Abstract
Background
Ofloxacin is a fluoroquinolone (FQ) used for the treatment of leprosy. FQs are known to interact with both A and B subunits of DNA gyrase and inhibit supercoiling activity of this enzyme. Mutations conferring FQ resistance have been reported to be found only in the gene encoding A subunit of this enzyme (gyrA) of M. leprae, although there are many reports on the FQ resistance-associated mutation in gyrB in other bacteria, including M. tuberculosis, a bacterial species in the same genus as M. leprae.
Methodology/Principal Findings
To reveal the possible contribution of mutations in gyrB to FQ resistance in M. leprae, we examined the inhibitory activity of FQs against recombinant DNA gyrases with amino acid substitutions at position 464, 502 and 504, equivalent to position 461, 499 and 501 in M. tuberculosis, which are reported to contribute to reduced sensitivity to FQ. The FQ-inhibited supercoiling assay and FQ-induced cleavage assay demonstrated the important roles of these amino acid substitutions in reduced sensitivity to FQ with marked influence by amino acid substitution, especially at position 502. Additionally, effectiveness of sitafloxacin, a FQ, to mutant DNA gyrases was revealed by low inhibitory concentration of this FQ.
Significance
Data obtained in this study suggested the possible emergence of FQ-resistant M. leprae with mutations in gyrB and the necessity of analyzing both gyrA and gyrB for an FQ susceptibility test. In addition, potential use of sitafloxacin for the treatment of problematic cases of leprosy by FQ resistant M. leprae was suggested.
Author Summary
Leprosy is one of the oldest human infectious diseases, which remains a public health problem with more than 200,000 new cases every year worldwide. Since the late 1990s, multi-drug resistant leprosy, resistant to rifampicin and dapsone, has emerged and the importance of ofloxacin has increased. However, their use for leprosy and other infectious diseases has already elicited ofloxacin resistant leprosy cases. Hence, early detection of ofloxacin resistance is essential for proper treatment. This study, by utilizing recombinant technology, predicted the future emergence of ofloxacin resistant Mycobacterium leprae with mutations that have not yet been reported. The data are useful for predicting ofloxacin resistance and, hence, able to contribute to the proper treatment of leprosy through suggesting the importance of analyzing gene mutations for FQ susceptibility testing.
Introduction
Leprosy is one of the oldest human infectious diseases and remains a public health problem. At the beginning of 2011, the number of registered leprosy cases was 192,246, and that of new cases reported during 2010 was 228,474, mainly from Asian, Latin American, and African countries [1]. Multibacillary leprosy is usually treated by administering dapsone (DDS), clofazimine (CLF), and rifampicin (RIF) in combination, where single skin lesion paucibacillary leprosy is recommened to be treated by administering RIF, ofloxacin (OFX), and minocycline (MIN) [2]. Since the late 1990s, multi-drug resistant (MDR) isolates of M. leprae, resistant to RIF and DDS, have emerged and the importance of OFX has been a focus for the treatment of MDR-leprosy [3]; however, their use not only for leprosy but also for other infectious diseases including tuberculosis has already led to OFX resistance in M. leprae [4]–[8]. Hence, early prediction of FQ resistance seems to be essential for the proper treatment of leprosy.
OFX is a fluoroquinole (FQ) and FQs inhibit type II DNA topoisomerases, including DNA gyrase and topoisomerase IV [9]. FQ resistance is given mainly by amino acid substitutions in the quinolone resistance-determining regions (QRDRs) located on the N- and C-terminal domains of A (GyrA) and B (GyrB) subunits of DNA gyrase and, less prominently, amino acid substitution in the QRDR on the N- and C-terminal domains of A (ParC) and B (ParE) subunits of topoisomerase IV has been reported [10]. M. leprae has only DNA gyrase [11], which is therefore the sole target of FQs. Genetic analysis of M. leprae clinical isolates revealed reduced FQ sensitivity associated with amino acid substitutions only at position 89 or 91 and 205 in GyrA and GyrB, respectively [4]–[8], [12]. In the latter study, the contribution of amino acid substitution in GyrA at position 89 or 91 to reduced FQ sensitivity was confirmed by an in vitro analysis [13]. In addition, the effect of amino acid substitution at position 95 in GyrA was predicted [14]. In contrast, amino acid substitution in GyrB at position 205, reported by You et al. [8], was revealed not to affect FQ sensitivity by an in vitro study [13]. Reduced FQ sensitivity associated with amino acid substitutions has been frequently reported in GyrA in M. tuberculosis; however, those in GyrB have been reported less frequently (Figure 1) [10], [15]. According to the reports, important residues of GyrB in M. tuberculosis were thought to be at codon 461, 499 and 501 (with a counting system proposed by Maruri et al. [10]). Notably, amino acid substitutions at position 499 and 501 in M. tuberculosis showed a correlation with reduced FQ susceptibility by an in vitro assay [15]–[18]. Lack of the detection of FQ-resistant M. leprae carrying GyrB amino acid substitutions is due to the low number of FQ resistant cases analyzed. Hence, it is highly important to elucidate the contribution of amino acid substitutions in GyrB to FQ resistance utilizing recombinant technology and in vitro assay.
