Abstract
Chronic obstructive pulmonary disease (COPD) is an inflammatory disorder characterized by incompletely reversible airflow obstruction. Bacterial infection of the lower respiratory tract contributes to approximately 50% of COPD exacerbations. Even during periods of stable lung function, the lung harbors a community of bacteria, termed the microbiome. The role of the lung microbiome in the pathogenesis of COPD remains unknown. The COPD lung microbiome, like the healthy lung microbiome, appears to reflect microaspiration of oral microflora. Here we describe the COPD lung microbiome of 22 patients with Moderate or Severe COPD compared to 10 healthy control patients. The composition of the lung microbiomes was determined using 454 pyrosequencing of 16S rDNA found in bronchoalveolar lavage fluid. Sequences were analyzed using mothur, Ribosomal Database Project, Fast UniFrac, and Metastats. Our results showed a significant increase in microbial diversity with the development of COPD. The main phyla in all samples were Actinobacteria, Firmicutes, and Proteobacteria. Principal coordinate analyses demonstrated separation of control and COPD samples, but samples did not cluster based on disease severity. However, samples did cluster based on the use of inhaled corticosteroids and inhaled bronchodilators. Metastats analyses demonstrated an increased abundance of several oral bacteria in COPD samples.
Introduction
Chronic obstructive pulmonary disease (COPD), a chronic inflammatory lung disorder characterized by non-reversible airflow limitation, is presently the third-leading cause of death in the United States. Cigarette smoking is the principal cause of COPD in industrialized nations, but only approximately 20% of adults with substantial tobacco exposure develop clinically significant COPD. Some patients with advanced COPD are prone to exacerbations, which are characterized by worsening dyspnea, wheezing, cough, and sputum production [1]. Therefore, COPD remains a heterogeneous disease with respect to disease susceptibility and progression. The pathogenesis of COPD likely involves many as-yet undescribed mediators of inflammation, with bacterial infection or colonization likely playing a role [2].
Traditional microbial culture techniques have demonstrated that approximately 50% of COPD exacerbations are associated with pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. These organisms can often be found colonizing the airways of COPD patients between exacerbations [3]. The term lung microbiome has been used to describe this community of organisms inhabiting the lung. Since many of these bacteria persist in the airways of patients with COPD, their presence may promote a chronic inflammatory state that drives COPD pathogenesis.
In the past, studies of the microbiome relied on culture-based systems. New techniques for describing the microbiome using 16S rRNA pyrosequencing have allowed us to taxonomically classify and describe the human microbiome without the biases inherent in microbial culture techniques [4]–[6]. Charlson et al. demonstrated the presence of 16S rDNA sequences in the bronchoalveolar lavage fluid (BALF) of healthy volunteers. The authors noted a significant correlation between each subject’s oropharyngeal and lung microbiomes, but no consistent lung-specific microbiome was found across subjects. The authors concluded that the normal lung microbiome consisted of organisms that gained access to the lower respiratory tract through microaspiration or bronchoscopic carryover [7].
Accurate descriptions of the microbiome have allowed us to study the interactions between the microbiome and the host immune system. The microbiome in early childhood may play a role in the development of asthma [8], while specific components of the microbiome are associated with chronic asthma in adulthood [9]. As has been described by the “hygiene hypothesis” for asthma pathogenesis, exposure to a normal commensal microbiome may promote immune tolerance, which is necessary for normal immune system maturation and control of inflammation [10]. An analogous process may be at play in the pathogenesis of other lung diseases that involve the interplay between the microbiome, immune tolerance, and infection. For instance, Herbst et al. have shown that the normal mouse microbiome is necessary for normal maturation, recruitment and control of allergic airway inflammation [11], while Ichinohe et al. showed that the mouse microbiome helps regulate the immune function necessary to respond to Influenza A virus infection [12].
The lung microbiomes of healthy smokers as well as patients with COPD have been described and reviewed [2], [13]. Huang et al. showed that patients experiencing COPD exacerbations requiring ventilator support and broad-spectrum antibiotics maintained diverse lung microbiomes [14]. Erb-Downward et al. showed that the microbiomes of 2 patients with Moderate or Severe COPD had lower bacterial diversity scores than healthy smokers and healthy non-smokers. They described a core COPD lung microbiome that included Pseudomonas, Streptococcus, Prevotella, Fusobacterium, Haemophilus, Veillonella, and Porphyromonas. In addition, the lung microbiomes of Very Severe COPD patients were sampled intensively at the time of transplantation. They noted striking differences in the microbiomes at adjacent lung sites, driven by the dominance of Pseudomonas, Haemophilus, and Stenotrophomonas in each sample [15]. Sze et al. evaluated the lung tissue microbiomes of 8 Very Severe COPD patients at the time of lung transplantation. They noted increased bacterial diversity in the COPD patients compared to controls. COPD patients had an increase in the phylum Firmicutes, attributable to an increase in Lactobacillus [16].
