Abstract
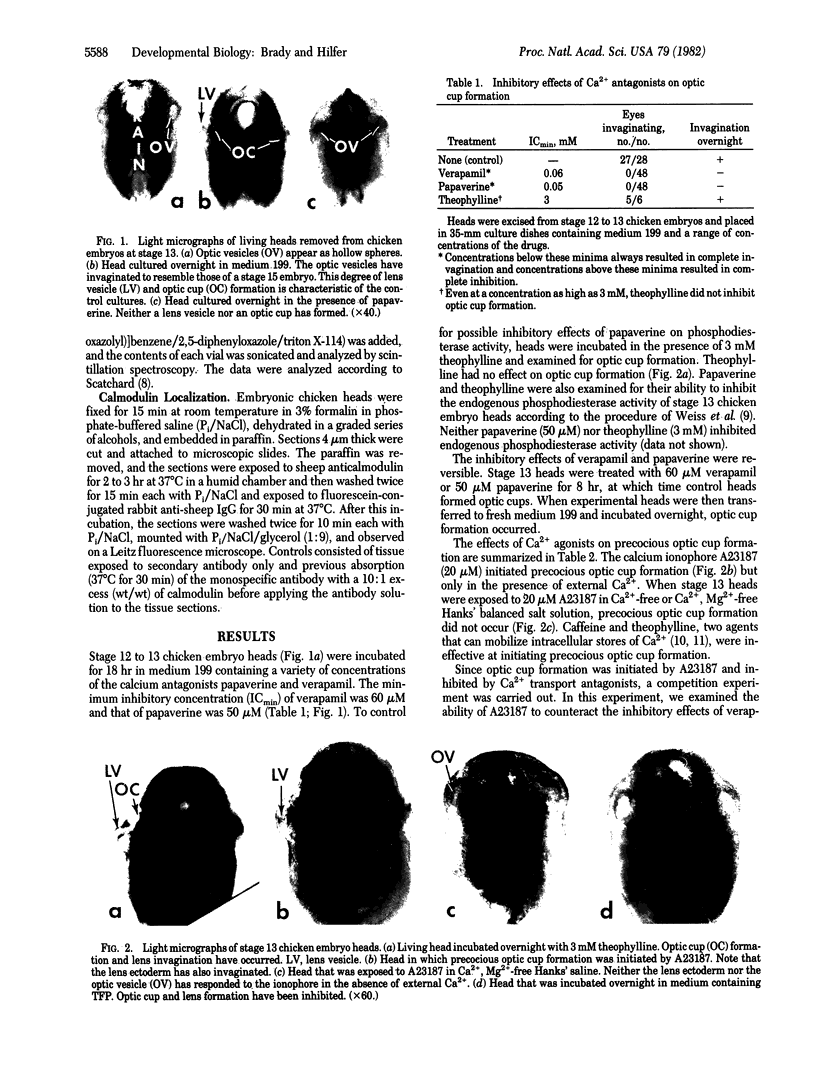
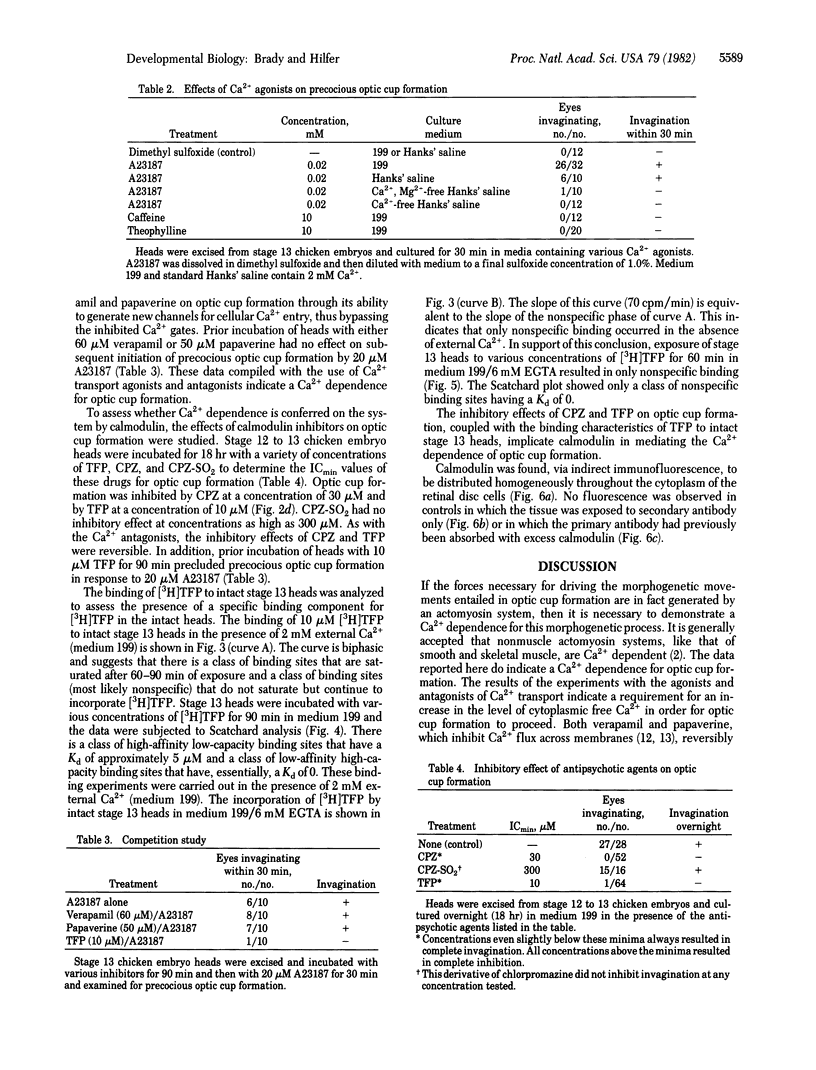
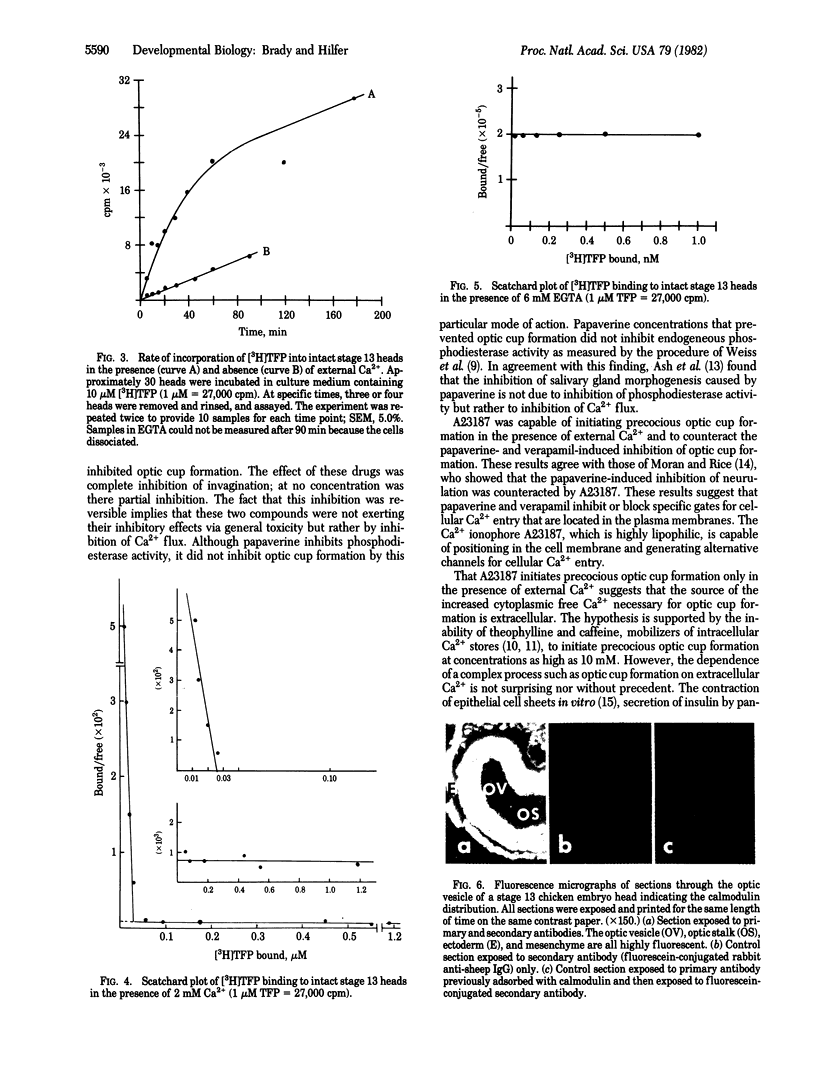
Invagination of the optic vesicle to form the optic cup is an important event in the formation of the eye in the early embryo. To obtain support for earlier conclusions that a contractile process is involved, calcium dependency of optic cup formation was tested. Heads were excised from chicken embryos at the optic vesicle stage of development (stage 13) and incubated in nutrient medium containing antagonists or agonists of calcium transport. Invagination was reversibly inhibited by the Ca2+ antagonists verapamil and papaverine. It was initiated in a precocious fashion by the Ca2+ ionophore A23187 but only in the presence of external Ca2+. Neither caffeine, theophylline, nor A23187 (in the absence of external Ca2+) were able to initiate precocious optic cup formation. Trifluoperazine and chlorpromazine reversibly inhibited optic cup formation while chlorpromazine sulfoxide had no effect at the concentrations used. The binding of [3H]trifluoperazine to isolated stage 13 heads revealed a class of