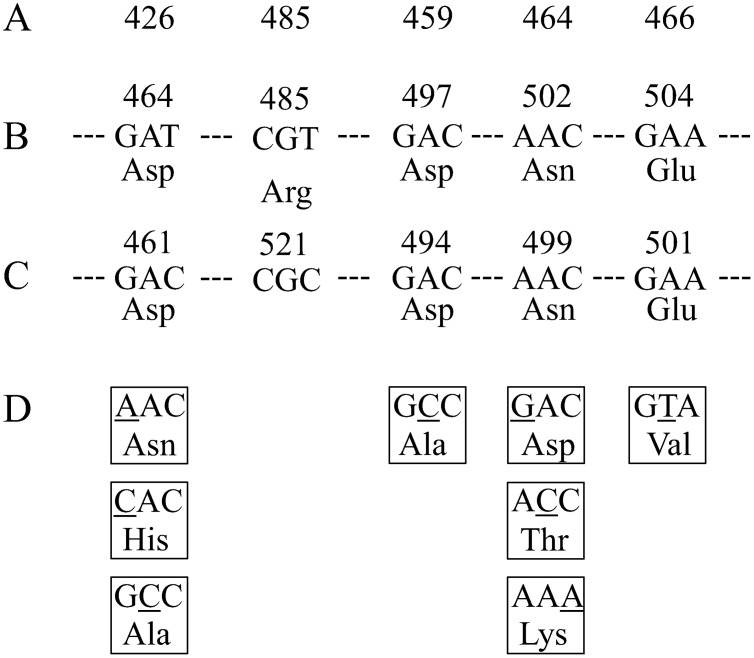
Figure 1. Nucleotide and amino acid sequences of QRDR of M. leprae and M. tuberculosis gyrB and mutations found in FQ-resistant isolates.
(A) amino acid number of GyrB in E. coli, (B) Amino acid number, nucleotide sequences and amino acid sequence of WT M. leprae GyrB QRDR, (C) Amino acid number, nucleotide sequences and of WT M. tuberculosis GyrB QRDR, (D) Altered amino acids and corresponding nucleotide substitutions found in higher rate in FQ-resistant M. tuberculosis isolates.
On the basis of reports on M. tuberculosis, we selected target amino acid substitutions at position 464, 502 and 504 in M. leprae GyrB, equivalent to position 461, 499 and 501 in M. tuberculosis, to reveal the significance of these amino acid substitutions for reduced FQ sensitivity, and conducted the FQ-inhibited supercoiling assay and FQ-mediated DNA cleavage assay using recombinant DNA gyrase.
Methods
Drugs and kits
Ofloxacin (OFX), ciprofloxacin (CIP) and levofloxacin (LVX) were purchased from LKT Laboratories, Inc. (St. Paul, MN); moxifloxacin (MXF) was from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada); sitafloxacin (SIT) was from Daiichisankyo Pharmaceutical, Co., Ltd. (Tokyo, Japan); ampicillin and kanamycin were purchased from Meiji Seika Pharma Ltd. (Tokyo, Japan). Oligonucleotide primers were synthesized by Life Technologies Corp. (Carlsbad, CA). Restriction enzymes were obtained from New England Biolabs, Inc. (Ipswich, MA). The supercoiling assay kit and supercoiled and relaxed pBR322 DNA were purchased from John Innes Enterprises Ltd. (Norwich, United Kingdom).
Bacterial strains and plasmid
The Thai-53 strain of M. leprae [19], maintained at the Leprosy Research Center, National Institute of Infectious Diseases (Tokyo, Japan), was used to prepare M. leprae DNA. Escherichia coli strains TOP-10 (Life Technologies Corp.), Rosetta-gami 2, and BL21 (DE3) pLysS (Merck KGaA, Darmstadt, Germany) were used for cloning and protein expression. pET-20b (+) (Merck KGaA) vector was used to construct expression plasmids for M. leprae DNA gyrases.
Construction of expression plasmids
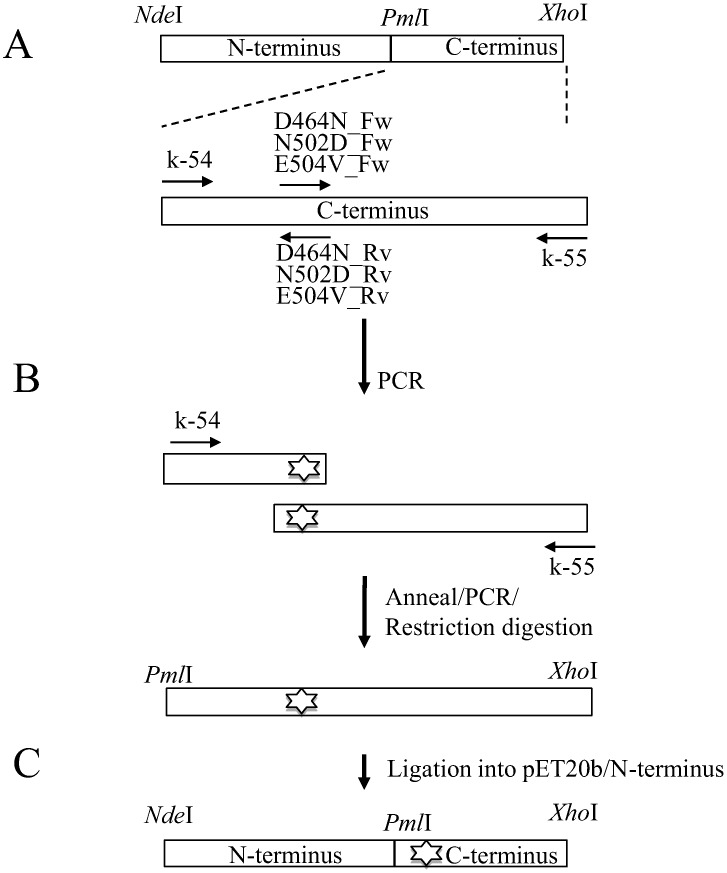
Wild-type (WT) recombinant GyrA and GyrB expression plasmids were constructed as described previously [14], [16]. Mutations were introduced into the WT gyrB gene by PCR using pairs of complementary primers containing the mutations of interest (Table 1). All PCR reactions were carried out in a thermal cycler (Life Technologies Corp.) under the following conditions: pre-denaturation at 95°C for 2 min; 35 cycles of denaturation at 95°C for 10 s, annealing at 50–60°C for 15 s, and extension at 68°C for 1 to 3 min, and then a final extension at 68°C for 5 min. The gyrB C-terminal cassettes with base substitutions were digested with Pml I and Xho I, ligated into WT gyrB expression plasmid, and digested with the same restriction endonucleases to obtain mutant gyrB expression plasmid (Figure 2). The nucleotide sequences of the DNA gyrase genes in the plasmids were confirmed using a BigDye Terminator (version 3.1) cycle sequencing kit and an ABI Prism 3130xI genetic analyzer (Life Technologies Corp.) according to the manufacturer's protocol.