We hypothesize that alterations in the COPD lung microbiome and/or its interactions with the host immune system may lead to disordered immune tolerance and the development of an inflammatory state that accelerates the progression of COPD. We undertook our study to evaluate the lung microbiomes of a large number of patients with Moderate or Severe COPD and compare them with the microbiomes of control patients. We chose to use COPD patients without a recent exacerbation to determine if the microbiome became more diverse during disease progression and limit the microbiome-altering effects of steroids and antibiotics.
Results
Thirty-two samples from 3 groups (10 Control samples, 14 Moderate COPD samples, and 8 Severe COPD samples) were submitted for 454 pyrosequencing. Over 460,000 sequences were obtained, with each sample averaging 14,451 sequences after trimming and quality control filtering (Table 1). The number of operational taxonomic units (OTUs) observed at 97% identity ranged from 3-119. There were statistically significant differences in the numbers of sequences obtained from the Control and COPD groups as indicated by a p-value of 0.0326. Using the Bonferroni method for post hoc comparisons we found that this is driven by the smaller number of sequences in the Severe COPD group, as compared to the Moderate COPD group. There was no difference in the number of OTUs obtained per sample between the groups (p = 0.36). Shannon and Simpson (1-D) diversity indices demonstrated that the Severe COPD group was the most diverse, followed by the Moderate COPD group; the Control group was the least diverse. These differences in the diversity indices were significantly different among the Control and COPD groups (Shannon p = 0.0082, Simpson p = 0.0167 by the Kruskal Wallace test) and in post hoc comparisons using the Bonferroni method we found that the differences were driven by differences among the Control and Severe COPD groups (for the Simpson index the difference between the control group and the Severe COPD group just missed the cutoff). However, when we statistically control for the effect of age we find that there is not a significant difference among the groups (the Shannon index p-values for Control and Moderate COPD patients and Control and Severe COPD patients are p = 0.49 and p = 0.73, respectively, while the p-values for the Simpson index are p = 0.30 and p = 0.89, respectively), but age is associated with diversity (p = 0.0163 for the Shannon index and p = 0.0062 for the Simpson index). Moderate COPD patients were older than severe COPD patients (p = 0.0241 by Wilcoxon’s test).
Table 1. Patient Characteristics and Sequencing Results.
| Sample | Age | Gender | Smoking Status* | Trimmed Sequences | OTUs | Shannon Index | Simpson (1-D) Index |
| Control 4 | 29 | Male | Nonsmoker | 5,845 | 34 | 0.11 | 0.03 |
| Control 5 | 48 | Female | Nonsmoker | 19,756 | 17 | 0.72 | 0.50 |
| Control 7 | 50 | Female | Nonsmoker | 11,102 | 16 | 0.02 | 0.00 |
| Control 8 | 42 | Male | Smoker | 21,438 | 50 | 0.34 | 0.11 |
| Control 9 | 28 | Male | Nonsmoker | 20,010 | 47 | 0.39 | 0.13 |
| Control 10 | 46 | Female | Smoker | 19,824 | 73 | 1.35 | 0.68 |
| Control 12 | 46 | Male | Smoker | 22,012 | 20 | 0.34 | 0.17 |
| Control 14 | 33 | Female | Nonsmoker | 9,340 | 45 | 0.62 | 0.33 |
| Control 19 | 48 | Male | Nonsmoker | 14,210 | 29 | 1.20 | 0.55 |
| Control 23 | 25 | Male | Smoker | 23,422 | 14 | 0.30 | 0.15 |
| Control Average | 39.5±9.7 | 16,696±6105# | 34.5±18.2 | 0.54±0.44† | 0.27±0.24† | ||
| Moderate 18 | 71 | Male | Nonsmoker | 15,670 | 14 | 0.78 | 0.49 |
| Moderate 22 | 69 | Male | Nonsmoker | 12,280 | 45 | 0.83 | 0.45 |
| Moderate 43 | 63 | Male | Nonsmoker | 12,996 | 27 | 0.15 | 0.05 |
| Moderate 55 | 76 | Male | Nonsmoker | 25,174 | 99 | 1.77 | 0.70 |
| Moderate 60 | 73 | Female | Nonsmoker | 13,470 | 109 | 2.51 | 0.83 |
| Moderate 74 | 66 | Male | Nonsmoker | 10,898 | 28 | 1.63 | 0.72 |
