Abstract
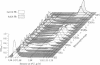
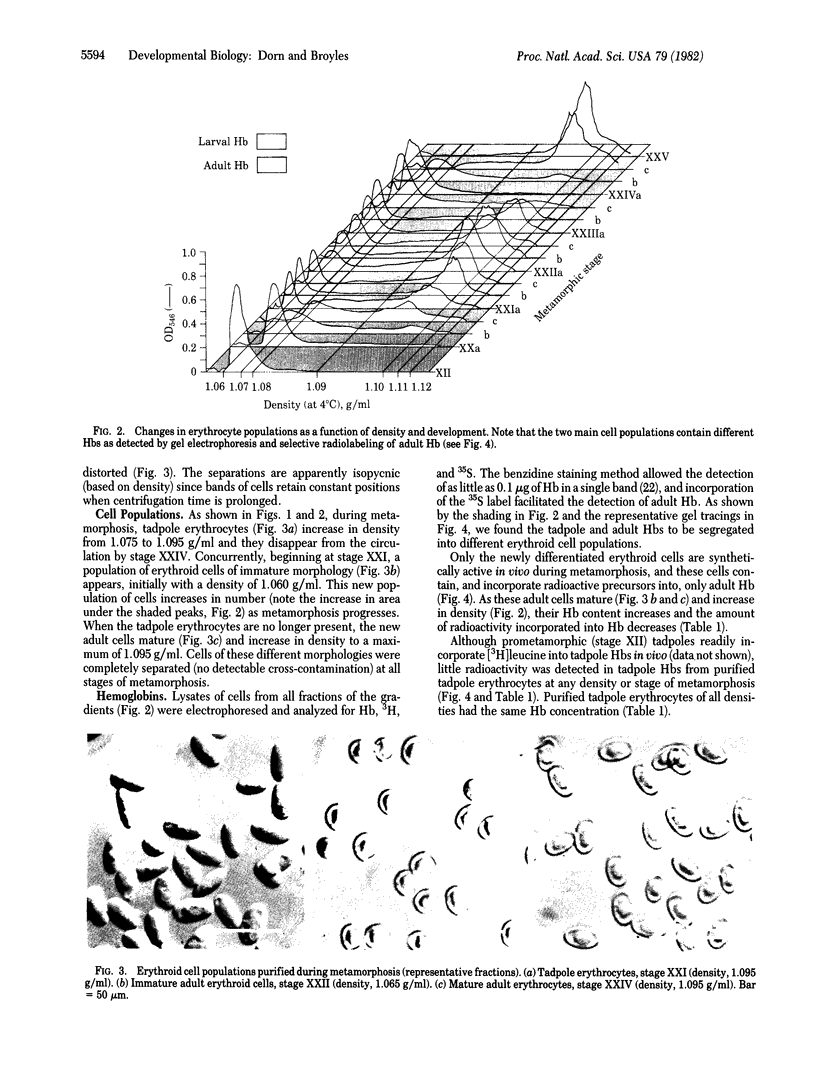
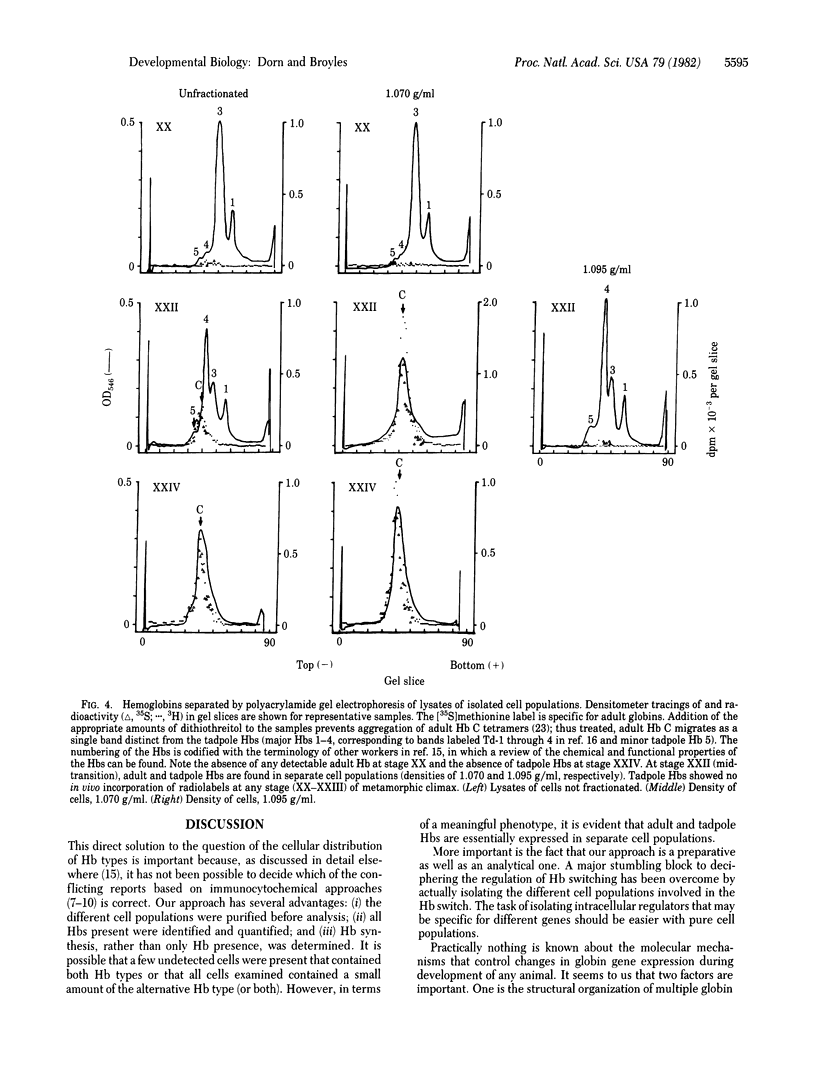
Anurans (frogs and toads) switch from tadpole to adult hemoglobin synthesis during metamorphosis. A number of workers have attempted to determine whether tadpole and adult Hb types are expressed in the same or different erythroid cells during the switch. If the different Hb types are found in different cells during the transition, the switch in globin gene expression occurs at an early stage of cellular differentiation. Previous studies, in which immunocytochemical techniques were used to approach this question, are in conflict in regard to the metamorphic Hb switch of the North American bullfrog Rana catesbeiana. We have purified newly differentiating erythroid cells from the blood of metamorphosing tadpoles by using Percoll gradients. These new cells have an immature morphology, are very active in the synthesis of adult Hb, and contain no detectable tadpole Hb. The tadpole cells have no detectable adult Hb, are synthetically inactive, increase in density during the switch, and are then cleared from the circulation. Thus, only adult Hb expression is detected in newly differentiating erythroid cells during metamorphosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal S. J., Riggs A. The hemoglobins of the bullfrog, Rana catesbeiana. I. Purification, amino acid composition, and oxygen equilibria. J Biol Chem. 1969 May 10;244(9):2372–2383. [PubMed] [Google Scholar]
- Benbassat J. The transition from tadpole to frog haemoglobin during natural amphibian metamorphosis. II. Immunofluorescence studies. J Cell Sci. 1974 Oct;16(1):143–156. doi: 10.1242/jcs.16.1.143. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles R. H., Deutsch M. J. Differentiation of red blood cells in vitro. Science. 1975 Oct 31;190(4213):471–473. doi: 10.1126/science.1080882. [DOI] [PubMed] [Google Scholar]