Table 1. Nucleotide sequences of primers used in this study.
| Primer name | Primer sequence (Nucleotide Position) |
| k-54 | 5′-CGTAAAGCACGTGAGTTAGTGCGTCGAAAAAGTGCC-3′ (1270–1305) |
| k-55 | 5′-GGCTCGAGCTAATGATGATGATGATGATGGACATCCAGGAAACGAACATCC-3′ (2013–2037) |
| D464N_Fw | 5′-A GTG GAA GGT AAT TCG GCT GGT G |
| D464N_Rv | 5′-C ACC AGC CGA ATT ACC TTC CAC T |
| N502D_Fw | 5′-A GTG CTA AAG GAC ACC GAA GTT C |
| N502D_Rv | 5′-G AAC TTC GGT GTC CTT TAG CAC T |
| E504V_Fw | 5′-A AAG AAC ACC GTA GTT CAA GCA A |
| E504V_Rv | 5′-T TGC TTG AAC TAC GGT GTT CTT T |
Mutated codons are indicated in bold face.
Figure 2. Construction of WT and mutant DNA gyrase expression plasmid.
(A) Primer pairs k-54+D464N_Rv, N502D_Rvor E504V_Rv (Table 1) were used for amplifying the DNA fragment encoding N-terminus half (amino acid 424 to 467, 505 or 507, respectively) of C-terminus region of GyrB carrying Asp464Asn, Asn502Asp and Glu504Val, respectively. Primer pairs k-55+D464N_Fw, N502D_Fw or E504V_Fw (Table 1) were used for amplifying the DNA fragment encoding the C-terminus half (amino acid 461, 499 or 501 to 678, respectively) of the C-terminus region of GyrB carrying Asp464Asn, Asn502Asp and Glu504Val, respectively. (B) To complete the C-terminus region encoding cassette, DNA fragments encoding the N-terminus half and C-terminus half of the C-terminus region of GyrB were annealed and reamplified by PCR using the primer pair of k-54 and k-55. (C) The mutated gyrB-C cassettes were digested with PmlI and XhoI restriction endonucleases and ligated into the expression plasmid containing the WTgyrBN-terminus region DNA fragment digested by the same enzymes.
Expression and purification of recombinant DNA gyrase subunits
Recombinant DNA gyrase subunits were expressed and purified as previously described [13], [14], [16], [20]. Briefly, expression plasmids carrying the gyrA and gyrB of M. leprae were transformed into E. coli Rosetta-gami 2 and BL21 (DE3) pLysS, respectively. The transformants were grown in Luria-Bertani (LB) medium in the presence of 100 µg/mL Ampicillin to the log phase and the expression of DNA gyrase was induced with the addition of 1 mM isopropyl-beta-D-thiogalactopyranoside (Wako Pure Chemical Industries Ltd., Osaka, Japan), followed by further incubation at 14°C for 16 h. The harvested E. coli were lysed by sonication (Sonifier 250; Branson, Danbury, CT) and the recombinant DNA gyrase subunits in supernatants after centrifugation (10,000× g for 30 min) were purified by Ni-NTA Agarose resin (Life Technologies Corp.) column chromatography and dialyzed against DNA gyrase dilution buffer (50 mM Tris-HCl pH 7.5, 100 mM KCl, 2 mM DTT, 1 mM EDTA). The purified protein fractions were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
DNA supercoiling assay and inhibition by FQs
ATP-dependent and FQ-inhibited DNA supercoiling assays were performed according to previous reports [13], [14], [16], [20]. DNA supercoiling activity was examined with reaction mixture consisting of DNA gyrase reaction buffer, relaxed pBR322 DNA (0.3 µg), and GyrA and GyrB subunits (50 ng each) in a total volume of 30 µl. Reactions were run at 30°C for 1.5 h followed by stopping with the addition of 30 µl chloroform/iso-amyl alcohol (24∶1 mixture) and 3 µl of 10× DNA loading solution. The total reaction mixtures were subjected to electrophoresis on 1% agarose gels in 1× Tris-borate-EDTA (TBE) buffer and stained by ethidium bromide (0.7 µg/ml). The extent of supercoiled DNA was quantified with ImageJ (http://rsbweb.nih.gov/ij) and the inhibitory effects of FQs on DNA gyrase were assessed by determining the drug concentration required to inhibit the supercoiling activity of the DNA gyrase by 50% (IC50s) in the presence or absence of serial two-fold increases in the concentrations of OFX, MXF, SIT, CIP and LVX. Enzymatic assays were performed at least three times to confirm the reproducibility.