| Moderate 86 | 78 | Male | Nonsmoker | 20,166 | 41 | 1.76 | 0.76 |
| Moderate 93 | 76 | Male | Nonsmoker | 5,070 | 26 | 0.74 | 0.41 |
| Moderate 95 | 77 | Male | Nonsmoker | 11,221 | 93 | 2.74 | 0.87 |
| Moderate 112 | 64 | Male | Nonsmoker | 14,276 | 44 | 1.48 | 0.57 |
| Moderate 138 | 73 | Male | Nonsmoker | 13,828 | 49 | 1.86 | 0.78 |
| Moderate 146 | 75 | Male | Nonsmoker | 19,391 | 119 | 2.47 | 0.87 |
| Moderate 184 | 55 | Male | Nonsmoker | 16,900 | 11 | 0.02 | 0.00 |
| Moderate 190 | 60 | Male | Nonsmoker | 17,468 | 47 | 1.62 | 0.68 |
| Moderate COPD Average | 69.7±7.1§ | 14,915±4843 | 53.7±34.7 | 1.45±0.85 | 0.58±0.28 | ||
| Severe 13 | 62 | Male | Nonsmoker | 11,325 | 35 | 1.38 | 0.66 |
| Severe 52 | 70 | Female | Nonsmoker | 8,383 | 56 | 2.59 | 0.90 |
| Severe 64 | 65 | Male | Nonsmoker | 11,130 | 45 | 2.00 | 0.79 |
| Severe 72 | 65 | Male | Nonsmoker | 12,175 | 42 | 1.09 | 0.45 |
| Severe 73 | 57 | Male | Nonsmoker | 9,654 | 89 | 2.01 | 0.76 |
| Severe 85 | 64 | Male | Nonsmoker | 10,201 | 89 | 2.73 | 0.87 |
| Severe 153 | 59 | Male | Nonsmoker | 11,570 | 85 | 2.09 | 0.78 |
| Severe 166 | 59 | Male | Nonsmoker | 12,214 | 3 | 0.00 | 0.00 |
| Severe COPD Average | 62.6±4.2§ | 10,832±1330 | 55.5±28.7 | 1.74±0.89 | 0.65±0.30 | ||
| All COPD Average | 67.1±7.0 | 13,430±4376# | 54.4±33.4 | 1.55±0.85† | 0.61±0.28† |
All COPD patients were non-smokers for at least 6 months prior to study entry.
Fewer sequences were obtained from COPD samples than from Control samples (p = 0.0326); this association was driven by the lower number of sequences in the Severe COPD (compared to Moderate COPD) group. This was not associated with a difference in the number of OTUs obtained.
COPD samples were significantly more diverse than Control Samples (p = 0.0082 Shannon, p = 0.0167 Simpson). This severity effect disappears when we control for age.
Moderate COPD patients are older than Severe COPD patients (p = 0.0241).
Rarefaction curves were calculated for all samples, and showed that with very few exceptions, additional sampling would not have provided additional OTUs (Figure S1). A Venn diagram was created to illustrate the similarities between each group (Figure S2). All sequences in each subject group were combined, with 285 OTUs observed in the Control group, 412 OTUs in the Moderate COPD group, and 253 OTUs in the Severe COPD group. Significant overlap was observed between the Severe and Moderate COPD groups, with 56% and 34% of OTUs shared between the two, respectively. In contrast, only 17% of OTUs were shared between Control and Moderate COPD, and only 23% of OTUs were shared between Control and Severe COPD groups. Only 6.3% of all OTUs were found in common in all 3 groups.
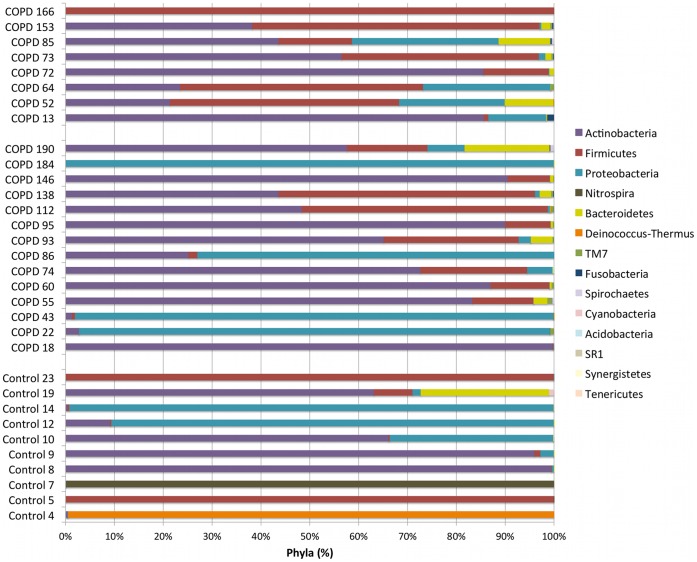
Sequences were submitted to RDP Classifier for taxonomic identification with a bootstrap cutoff of 50%. Phylum-level classification for each sample is provided in Figure 1. The most common phylum in all samples was Actinobacteria, followed by Firmicutes, Proteobacteria, Nitrospira, and Bacteroidetes. Most control samples contained a mix of Actinobacteria, Firmicutes and Proteobacteria. Two samples (Control 4 and 7), both with low diversity indices and a moderate number of OTUs, were unexpectedly dominated by the phyla Deinococcus-Thermus or Nitrospira, respectively. The corresponding genera Deinococcus and Nitrospira have been isolated from numerous environmental sites, but have not been isolated from humans. The Moderate COPD group contained mostly Actinobacteria and Proteobacteria. Two samples (Moderate 43 and 184) with low diversity indices were dominated by Proteobacteria. The Severe COPD group contained mostly Actinobacteria and Firmicutes. One sample (Severe 166) with a low diversity index was dominated by Firmicutes. It appeared that Severe COPD samples contained more Firmicutes and less Actinobacteria and Proteobacteria than the Moderate COPD samples; however, statistical analysis did not demonstrate a significant association.