- Broyles R. H., Frieden E. Sites of haemoglobin synthesis in amphibian tadpoles. Nat New Biol. 1973 Feb 14;241(111):207–209. doi: 10.1038/newbio241207a0. [DOI] [PubMed] [Google Scholar]
- Broyles R. H., Johnson G. M., Maples P. B., Kindell G. R. Two erythropietic microenvironments and two larval red cell lines in bullfrog tadpoles. Dev Biol. 1981 Jan 30;81(2):299–314. doi: 10.1016/0012-1606(81)90293-1. [DOI] [PubMed] [Google Scholar]
- Broyles R. H., Pack B. M., Berger S., Dorn A. R. Quantification of small amounts of hemoglobin in polyacrylamide gels with benzidine. Anal Biochem. 1979 Apr 1;94(1):211–219. doi: 10.1016/0003-2697(79)90811-x. [DOI] [PubMed] [Google Scholar]
- Chapman B. S., Tobin A. J. Distribution of developmentally regulated hemoglobins in embryonic erythroid populations. Dev Biol. 1979 Apr;69(2):375–387. doi: 10.1016/0012-1606(79)90298-7. [DOI] [PubMed] [Google Scholar]
- Dolan M., Sugarman B. J., Dodgson J. B., Engel J. D. Chromosomal arrangement of the chicken beta-type globin genes. Cell. 1981 Jun;24(3):669–677. doi: 10.1016/0092-8674(81)90093-3. [DOI] [PubMed] [Google Scholar]
- Farquhar M. N., Papayannopoulou T., Brice M., Kan Y. W., Stamatoyannopoulos G. Cellular regulation of fetal hemoglobin synthesis in man. Investigation of gamma and beta mRNA accumulation in clonal erythroid cultures initiated from erythroid progenitors derived from fetuses, neonates, and adult individuals. Dev Biol. 1980 Nov;80(1):64–78. doi: 10.1016/0012-1606(80)90499-6. [DOI] [PubMed] [Google Scholar]
- Forman L. J., Just J. J. The life span of red blood cells in the amphibian larvae, Rana catesbeiana. Dev Biol. 1976 Jun;50(2):537–540. doi: 10.1016/0012-1606(76)90173-1. [DOI] [PubMed] [Google Scholar]