FQ-mediated DNA cleavage assay
DNA cleavage assays were also carried out as described in previous reports [13], [14], [16], [20], [21]. Briefly, the reaction mixture (total volume 30 µl) contained DNA gyrase assay buffer, purified DNA gyrase subunits, supercoiled pBR322 DNA (0.3 µg) and increasing concentrations of OFX, MXF, SIT, CIP and LVX. After incubation for 2 h at 30°C, cleavage reactions were stopped by adding 3 µl of 2% SDS and 3 µl proteinase K (1 mg/ml). After subsequent incubation for 30 min at 30°C, proteinase K reactions were stopped by the addition of 3 µl of 0.5 mM EDTA, 30 µl chloroform/iso-amyl alcohol (24∶1 mixture) and 3 µl of 10× DNA loading dye. The total reaction mixtures were subjected to electrophoresis in 0.8% agarose gels in 1× TBE buffer, followed by ethidium bromide staining. The extent of DNA cleavage was quantified with ImageJ (http://rsbweb.nih.gov/ij) and the FQ concentrations required to induce 25% of the maximum DNA cleavage (CC25s) were determined.
Results
Construction and purification of recombinant WT and mutant DNA gyrase subunits
The WT GyrA and GyrB expression plasmids constructed in our previous work [14] were used. DNA fragments with mutations causing amino acid substitutions at position 464, 502 and 504 in GyrB were amplified from WT GyrB expression plasmid [14] and introduced into expression vector pET-20b (+). Recombinant GyrA and GyrB were expressed as C-terminus hexa-histidine tagged protein for ease of purification, as the His-tag has been shown not to interfere with the catalytic functions of GyrA and GyrB [13]–[16], [20], [22]. Expressed recombinant WT and mutant DNA gyrase subunits were purified as 0.4 to 1.7 mg soluble His-tagged protein with molecular weights of 80 kDa and 75 kDa for GyrA and GyrB, respectively, from 500 ml cultures. The purity of recombinant proteins was confirmed by SDS-PAGE (Figure S1). All of the recombinant proteins were obtained with high purity (>90–95%).
ATP-dependent DNA supercoiling activities of WT and mutant DNA gyrases
Combinations of WT GyrA and WT or mutant GyrBs (GyrB-Asp464Asn, GyrB-Asn502Asp or GyrB-Glu504Val) were examined for DNA supercoiling activities using relaxed pBR322 DNA as a substrate in the presence or absence of ATP (Figure S2). DNA supercoiling activities were observed in the presence of ATP and recombinant DNA gyrase subunits (Figure S2 A–D, lane 3), while neither subunit alone exhibited DNA supercoiling activity (Figure S2 A–D, lane 4, 5). In addition, no supercoiling activity was observed when ATP was omitted from the reaction condition (Figure S2 A–D, lane 6). Consequently, ATP-dependent DNA supercoiling activities were confirmed with WT and three mutant DNA gyrases.
IC50s of five FQs for WT and mutant DNA gyrases
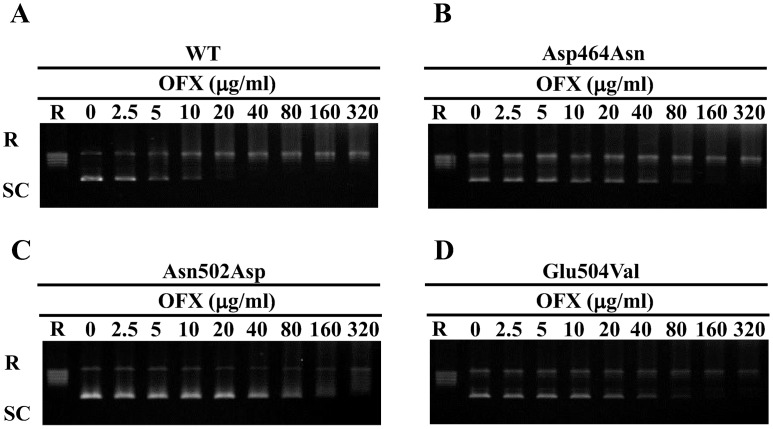
FQs-inhibited DNA supercoiling activities were assessed for the determination of IC50s. Figure 3 shows a representative result of the inhibitory effect of OFX and the results for the other FQs are presented in Figure S3. Results show the dose-dependent inhibition of five FQs against WT and mutant DNA gyrases, as summarized in Table 2. The five FQs inhibited the DNA supercoiling activities of WT DNA gyrase at low concentration (Table 2).
Figure 3. OFX-inhibited DNA supercoiling assay.
Relaxed pBR322 (0.3 mg) was incubated with GyrA (50 ng) and GyrB (50 ng) in the presence of the indicated concentration of OFX. FQ-inhibited supercoiling activity assay was performed in combination of WTGyrA+WTGyrB (A), GyrB-Asp464Asn (B), GyrB-Asn502Asp (C) and GyrB-Glu504Val (D). R and SC denote relaxed and supercoiled pBR322 DNA, respectively.