Figure 1. Taxonomic Identification at the Phylum Level.
All sequences were submitted to RDP Classifier for taxonomic identification with a bootstrap cutoff of 50%. Taxonomic results at the phylum level are displayed for each sample with Control samples at the bottom, Moderate COPD samples in the middle, and Severe COPD samples at the top. The legend is organized from most (top) to least abundant (bottom) phyla.
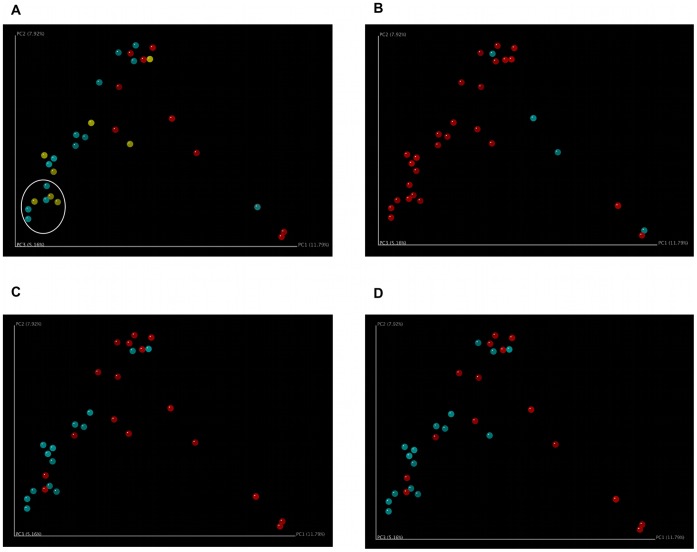
In order to evaluate the similarities between our samples, principal coordinate analysis (PCoA) was performed using Fast UniFrac. This analysis revealed clustering of control and COPD samples. No separation between Moderate COPD and Severe COPD samples was observed (Figure 2A). We identified 7 COPD samples that clustered most distinctly from the control samples and labeled them “left lower quadrant” samples (LLQ, circled). These 7 samples were almost evenly divided between Moderate and Severe samples and included COPD 55, 73, 85, 93, 138, 146, and 153.
Figure 2. Principal Coordinate Analysis Demonstrates Clustering of COPD Samples, Inhaled Corticosteroid Users, and Inhaled Bronchodilator Users.
Principal coordinate analysis was performed using mothur and Fast UniFrac, and the results for principal coordinates 1 and 2 are shown. A. Control, Moderate COPD, and Severe COPD. Control samples (red) cluster separately from Moderate COPD (blue) and Severe COPD (yellow) samples. Moderate and Severe COPD samples do not cluster separately. Seven COPD samples that separate the most from the control samples are circled and designated “left lower quadrant” (LLQ) samples for further analysis. B. Smokers and Non-Smokers. Smokers (blue) do not cluster separately from Non-Smokers (red). All of the COPD patients had been non-smokers for at least 6 months prior to bronchoscopy. C. Inhaled Corticosteroid Users and Non-Users. Inhaled corticosteroid users (blue, 14 of 22 COPD patients) are more likely to cluster near the intersection of principal coordinates 1 and 2 than non-users (red). D. Inhaled Bronchodilator Users and Non-Users. Inhaled bronchodilator users (blue, 16 of 22 COPD patients) are more likely to cluster near the intersection of principal coordinates 1 and 2 than non-users (red). All patients who received inhaled corticosteroids also received inhaled bronchodilators.