- Jurd R. D., Maclean N. An immunofluorescent study of the haemoglobins in metamorphosing Xenopus laevis. J Embryol Exp Morphol. 1970 Apr;23(2):299–309. [PubMed] [Google Scholar]
- Just J. J., Sperka R., Strange S. A quantitative analysis of plasma osmotic pressure during metamorphosis of the bullfrog, Rana catesbeiana. Experientia. 1977 Nov 15;33(11):1503–1505. doi: 10.1007/BF01918836. [DOI] [PubMed] [Google Scholar]
- Leder A., Swan D., Ruddle F., D'Eustachio P., Leder P. Dispersion of alpha-like globin genes of the mouse to three different chromosomes. Nature. 1981 Sep 17;293(5829):196–200. doi: 10.1038/293196a0. [DOI] [PubMed] [Google Scholar]
- Patient R. K., Elkington J. A., Kay R. M., Williams J. G. Internal organization of the major adult alpha- and beta-globin genes of X. laevis. Cell. 1980 Sep;21(2):565–573. doi: 10.1016/0092-8674(80)90494-8. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, weir E. E., Zettergren J. G. Problems connected with the separation of different kinds of cells. Int Rev Exp Pathol. 1975;14:91–204. [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Shortman K. Physical procedures for the separation of animal cells. Annu Rev Biophys Bioeng. 1972;1:93–130. doi: 10.1146/annurev.bb.01.060172.000521. [DOI] [PubMed] [Google Scholar]
- Stratton L. P., Frieden E. Autoradiographic detection of reactive protein-SH and its application to anuran haemoglobin chains. Nature. 1967 Dec 2;216(5118):932–934. doi: 10.1038/216932a0. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Red cell ferritin content during the hemoglobin transition of amphibian metamorphosis. Dev Biol. 1973 Oct;34(2):282–288. doi: 10.1016/0012-1606(73)90357-6. [DOI] [PubMed] [Google Scholar]
- Watt K. W., Riggs A. Hemoglobins of the tadpole of the bullfrog, Rana catesbeiana. Structure and function of isolated components. J Biol Chem. 1975 Aug 10;250(15):5934–5944. [PubMed] [Google Scholar]