Table 2. IC50s and CC25s of FQs against WT and mutant DNA gyrases.
| Drug | IC50 (µg/ml) | CC25 (µg/ml) | ||||||
| WT | Asp464Asn | Asn502Asp | Glu504Val | WT | Asp464Asn | Asn502Asp | Glu504Val | |
| OFX | 5.7±0.8 | 53.9±9.0 | 106.6±25.1 | 34.6±4.3 | 2.4±0.2 | 32.7±6.3 | 78.2±12.6 | 30.0±7.9 |
| MXF | 1.7±0.3 | 4.1±0.4 | 17.8±2.6 | 13.9±0.6 | 0.6±0.0 | 3.3±0.9 | 15.3±2.6 | 9.6±1.7 |
| SIT | 0.5±0.1 | 1.8±0.3 | 1.6±0.6 | 1.7±0.2 | 0.2±0.0 | 0.9±0.0 | 1.0±0.2 | 0.7±0.1 |
| CIP | 2.3±0.3 | 11.3±2.7 | 257.9±46.1 | 49.3±9.4 | 0.9±0.2 | 6.5±0.6 | 42.5±13.6 | 24.7±0.5 |
| LVX | 4.5±0.3 | 32.9±3.2 | 46.8±1.1 | 19.9±2.9 | 1.4±0.1 | 18.6±4.9 | 51.7±10.6 | 9.3±0.7 |
CC25s of five FQs for WT and mutant DNA gyrases
DNA cleavage assay was performed in the presence of increasing concentrations of FQs to estimate CC25s. Figure 4 presents the results of a representative DNA cleavage assay using OFX, and Figure S4 shows those using other FQs. Table 2 shows the CC25s of FQs for WT and mutant DNA gyrases. Highest CC25s of FQs were observed for GyrB-Asn502Asp DNA gyrase.
Figure 4. OFX-mediated DNA cleavage assay.
Supercoiled pBR322 (0.3 mg) was incubated with GyrA (50 ng) and GyrB (50 ng) in the presence of the indicated concentration of OFX. DNA cleavage assay was performed in combination of WT GyrA+WT GyrB (A), GyrB-Asp464Asn (B), GyrB-Asn502Asp (C) and GyrB-Glu504Val (D). R, L and SC denote relaxed, linear and supercoiled pBR322 DNA, respectively.
Discussion
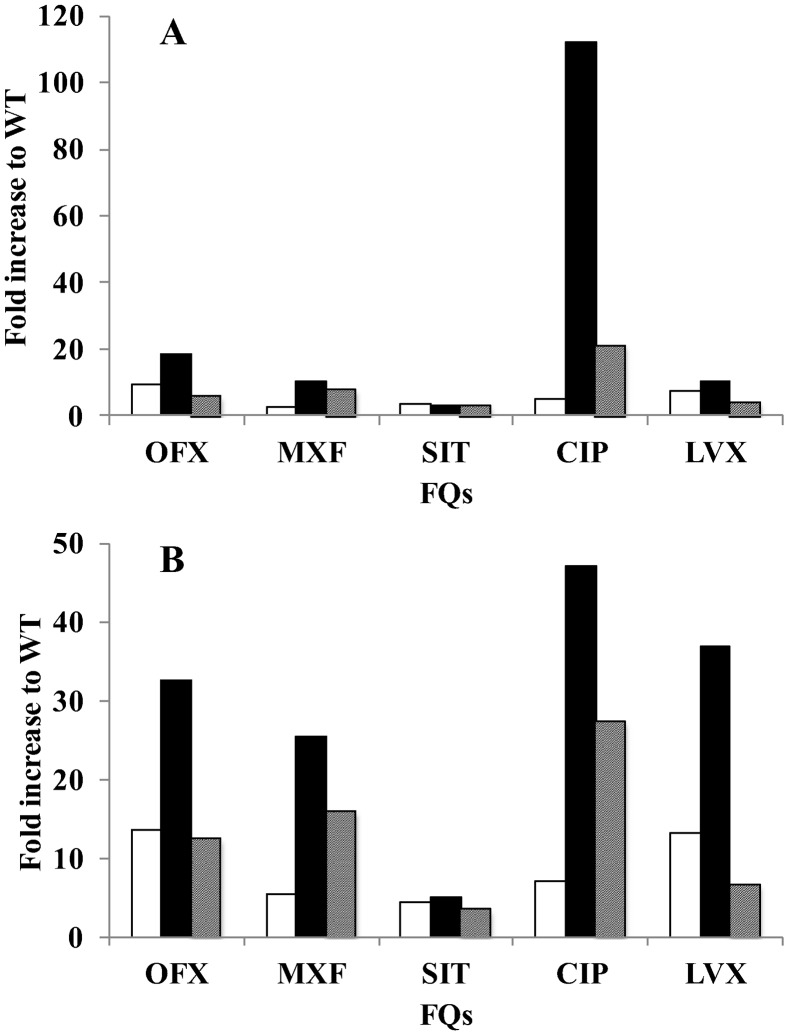
We focused on amino acid substitutions at position 464, 502 and 504 in GyrB in M. leprae equivalent to 461, 499 and 501, respectively, in M. tuberculosis, as amino acid substitutions at these positions in M. tuberculosis are known to contribute to FQ resistance [13]–[16], [20], [22], [23]. We carried out a FQ-mediated supercoiling activity inhibition assay and a DNA cleavage assay using recombinant WT and mutant DNA gyrases at 30°C, the optimal temperature of M. leprae growth [24], and calculated IC50s and CC25s of five FQs, including OFX, MXF, SIT, CIP and LVX. All FQs inhibited DNA supercoiling activities of WT DNA gyrase at low concentration (Table 2). In strong contrast, three mutant DNA gyrases showed reduced sensitivity to all five FQs. GyrB-Asn502Asp DNA gyrase exhibited the lowest FQ sensitivity among the three mutant DNA gyrases. IC50s of OFX, MXF, SIT, CIP and LVX for GyrB-Asp464Asn, Asn502Asp and Glu504Val DNA gyrases were 2.4- to 9.5-fold, 3.2- to 112.1-fold and 3.4- to 21.4-fold higher than those for WT DNA gyrase (Figure 3, 5, S3 and Table 2). A similar tendency was observed in the DNA cleavage assay. Namely, CC25s of OFX, MFX, SIT, CIP and LVX for GyrB-Asp464Asn, Asn502Asp and Glu504Val DNA gyrases were 4.5- to 13.6-fold, 5.0- to 47.2-fold and 3.5- to 27.4-fold higher than for WT DNA gyrase (Figure 4, 5, S4, Table 2). These results suggested the contribution of these amino acid substitutions in GyrB to reduced FQ sensitivity and the possible emergence of M. leprae with mutant GyrB, although previously identified Asp to Asn amino acid substitution in GyrB at position 205 [8] was revealed not to have an effect on FQ susceptibility [13]. It is noteworthy that mutant DNA gyrases exhibited a similar sensitivity pattern to those reported for mutant GyrB in M. tuberculosis. M. leprae GyrB-Asn502Asp DNA gyrase had lower FQ sensitivity than GyrB-Asp426Asn and GyrB-Glu504Val DNA gyrase, as has been shown in M. tuberculosis [15]–[18]. The high homology of the entire GyrB and full sequence match in QRDR between M. leprae and M. tuberculosis might lead to a similar tendency of FQ sensitivity. It is interesting that the Asp to Asn amino acid substitution in E. coli at position equivalent to 464 in M. leprae showed enhancing effect on CIP resistance [25] where Glu to Asp or Ala amino acid substitution in Streptococcus pneumonia at position equivalent to 504 in M. leprae showed little or reducing effect on CIP resistance, respectively [26]. Overall or QRDR structure of GyrB might affect the acquisition of FQ resistance.