Using the clinical information available on these subjects, we also analyzed the data for clustering based on other clinical parameters. We were specifically interested in the potential effects of tobacco exposure or immunosuppressant drugs such as steroids on the lung microbiome. All of the COPD subjects were non-smokers for at least 6 months prior to bronchoscopy, but 4 of our control subjects were smokers. PCoA did not demonstrate any clustering among the 4 control subjects who were active tobacco users at the time of bronchoscopy (Figure 2B). We then turned our attention to the potential effect of steroid use on the microbiome. None of the COPD patients had used systemic steroids in the 2 months prior to bronchoscopy. However, 14 of the 22 COPD patients were using inhaled corticosteroids (ICS) while none of the control subjects were using ICS or systemic steroids. PCoA demonstrated clustering of the ICS users (Figure 2C). We were also interested in the effects of other lung medications, including inhaled bronchodilators (IBD). Of the 16 COPD patients using IBD, 14 were also using ICS. PCoA did demonstrate clustering of IBD users (Figure 2D), but given the high degree of overlap between the ICS- and IBD-using populations, we are unable to determine which medication drives this association. We also analyzed the PCoA data for clustering based on age, gender, percent of lung tissue with emphysema (determined based on CT scanning), and theophylline use. No clustering based on these clinical parameters was observed (Figure S3A–D).
To determine the taxa responsible for the clustering observed on PCoA analysis, we used Metastats to detect differentially abundant features between samples. We compared COPD vs. Control samples, ICS users vs. non-users, IBD users vs. non-users, and samples in the left lower quadrant (LLQ, circled in Figure 2A) vs. all others (Table 2). In each comparison, separate analyses were performed at each taxonomic level. In order to control the false discovery rate at 10%, we reported only taxa with q-values <0.10 (it transpires that all of these comparisons also had p-values less than 0.05). We primarily focused our discussion on organisms that were differentially abundant in 3 or more of our 4 analyses, and on organisms that were differentially abundant at multiple corresponding taxonomic levels.
Table 2. Metastats Analysis of Differential Abundance.
| Phylum | Class | Order | Family | Genus |
| Actinobacteria | Actinobacteria | ↑CoribacterialesAD | ↑CoriobacteriaceaeA | ↑AtopobiumA |
| ↑CryptobacteriumABC | ||||
| ↑OlsenellaA | ||||
| ↓RubrobacteralesC | ↓RubrobacterineaceaeC | ↓RubrobacterC | ||
| Actinomycetales | Nocardioidaceae | ↑NocardioidesABC | ||
| ↑MicrococcaceaeAC | ↓ArthrobacterA | |||
| ↑RothiaAC | ||||
| Propionibacteriaceae | ↓PropionibacteriumB | |||
| ↑KineosporiaceaeD | ||||
| ↑CellulomonadaceaeC | ↑TropherymaC | |||
| ↑ActinomycetaceaeA | ↑ActinomycesA | |||
| ↓GeodermatophilaceaeAC | ↓ModestobacterAC | |||
| ↓NakamurellaceaeABC | ↓HumicoccusABC | |||
| Bifidobacteriales | Bifidobacteriaceae | ↑BifidobacteriumA | ||
| Firmicutes | Bacilli | Bacillales | Bacillalaceae | ↑Pontibacillus BC |
| ↓ThermoactinomycetaceaeABC | ↓ThermoactinomycesABC | |||
| ↑LactobacillalesAD | ↑StreptococcaceaeAD | ↑StreptococcusA | ||
| ↑AerococcaceaeABC | ↑AbiotrophiaABC | |||
| Erysipelotrichi | ↑ErysipelotrichalesA | ↑ErysipelotrichaceaeA | ↑Bulleidia/↑SolobacteriumA | |
| Negativicutes | Selenomonadales | ↑VeillonellaceaeA | ↑DialisterA | |
| ↑VeillonellaA | ||||
| ↑SelenomonasA | ||||
| ↑CentipedaA | ||||
| Clostridia | Clostridiales | ↑LachnospiraceaeA | ↑CatonellaA | |
| ↑OribacteriumA | ||||
| ↑ButyrivibrioA | ||||
| ↑EubacteriaceaeA | ↑EubacteriumA | |||
| Clostridiaceae | ↑AnaerosporobacterA | |||
| ↑Clostridium BC | ||||
| ↓AnaerobacterA | ||||
| Peptostreptococcaceae | ↓SporacetigeniumBC | |||
| ↑PeptostreptococcusA | ||||
| Unclassified Clostridales | ↑HowardellaD | |||
| Clostridales incerta sedis | ↑ParvimonasA | |||
| ↑FusobacteriaA | ↑FusobacteriaA | ↑FusobacterialesA | ↑LeptotrichiaceaeA | ↑LeptotrichiaA |
| ↑FusobacteriaceaeA | ↑FusobacteriumA | |||
| Proteobacteria | α-proteobacteria | ↑RhodospirillalesA | ↑BradyrhizobiaceaeBC | ↑Balneimonas ABC |
| Sphingomonadales | ↑SphingomonadaceaeB | |||
| β-proteobacteria | ↑NeisseralesA | Neisseriaceae | ↑KingellaABC | |
| γ-proteobacteria | ↑Aeromonadales ABC | ↑Aeromonadaceae ABC | ↑Aeromonas AB | |
| Pseudomonadales | Pseudomonadaceae | ↑AzomonasBC | ||
