Abstract
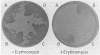
There are seven ribosomal RNA operons (rrn operons) in Escherichia coli. A single rrn operon was amplified by use of a multicopy recombinant plasmid containing a complete rrnH operon. rrnH thereby has the potential to contribute a greater fraction of the rRNA found in ribosomes. Erythromycin-resistant mutants were isolated from cells containing the plasmid, and at least one mutation to resistance was shown to reside in rrnH on the plasmid. Erythromycin resistance was retained when a major deletion was introduced into the 16S rRNA gene and was abolished by deletions that affect the 16S and 23S rRNA genes but do not alter the 5S rRNA gene or non-rrnH DNA. Cell-free S30 protein-synthesizing extracts from cells containing the mutant plasmid have an increased resistance to erythromycin. The selection procedure used to isolate erythromycin-resistance mutations in rrnH may allow, with minor modifications, the isolation of mutations in rrn operons that change resistance of the ribosome to other antibiotics or that alter other properties of ribosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Graham M. Y., Weisblum B. 23S ribosomal ribonucleic acid of macrolide-producing streptomycetes contains methylated adenine. J Bacteriol. 1979 Mar;137(3):1464–1467. doi: 10.1128/jb.137.3.1464-1467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Fallon A. M., Nomura M. Identification and organization of ribosomal protein genes of Escherichia coli carried by lambdafus2 transducing phage. J Biol Chem. 1977 Oct 25;252(20):7323–7336. [PubMed] [Google Scholar]
- Kenerley M. E., Morgan E. A., Post L., Lindahl L., Nomura M. Characterization of hybrid plasmids carrying individual ribosomal ribonucleic acid transcription units of Escherichia coli. J Bacteriol. 1977 Dec;132(3):931–949. doi: 10.1128/jb.132.3.931-949.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D. Cell-free protein synthesis directed by coliphage MS2 RNA: synthesis of intact viral coat protein and other products. J Mol Biol. 1965 Sep;13(2):521–531. doi: 10.1016/s0022-2836(65)80114-0. [DOI] [PubMed] [Google Scholar]
- Pardo D., Rosset R. A new ribosomal mutation which affects the two ribosomal subunits in Escherichia coli. Mol Gen Genet. 1977 Jun 8;153(2):199–204. doi: 10.1007/BF00264736. [DOI] [PubMed] [Google Scholar]
- Pestka S. Binding of [14C]erythromycin to Escherichia coli ribosomes. Antimicrob Agents Chemother. 1974 Oct;6(4):474–478. doi: 10.1128/aac.6.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhl R., Nierhaus K. H. Assembly map of the large subunit (50S) of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):729–733. doi: 10.1073/pnas.79.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman L., Apirion D. Binding of erythromycin to the 50S ribosomal subunit is affected by alterations in the 30S ribosomal subunit. Mol Gen Genet. 1976 Feb 2;143(3):301–306. doi: 10.1007/BF00269407. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Sekiya T., Mori M., Takahashi N., Nishimura S. Sequence of the distal tRNA1Asp gene and the transcription termination signal in the Escherichia coli ribosomal RNA operon rrnF(or G). Nucleic Acids Res. 1980 Sep 11;8(17):3809–3827. doi: 10.1093/nar/8.17.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Nierhaus K. H. Proteins fro Escherichia coli ribosomes involved in the binding of erythromycin. J Mol Biol. 1978 Dec 5;126(2):185–193. doi: 10.1016/0022-2836(78)90358-3. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Davis B. D. Cyclic blockade of initiation sites by streptomycin-damaged ribosomes in Escherichia coli: an explanation for dominance of sensitivity. J Mol Biol. 1973 Apr 5;75(2):377–390. doi: 10.1016/0022-2836(73)90028-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Corcoran J. W. Antibiotic glycosides. VI. Definition of the 50s ribosomal subunit of Bacillus subtilis 168 as a major determinant of sensitivity to erythromycin A. Biochemistry. 1967 Aug;6(8):2578–2585. doi: 10.1021/bi00860a040. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G., Apirion D., Rosen L., Tanaka K., Tamaki M., Takata R., Dekio S., Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973 Dec 20;127(2):175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]
- Yamada T., Mizugichi Y., Nierhaus K. H., Wittmann H. G. Resistance to viomycin conferred by RNA of either ribosomal subunit. Nature. 1978 Oct 5;275(5679):460–461. doi: 10.1038/275460a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Tandem promoters direct E. coli ribosomal RNA synthesis. Cell. 1979 May;17(1):225–234. doi: 10.1016/0092-8674(79)90310-6. [DOI] [PubMed] [Google Scholar]