Figure 5. Increased IC50s and CC25s of FQs for mutant DNA gyrases.
IC50s and CC25s were calculated by the quinolone-inhibited supercoiling assay and FQ-mediated cleavage assay, respectively. Fold increase of each FQ for mutant DNA gyrases was plotted. (A) IC50s, (B) CC25s. Open, closed and hatched bar denotes the value for GyrB- GyrB-Asp464Asn, GyrB-Asn502Asp and GyrB-Glu504Val DNA gyrase, respectively.
IC50s of FQs were 8 to 40 times higher than the minimum inhibitory concentrations (MICs) in M. tuberculosis [17], [18], [22]. This non-proportionality presumably reflects basic differences in the cell-permeating properties and the accumulation of different FQs [22]. We investigated the inhibitory effects of OFX, GAT, MXF, LVX and SIT against WT and mutant DNA gyrases. IC50s of OFX for WT DNA gyrase was 5.7 µg/ml (Table 2) and it seemed reasonable that OFX has been used by a single application of 400 to 600 mg for leprosy patients with a single lesion and two or three doses of 400 to 600 mg in combination with first-line drugs, DDS and RIF [27] for the treatment of patients with MDR leprosy. On the contrary, IC50s of OFX for GyrB-Asp464Asn, Asn502Asp and Glu504Val showed 9.5, 18.7 and 6.1 fold higher concentration comparing to WT DNA gyrase, respectively, and OFX seems not to have the ability to inhibit M. leprae with DNA gyrase with these mutations. On the other hand, the order of inhibitory activity was SIT>MXF>CIP>LVX>OFX. Namely, SIT most effectively inhibited WT and mutant DNA gyrases among five FQs. IC50s of SIT for WT was 0.5 µg/ml and the increase was 3.6-, 3.2- and 3.4-fold for GyrB-Asp464Asn, GyrB-Asn502Asp and GyrB-Glu504Val DNA gyrases, respectively. In addition, the maximum serum concentration (Cmax) of OFX, SIT, CIP and LVX in 100 mg dosage was determined in clinical trials to be 0.95, 1.00, 1.33 and 1.22 µg/ml, respectively [28]–[31], and that of MFX in 400 mg dose to be 4.13 [32]. SIT might strongly inhibit M. leprae carrying GyrB-Asp464Asn, Asn502Asp and Glu504Val DNA gyrase as well as that carrying GyrA-Ala90Val, Asp95Gly, and Asp95Asn [14], [16], [20]. Thus, SIT is a promising candidate for the treatment of leprosy caused by OFX-resistant M. leprae with these problematic gyrases. Although SIT is now only approved in Japan and mild gastrointestinal disorders as adverse reactions have been reported, our data in this study might encourage the use of SIT for OFX-resistant leprosy.
In conclusion, we revealed the contribution of Asp464Asn, Asn502Asp and Glu504Val amino acid substitution to reduced sensitivity to FQ in M. leprae by an in vitro assay. This suggested the possible emergence of FQ-resistant M. leprae carrying GyrB with these amino acid substitutions in the future. Hence we would like to propose the analysis of these amino acid substitutions in GyrB to detect FQ-resistant leprosy. Additionally, effectiveness of sitafloxacin to the mutant DNA gyrases suggested the potential use of this FQ for the treatment of ofloxacin resistant cases.
Supporting Information
SDS-PAGE analysis of purified M. leprae DNA gyrases. The His-tagged recombinant DNA gyrases were over expressed in E. coli and purified by Ni-NTA affinity resin chromatography. Lanes: M: Protein marker (NEB), 1: WTGyrA, 2: WTGyrB, 3: GyrB-Asp464Asn, 4: GyrB-Asn502Asp, 5: GyrB-Glu504Val. 300 ng of each protein was loaded on 5–20% gradient polyacrylamide gel.