Ca2+-dependent binding sites having a Kd similar to that of calmodulin. These results indicate a Ca2+-dependence for optic cup formation and that the source of the Ca2+ may be extracellular. This Ca2+ dependence probably is conferred to the system by calmodulin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Spooner B. S., Wessells N. K. Effects of papaverine and calcium-free medium on salivary gland morphogenesis. Dev Biol. 1973 Aug;33(2):463–469. doi: 10.1016/0012-1606(73)90151-6. [DOI] [PubMed] [Google Scholar]
- Brisson G. R., Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. VII. A proposed site of action for adenosine-3',5'-cyclic monophosphate. J Clin Invest. 1972 Feb;51(2):232–241. doi: 10.1172/JCI106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen I., De Vries A. Platelet contractile regulation in an isometric system. Nature. 1973 Nov 2;246(5427):36–37. doi: 10.1038/246036a0. [DOI] [PubMed] [Google Scholar]
- Devis G., Somers G., Malaisse W. J. Stimulation of insulin release by calcium. Biochem Biophys Res Commun. 1975 Nov 17;67(2):525–529. doi: 10.1016/0006-291x(75)90843-8. [DOI] [PubMed] [Google Scholar]
- Elferink J. G. Chlorpromazine inhibits phagocytosis and exocytosis in rabbit polymorphonuclear leukocytes. Biochem Pharmacol. 1979 Apr 1;28(7):965–968. doi: 10.1016/0006-2952(79)90287-9. [DOI] [PubMed] [Google Scholar]
- Gautvik K. M., Iversen J. G., Sand O. On the role of extracellular Ca2+ for prolactin release and adenosine 3':5'-monophosphate formation induced by thyroliberin in cultured rat pituitary cells. Life Sci. 1980 Mar 24;26(12):995–1005. doi: 10.1016/0024-3205(80)90122-8. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Auersperg N. Calcium in epithelial cell contraction. J Cell Biol. 1980 May;85(2):325–336. doi: 10.1083/jcb.85.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D., Rice R. W. Action of papaverine and ionophore A23187 on neurulation. Nature. 1976 Jun 10;261(5560):497–499. doi: 10.1038/261497a0. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Weiss B., Lehne R., Strada S. Rapid microassay of adenosine 3',5'-monophosphate phosphodiesterase activity. Anal Biochem. 1972 Jan;45(1):222–235. doi: 10.1016/0003-2697(72)90022-x. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]






