to organ lung morphogenesis, the Wnt pathway has recently been linked to the pathogenesis of lung cancers.1,2 In particular, in this study, we hypothesized that Klotho inhibits Wnt signaling activity, which can reduce transcription of Wnt target genes and growth of both A549 and H1975 cells. It has been reported that the Klotho also involved in melanoma progression by regulating Wnt5a dependent non-canonical Wnt signaling.19 According to our results, we found the active form of β-catenin could rescue Klotho derived effects upon A549 and H1975 sufficiently. So we imagine that in the case of lung cancer, Klotho mainly regulates the canonical Wnt signaling pathway.
In all, these findings provide insights into the mechanistic link between Klotho and lung cancer and suggest that Klotho could works as a tumor suppressor in lung cancer. These results suggest Klotho or KL-S could be served as potential strategy for the development of novel therapeutic interventions for lung cancers. Since this study was only on the cell level, further studies will need to identify additional details about this association.
Materials and Methods
Cell culture
Human lung cancer cell lines A549, H1975 and HEK-293 cells are all purchased from ATCC, cultured in Dulbecco’s modified Eagle’s medium (Invitrogen), supplemented with 10 mM HEPES (Invitrogen), 5 mM L-glutamine (Invitrogen), and 10% fetal bovine serum (Invitrogen) in a humidified atmosphere of sterile air, 5.0% CO2 at 37°C.
Plasmid constructs and transfection
The Myc-Flag tagged human Klotho expression vector was prepared as described previously.10 The Flag-tagged human Klotho-S expression vector was subcloned into pCDNA3.1 expression plasmid (Invitrogen). The pSuper vectors (OligoEngine) were used to transcribe functional small interfering RNA (siRNA). In the vectors, oligonucleotides targeting Klotho were inserted into the downstream of H1 promoter, with their veracity confirmed by double digestion and sequencing. The target sequence was as follow: CCTAAGCTCTCACTGGATC, The expression levels of proteins in A549 cells transfected with the resulting siRNA or the scramble siRNA were analyzed by immunoblotting. Transient transfection of constructs in A549 cells was performed using Lipofect Amine TM 2000 Reagent (Invitrogen), according to the manufacturer’s recommendation.
MTT assay for cell viability
Cell viability was assessed by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) assay according to the manufacturer’s recommendation. Different groups (including transfection group) of A549 cells were cultured for 24, 48, 72 and 96 h, respectively. The absorbance was evaluated by OD values at 570 nm using a microplate reader (BioRad Co.).
Assessment of apoptosis
In situ detection of cells with DNA strand breaks (apoptosis) was performed on sides by the terminal deoxynucleotidyl transferase (TdT)-mediated dUDP nick-end labeling (TUNEL) technique using In Situ Cell Death Detection Kit (Roche). A549 cells were seeded onto sterile glass coverslips in a 6-well plate, then transfected with indicated constructs. After 48 h, the cells were assessed by TUNEL assay, according to the manufacturer’s recommendation. The samples were analyzed under fluorescent microscope with 495 nm blue light activation and 515–565 nm green light detection.
RNA extraction and RT-PCR
Total RNA was isolated using TRIZOL Reagent (Tiangen Corp.) with DNaseI digestion (Promega Corp.). PCR experiments were performed in 25 μl system applying 2 × PCR Taq MasterMix (TIANGEN Corp.). The specific primer sequences were as follows:
Klotho (374 bp), sense: 5' –CGACCACTTCAGGGATTACGC-3'; antisense: 5'-GGATAGTCACCATCAATAAATACGG-3'; CyclinD1 (322 bp), sense: 5'-CCGCTGGCCATGAACTACCT-3'; antisense: 5'-ACGAAGGTCTGCGCGTGTT-3' C-Myc (110 bp), sense: 5'-TCAAGAGGCGAACACACAAC -3'; antisense: 5'-GGCCTTTTCATTGTTTTCCA -3' Wnt3a (166 bp), sence: 5'-TCCACGCCATTGCCTCAG-3'; antisense: 5'-CGAGACACCATCCCACCAAA-3'.
Western blot
Protein level was measured by western blot. Briefly, equal amounts of protein (10 µg per lane) were separated by SDS-PAGE electrophoresis, and transferred to PVDF membrane (Bio-Rad Inc.). The membranes were incubated in blocking buffer (0.2 mM Tris, 137 mM NaCl, 5% no-fat milk, and 0.1% Tween-20) for an hour and then probed at 4°C overnight with KL antibody. The membranes were rinsed with washing buffer (0.1% Tween 20, 0.2 mM Tris, and 137 mM NaCl) and incubated with HRP-conjugated secondary antibody (1:5,000) for an hour at room temperature, followed by chemiluminescent detection. Antibodies against Klotho (Santa Cruz), total β-catenin (Cell Signaling), Wnt3a (Abcam) and active β-catenin (Millipore) were used to probe the blot.