(TIF)
DNA supercoiling assay. Supercoiling activities of WT DNA gyrase (A), DNA gyrases bearing GyrB-Asp464Asn (B), Asn502Asp (C) and Glu504Val (D) were analyzed. Relaxed pBR322 (0.3 mg) was incubated with GyrA (50 ng) or GyrB (50 ng) or both. Lanes: 1: relaxed pBR322 alone, 2: relaxed pBR322 and ATP, 3: relaxed pBR322, ATP, GyrA and GyrB, 4: relaxed pBR322, ATP and GyrA, 5: relaxed pBR322, ATP and GyrB, 6: relaxed pBR322, GyrA and GyrB.
(TIF)
Inhibitory activities of (A) MXF, (B) SIT, (C) CIP and (D) LVX on supercoiling activities against M. leprae WT and mutant DNA gyrases. Relaxed pBR322 DNA (0.3 mg) was incubated with 50 ng each of GyrA and GyrB in the absence or presence of the indicated concentration (in mg/ml) of three FQs. The reactions were stopped, and the DNA products were analyzed by electrophoresis on 1% agarose gel. R and SC denote relaxed and supercoiled pBR322 DNA, respectively.
(TIF)
DNA cleavage activity of (A) MXF, (B) SIT, (C) CIP and (D) LVX against M. leprae WT and mutant DNA gyrases. Supercoiled pBR322 DNA (0.3 mg) was incubated with 50 ng each of GyrA and GyrB in the absence or presence of the indicated concentration (in mg/ml) of three FQs. The reactions were stopped, and the processed DNA products were analyzed by electrophoresis on 1% agarose gel. R, L and SC denote relaxed, linear and supercoiled pBR322 DNA, respectively.
(TIF)
Acknowledgments
We thank Ms. Haruka Suzuki, Ms. Yukari Fukushima and Ms. Aiko Ohnuma for their technical support.
Funding Statement
This study was supported in part by J-GRID, the Japan Initiative for Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT), the Global Center of Excellence (COE) Program, “Establishment of International Collaboration Centers for Zoonosis Control” from MEXT, a grant from the U.S.-Japan Cooperative Medical Science Programs to Y.S., a Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) to Y.S. and C.N., and a Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labour, and Welfare of Japan to T.M. and Y.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organization (2011) Weekly epidemiological record 2011;. 86: 389–400. [Google Scholar]
- 2. Ji B, Grosset JH (1990) Recent advances in the chemotherapy of leprosy. Lepr Rev 61: 313–329. [DOI] [PubMed] [Google Scholar]
- 3. Katoch VM (2002) Advances in the diagnosis and treatment of leprosy. Expert Rev Mol Med 4: 1–14. [DOI] [PubMed] [Google Scholar]
- 4. Cambau E, Perani E, Guillemin I, Jamet P, Ji B (1997) Multidrugresistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae . Lancet 349: 103–104. [DOI] [PubMed] [Google Scholar]
- 5. Kim SK, Lee SB, Kang TJ, Chae GT (2003) Detection of gene mutations related with drug resistance in Mycobacterium leprae from leprosy patients using touch-down (TD) PCR. FEMS Immunol Med Microbiol 36: 27–32. [DOI] [PubMed] [Google Scholar]
- 6. Maeda S, Matsuoka M, Nakata N, Kai M, Maeda Y, et al. (2001) Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob Agents Chemother 45: 3635–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuoka M, Kashiwabara Y, Namisato M (2000) A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int J Lepr Other Mycobact Dis 68: 452–455. [PubMed] [Google Scholar]
- 8. You E-Y, Kang TJ, Kim S-K, Lee S-B, Chae G-T (2005) Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J Infect 50: 6–11. [DOI] [PubMed] [Google Scholar]
- 9. Champoux JJ (2001) DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem 70: 369–413. [DOI] [PubMed] [Google Scholar]
- 10. Maruri F, Sterling TR, Kaiga AW, Blackman A, va der Hijden YF, et al. (2012) A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob. Chemother 67: 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camus JC, Pryor MJ, Medigue C, Cole ST (2002) Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148 (Pt10) 2967–2973. [DOI] [PubMed] [Google Scholar]
- 12. Matsuoka M, Suzuki Y, Garcia IE, Fafutis-Morris M, Vargas-Gonzalez A, et al. (2010) Possible mode of emergence for drug-resistant leprosy is revealed by an analysis of saples from Mexico. Jpn J Infect Dis 63: 412–416. [PubMed] [Google Scholar]
- 13. Matrat S, Cambau E, Jarlier V, Aubry A (2008) Are all the DNA gyrase mutations found in Mycobacterium leprae clinical strains involved in resistance to fluoroquinolones? Antimicrob Agents Chemother 52: 745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yokoyama K, Kim H, Mukai T, Matsuoka M, Nakajima C, et al. (2012) Amino acid substitutions at position 95 in GyrA can add fluoroquinolone resistance to Mycobacterium leprae . Antimicrobe Agents Chemother 56: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aubry A, Veziris N, Cambau E, Truffot-Pernot C, Jarlier V, et al. (2006) Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob Agents Chemother 50: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H, Nakajima C, Yokoyama K, Rahim Z, Kim YU, et al. (2011) Impact of the E540V amino acid substitution in GyrB of Mycobacterium tuberculosis on quinolone resistance. Antimicrob Agents Chemother 55: 3661–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pantel A, Petrella S, Matrat S, Brossier F, Bastian S, et al. (2011) DNA gyrase inhibition assays are necessary to demonstrate fluoroquinolone resistance secondary to gyrB mutations in Mycobacterium tuberculosis . Antimicrob Agents Chemother 55: 4524–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pantel A, Petrella S, Veziris N, Brossier F, Bastian S, et al. (2012) Extending GyrB QRDR definition in Mycobacterium tuberculosis DNA gyrase for assessing FQ resistance in M. tuberculosis . Antimicrobe Agents Chemother 56: 1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuoka M (2010) The history of Mycobacterium leprae Thai-53 strain. Lepr Rev 81: 137. [PubMed] [Google Scholar]