| Enterobacteriales | Enterobacteriaceae | ↑SerratiaA | ||
| ↓CitrobacterABC | ||||
| ↑EnterobacterBC | ||||
| ↑CardiobacterialesD | ||||
| δ-proteobacteria | ↑Desulfobacterales ABC | ↑Desulfobulbaceae ABC | ↑DesulfobulbusAC | |
| ↑DesulfovibrionalesD | ||||
| ↑ε-proteobacteriaA | ↑CampylobacterialesA | ↑CampylobacteriaceaeA | ↑CampylobacterA | |
| Acidobacteria | ↑Group 3 ABC | |||
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | ↑DysgonomonasABC |
| ↑TannerellaA | ||||
| Prevotellaceae | ↑PrevotellaA | |||
| ↑HallellaA | ||||
| Unclassified Bacteroidales | ↑PhocaeicolaBCD | |||
| ↑Bacteroidales incerta sedisBCD | ||||
| Flavobacteria | Flavobacteriales | Flavobacteriaceae | ↑PlanobacteriumBC | |
| ↑CapnocytophagaAC | ||||
| Synergistetes | ↑SynergistiaD | ↑SynergistalesD | ||
| ↑SR1BC | ↑SR1 genera incertae sedis BC | |||
| ↑TM7A | ↑TM7 genera incerta sedisA |
Change in abundance in COPD (compared to Control).
Change in abundance in 14 subjects using inhaled corticosteroids (compared to all other COPD samples and controls).
Change in abundance in 16 subjects using inhaled bronchodilators (compared to all other COPD samples and controls).
Change in abundance in 7 COPD samples in the left lower quadrant (compared to all other COPD samples and controls).
Bolded Taxa were differentially expressed in Severe COPD (compared to Moderate COPD).
At the phylum level, several changes were noted. The anaerobic gram-negative phylum Fusobacteria was increased in the COPD samples, and this increase was reflected at all taxonomic levels down to the genera Leptotrichia and Fusobacterium, two bacteria found in the oral flora [17]. The candidate phyla SR1 and TM7 and their associated genera were increased in the IBD/ICS and COPD analyses, respectively.
At the class level, two changes were noted. The Epsilonproteobacteria were increased in the COPD analysis, and this extended to the genus Campylobacter. Although usually considered a gastrointestinal pathogen, lung infections have been reported due to aspiration of food [18]. Campylobacter was also recently found in the metagenome of cigarettes [19]. The class Synergistia and order Synergistales were increased in the LLQ analysis. These taxa contain the genus Jonquetella, a gram-negative anaerobe implicated in periodontal disease and wound infections [20].
At the order level, multiple changes were noted. Coribacteriales, a member of the gram-positive phylum Actinobacteria, were increased in the COPD and LLQ analyses. This increase was extended down to the genus level: Atopobium, a vaginal commensal and member of the oral flora [21] and Cryptobacterium, a cause of dental abscess [22]. Two orders in the gram-positive phylum Firmicutes were differentially abundant. The Lactobacillales were increased in the COPD and LLQ analyses, and this increase was extended to the genera Streptococcus and Abiotrophia, well-known members of the oral flora. The Erysipelotrichales were also increased in the COPD analysis. This increase was also reflected at multiple taxonomic levels including the genus Bulleidia (Solobacterium), an organism implicated in periodontal disease and dental abscesses [23]. The Aeromonadales, an order of the gram-negative class Gammaproteobacteria, were increased in the COPD, ICS, IBD, and Severe (vs. Moderate) COPD analyses. This increase extended to the genus Aeromonas, which is found in fresh and brackish water and causes diarrhea and wound infections, particularly in immunocompromised patients [24]. The order Desulfobacterales, in the gram-negative class Deltaproteobacteria, was increased in the COPD, ICS, IBD, and Severe (vs. Moderate) COPD analyses. This increase extended to the genus Desulfobulbus, which is associated with periodontitis [25].
Eight additional genera from 4 different phyla were differentially abundant in at least 3 of our 4 analyses. In the phylum Actinobacteria, we noted a decrease in Humicoccus and an increase in Nocardioides, which have both been found in environmental samples. Within Firmicutes, we noted a decrease in Thermoactinomyces, a potential cause of hypersensitivity pneumonitis [26], [27]. Within the phylum Proteobacteria, we saw increases in Balneimonas (found in the environment), as well as Kingella, a member of the oral flora and a cause of bacteremia and endocarditis [26], [27]. Citrobacter, a gastrointestinal pathogen, was decreased. Within the anaerobic phylum Bacteroidetes, we noted increases in the gastrointestinal pathogen Dysgonomonas [28] and Phocaeicola, a cause of brain abscess [29].