Scratch motility assay for motility and invasiveness
3 × 105 A549 cells were plated in a 6-well plate and grown to 80% confluency before the transfection. After 24 h, the monolayer was scratched with a pipette tip to create a cell free strip area, washed with PBS gentlely to remove floating cells and took photograph at three random locations (0 h). We set up cells transfected with scramble siRNA for control. The microscope photograph of migration was taken every 24 h for two days at the same locations.
Invasion assays
Transwell Boyden chambers (8 mm pores size filter insert) were coated with 2.5 mg/ml Matrigel (BD Biosciences). Cells (2 × 105) were seeded in DMEM medium in the upper chamber with serum-containing medium in the lower chamber. After incubation for 24 h, cells that invaded the Matrigel were stained with 4 μg/ml of calcein-PBS at 37°C for 1 h and subjected to scan fluorescence.
Statistical analysis
Statistical significance was assessed using Student’s t-test or analysis of variance (ANOVA). Data were presented as mean ± SEM, and p < 0.05 was considered significant. All the experiments were repeated in three parallels.
Supplementary Material
Acknowledgments
This work was a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and also supported by the National Natural Science Foundation of China (30971320).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cbt/article/21420
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/21420
References
- 1.Königshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42:21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 2.He B, Jablons DM. Wnt signaling in stem cells and lung cancer. Ernst Schering Found Symp Proc. 2006:27–58. doi: 10.1007/2789_2007_043. [DOI] [PubMed] [Google Scholar]
- 3.He B, Barg RN, You L, Xu Z, Reguart N, Mikami I, et al. Wnt signaling in stem cells and non-small-cell lung cancer. Clin Lung Cancer. 2005;7:54–60. doi: 10.3816/CLC.2005.n.022. [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 5.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 6.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–23. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 8.Kurosu H, Kuro-o M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17:368–72. doi: 10.1097/MNH.0b013e3282ffd994. [DOI] [PubMed] [Google Scholar]
- 9.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010;29:99. doi: 10.1186/1756-9966-29-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Jeong DJ, Kim J, Lee S, Park JH, Chang B, et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Wichert G, Haeussler U, Greten FR, Kliche S, Dralle H, Böhm BO, et al. Regulation of cyclin D1 expression by autocrine IGF-I in human BON neuroendocrine tumour cells. Oncogene. 2005;24:1284–9. doi: 10.1038/sj.onc.1208264. [DOI] [PubMed] [Google Scholar]
- 15.Borowiec AS, Hague F, Gouilleux-Gruart V, Lassoued K, Ouadid-Ahidouch H. Regulation of IGF-1-dependent cyclin D1 and E expression by hEag1 channels in MCF-7 cells: the critical role of hEag1 channels in G1 phase progression. Biochim Biophys Acta. 2011;1813:723–30. doi: 10.1016/j.bbamcr.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–68. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth AI, Stewart DB, Nelson WJ. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc Natl Acad Sci USA. 1999;96:4947–52. doi: 10.1073/pnas.96.9.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katoh M. Molecular cloning and characterization of human WNT3. Int J Oncol. 2001;19:977–82. doi: 10.3892/ijo.19.5.977. [DOI] [PubMed] [Google Scholar]
- 19.Camilli TC, Xu M, O’Connell MP, Chien B, Frank BP, Subaran S, et al. Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res. 2011;24:175–86. doi: 10.1111/j.1755-148X.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, et al. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res. 2011;17:4254–66. doi: 10.1158/1078-0432.CCR-10-2749. [DOI] [PubMed] [Google Scholar]
- 22.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–65. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Katsaros D, Wiley A, de la Longrais IA, Puopolo M, Yu H. Klotho expression in epithelial ovarian cancer and its association with insulin-like growth factors and disease progression. Cancer Invest. 2008;26:185–92. doi: 10.1080/07357900701638343. [DOI] [PubMed] [Google Scholar]
- 25.Usuda J, Ichinose S, Ishizumi T, Ohtani K, Inoue T, Saji H, et al. Klotho predicts good clinical outcome in patients with limited-disease small cell lung cancer who received surgery. Lung Cancer. 2011;74:332–7. doi: 10.1016/j.lungcan.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Chen B, Xu W, Liu S, Zhao W, Wu J. Combined effects of klotho and soluble CD40 ligand on A549 lung cancer cells. Oncol Rep. 2011;25:1465–72. doi: 10.3892/or.2011.1178. [DOI] [PubMed] [Google Scholar]
- 27.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/S0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–5. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 29.Velcheti V, Govindan R. Insulin-like growth factor and lung cancer. J Thorac Oncol. 2006;1:607–10. doi: 10.1097/01243894-200609000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn H, Konrad J, Holtz S, Salameh A, Gessner C, Hammerschmidt S, et al. Enhanced expression of VEGF following bFGF inhibition in non-small cell lung cancer cell lines. Lung Cancer. 2006;54:149–53. doi: 10.1016/j.lungcan.2006.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.