- 20. Matrat S, Petrella S, Cambau E, Sougakoff W, Jarlier V, et al. (2007) Expression and purification of an active form of the Mycobacterium leprae DNA gyrase and its inhibition by quinolones. Antimicrob Agents Chemother 51: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walton L, Elwell LP (1988) In vitro cleavable-complex assay to monitor antimicrobial potency of quinolones. Antimicrobe Agents Chemother 32: 1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aubry A, Pan XS, Fisher LM, Jarlier V, Cambau E (2004) Mycobacterium tuberculosis DNAgyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob Agents Chemother 48: 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piton J, Petrella S, Delatue M, Andre-Leroux G, Jarlier V, et al. (2010) Structural insights into the quinolone resistance mechanism of Mycobacterium tuberculosis DNA gyrase. Plos One 5: e12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepard CC (1965) Temperature optimum of Mycobacterium leprae in mice. J Bacteriol 90: 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heddle J, Maxwell A (2002) Quinolone-binding pocket of DNA gyrase: role of GyrB. Antimicrobe Agents Chemother 46: 1805–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan XS, Gould KA, Fisher LM (2009) Probing the differential interactions of quinazolinedione PD 0305970 and quinolones with gyrase and topoisomerase IV. Antimicrobe Agents Chemother 53: 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto M, Nogami R, Hatano K, Okano Y, Ishii N, et al. (2006) Guideline for the treatment of hansen's disease in Japan (Second edition). Jpn. J Leprosy 75: 191–226. [DOI] [PubMed] [Google Scholar]
- 28. Ichihara N, Tachizawa H, Tsumura M, Une T, Sato K (1984) Phase I study on DL-8280. Chemotherapy 32: 118–149. [Google Scholar]
- 29. Nakashima M, Uematsu T, Kanamaru M, Okazaki O, Hakusui H (1992) Phase I study of levofloxacin, (S)-(−)-ofloxacin. Jpn J Clin Pharmacol Ther 23: 515–520. [Google Scholar]
- 30. Nakashima M, Uematsu T, Kosuge K, Umemura K, Hakusui H, et al. (1995) Pharmacokinetics and tolerance of DU-6859a, a new fluoroquinolone, after single and multiple oral doses in healthy volunteers. Antimicrobe Agents Chemother 39: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yasunaga K, Ueno K, Watanabe K, Azuma J (1997) Phase I studies of intravenous ciprofloxacin (BAY q 3939). CLINICAL REPORT 31: 2433–2466. [Google Scholar]
- 32. Ohnishi N, Toyoki T, Yoshikawa K, Hashizume K, Tanigawa T, et al. (2005) Safety, pharmacokinetics and influence on the intestinal flora of BAY 12-8039 (moxifloxacin hydrochloride) after oral administration in healthy male subjects. Japanese Pharmacology & Therapeutics 33: 1029–1045. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE analysis of purified M. leprae DNA gyrases. The His-tagged recombinant DNA gyrases were over expressed in E. coli and purified by Ni-NTA affinity resin chromatography. Lanes: M: Protein marker (NEB), 1: WTGyrA, 2: WTGyrB, 3: GyrB-Asp464Asn, 4: GyrB-Asn502Asp, 5: GyrB-Glu504Val. 300 ng of each protein was loaded on 5–20% gradient polyacrylamide gel.
(TIF)
DNA supercoiling assay. Supercoiling activities of WT DNA gyrase (A), DNA gyrases bearing GyrB-Asp464Asn (B), Asn502Asp (C) and Glu504Val (D) were analyzed. Relaxed pBR322 (0.3 mg) was incubated with GyrA (50 ng) or GyrB (50 ng) or both. Lanes: 1: relaxed pBR322 alone, 2: relaxed pBR322 and ATP, 3: relaxed pBR322, ATP, GyrA and GyrB, 4: relaxed pBR322, ATP and GyrA, 5: relaxed pBR322, ATP and GyrB, 6: relaxed pBR322, GyrA and GyrB.
(TIF)
Inhibitory activities of (A) MXF, (B) SIT, (C) CIP and (D) LVX on supercoiling activities against M. leprae WT and mutant DNA gyrases. Relaxed pBR322 DNA (0.3 mg) was incubated with 50 ng each of GyrA and GyrB in the absence or presence of the indicated concentration (in mg/ml) of three FQs. The reactions were stopped, and the DNA products were analyzed by electrophoresis on 1% agarose gel. R and SC denote relaxed and supercoiled pBR322 DNA, respectively.
(TIF)
DNA cleavage activity of (A) MXF, (B) SIT, (C) CIP and (D) LVX against M. leprae WT and mutant DNA gyrases. Supercoiled pBR322 DNA (0.3 mg) was incubated with 50 ng each of GyrA and GyrB in the absence or presence of the indicated concentration (in mg/ml) of three FQs. The reactions were stopped, and the processed DNA products were analyzed by electrophoresis on 1% agarose gel. R, L and SC denote relaxed, linear and supercoiled pBR322 DNA, respectively.
(TIF)