We also performed Metastats analysis comparing our Moderate and Severe COPD samples (Supplementary Table 1). This analysis identified 9 differentially abundant genera, 3 of which were also identified as differentially abundant in our Metastats analysis of control and COPD samples.
Discussion
Presented here is an analysis of the lung microbiome in 22 patients with Moderate or Severe COPD compared to 10 control patients. This represents the largest analysis of the COPD microbiome yet published, and the only one to primarily include ambulatory patients with moderate or severe disease. Our results indicate a higher level of microbial diversity among the COPD subjects, but this was driven by differences in age between the Moderate and Severe COPD groups. This is in contrast to the findings of Erb-Downward et al. [15] in an earlier study of the COPD microbiome, who found that Moderate and Severe COPD patients had little bacterial diversity. We noted that several patients in each of our groups exhibited very low diversity scores, despite obtaining greater than 5,000 sequences per sample and rarefaction curves indicating thorough sampling of the microbiome. It seems likely that a minority of COPD patients and controls exhibit low microbial diversity, and these samples may skew the results of studies with small sample sizes. Our results are consistent with Sze et al., who showed that patients with Very Severe COPD maintained greater microbial diversity than control subjects [16]. Our data show that age, rather than severity of COPD, is associated with increased microbial diversity. This analysis excluded the control patients, who were not age-matched to the COPD patients. In our study, Moderate COPD patients were approximately 7 years older than Severe COPD patients. It is unclear to what extent patient age may reflect years since the diagnosis of COPD, as our clinical data do not include the subject’s age at COPD diagnosis.
Our data are consistent with the hypothesis originally proposed by Charlson et al. [7] suggesting that the lung microbiome appears to reflect microaspiration of the oral flora. We noted a significant overlap between Control and COPD sample taxa, although our PCoA was able to cluster Control and COPD samples, but not Moderate and Severe COPD samples, separately. Detailed analysis of clinical factors that may account for alterations in the lung microbiome indicated that use of inhaled corticosteroids or inhaled bronchodilators may have accounted for some of the clustering that we observed. The immunomodulatory effects of steroid exposure likely inhibit the immune response to the lung microbiome. This may allow for the persistence or expansion of the lung microbiome. We did not observe clustering based on tobacco exposure, although our study was hampered by relatively few subjects who were actively smoking at the time of bronchoscopy with an average of 17.5 pack-years of tobacco exposure. Based on our data, it does not appear that the microbiome shifted significantly as a result of tobacco exposure. Further longitudinal microbiome studies of smokers both before and after the development of COPD will be needed to address the question of whether or not tobacco exposure alters the microbiome in a way that predisposes smokers to the development of COPD.
A potential weakness of this study is the possibility that nasal or oral contamination of the bronchoscope, and thus the BALF samples, may have contributed to the observed results. This issue was extensively addressed by Charlson et al. [7] who noted that the bacteria in the BALF arise from upper respiratory tract bacteria, likely through a combination of microaspiration and bronchoscopic carryover during sampling. Determination of the relative contribution of carryover versus microaspiration on lung microbiome composition will require lung tissue microbiome determination, such as was done by Sze et al. [16]. This technique is unfortunately limited to patients who undergo lung explantation or lobectomy, which is not typically performed on patients with relatively stable COPD.
We detected multiple taxa that were differentially abundant in the COPD, inhaled corticosteroid, inhaled bronchodilator and “LLQ” microbiomes. Our findings reinforce the notion that microaspiration of oral flora is the source of the lung microbiome. We identified several common or rare lung pathogens (Rothia, Tropheryma, Actinomyces, Streptococcus, Peptostreptococcus, Serratia, Capnocytophaga), as well as known causes of bacteremia or endocarditis (Rothia, Tropheryma, Streptococcus, Peptostreptococcus, Leptotrichia, Kingella, Dysgonomonas) among the organisms identified by our analysis. Several anaerobes also were observed, including members of the phyla Fusobacteria, Bacteroidetes, and the genus Clostridium within the phylum Firmicutes. Although this is a surprising finding within the presumed aerobic environment of the lung, it is possible that these anaerobes can persist in an abnormal microenvironment of the COPD lung in a manner similar to that seen in lungs affected by cystic fibrosis.
Although other authors have described a “core” lung microbiome, we hesitate to interpret our data in this manner. Both our control and COPD samples included “outlier” samples with very low diversity indices and few OTUs identified despite robust sequencing. Data from healthy controls demonstrated that the lung microbiome composition was much more similar to the same subject’s oral microbiome than to the lung microbiomes of the other subjects [7]. Our data does not support the presence of a “core” microbiome that is stable across multiple subjects, and we suggest that the oral microbiome heavily influences the lung microbiome content.
Both Erb-Downward et al. and Sze et al. [16] published on the COPD microbiome of patients with Very Severe COPD at the time of lung explantation for lung transplant. Patients presenting for lung transplantation likely experience frequent COPD exacerbations, necessitating frequent systemic steroids and/or broad-spectrum antibiotics. They also likely have very abnormal lung anatomy due to long-standing lung disease. Their abnormal anatomy and use of medications that may alter the microbiome makes it difficult to extrapolate these results to patients with less-severe COPD. Our study is the first to describe the microbiome of a large group of COPD patients whose disease is relatively stable, with no systemic steroid or antibiotic use in the previous 2 months. They represent the best environment in which to study the interactions between the microbiome, the immune system, and COPD pathogenesis, as their disease is still evolving.
Our results, as well as other research on the lung microbiome, indicate that microaspiration of oral flora may serve as the source of the lung microbiome. Multiple bacteria in the COPD microbiome are also found in dental caries, dental abscesses, or periodontal disease. Epidemiologic studies have shown an association between poor oral health and COPD progression. Good oral health and regular professional dental cleaning has improved respiratory outcomes for patients, particularly those living in nursing homes. A recent meta-analysis has shown that periodontal disease may be associated with COPD [30]. As a large proportion of inhaled drugs are retained in the oral cavity, they may also interfere with oral physiology and the oral microbiome. Prolonged use of IBDs is associated with increased dental caries and increased gastroesophageal reflux, while ICSs are associated with increased gingivitis and oral thrush [31]. Ongoing research on the effect of oral health on COPD disease progression and exacerbations will likely further our understanding of the interactions between our oral microbiome, lung microbiome, and the progression of COPD.
Methods
Sample Selection
Frozen bronchoalveolar lavage fluid (BALF) samples from 22 patients who participated in the FORTE study were selected for our study [32]. Fourteen of the patients had moderate COPD and 8 had severe COPD, as detailed in Table 1. Patients in this study consented to bronchoscopy at study entry (all samples included in this study were obtained at study entry), and were excluded if they had had smoked or required systemic steroids in the past 6 months or antibiotics in the past 2 months. BALF samples were immediately frozen and maintained at −80°C until thawed for DNA extraction. We also obtained BALF samples from 10 healthy individuals (4 smokers, 6 non-smokers) with normal lung function defined as FEV1>80% predicted and FEV1/FVC >70. Standard clinical protocols were followed to prevent nasopharyngeal contamination of the BALF samples. Per the FORTE study protocol, the nasopharyngeal approach was used preferentially, with the oropharyngeal approach attempted if the nasopharyngeal technique failed. All patients provided informed consent and their identities were not provided to the research team. The institutional review board for human studies approved the protocols (IRB Study 0202M17621 and IRB study 0601E80869).
DNA Isolation, PCR Amplification, and Sequencing
BALF samples were thawed and 0.5 ml of fluid used for DNA isolation. We used a previously described protocol [33] for DNA isolation that included bead beating to lyse bacterial cells, followed by precipitation with isopropanol and digestion with RNase. Purified DNA was subjected to Multiple Displacement Amplification with REPLI-g (Qiagen, Valencia, CA), which provided highly uniform DNA amplification with minimal amplification bias [34]. REPLI-g was used to minimize PCR cycles, which may introduce bias. PCR amplification using 16S rRNA gene primers specific to the constant regions flanking the V3 region [35], [36] was performed using 20 cycles. The primer sequences were: GCCTCCCTCGCGCCATCAG - 10 base barcode - CCTACGGGAGGCAGCAG 3′ (forward) and 5′ GCCTTGCCAGCCCGCTCAG - ATTACCGCGGCTGCTGG 3′ (reverse). For each sample, a 10 base bar code was included to distinguish patient number and sampling time. Amplicons were gel purified and sequenced at the University of Illinois Urbana-Champaign on a Roche 454 FLX DNA sequencer using titanium chemistry. To minimize effects of random sequencing errors, we used RDP Pipeline [4] to eliminate (a) sequences that did not appropriately match the PCR primer and the barcode at the beginning of a read, (b) sequence reads with <50 bases after the proximal PCR primer if they terminated before reaching the distal primer, and (c) sequences that contained more than one undetermined nucleotide (N). Trim.seqs and chimera.uchime implemented in mothur [37] were used to truncate low-quality sequences and remove chimeras, respectively. Both primers were trimmed from high-quality reads before sequences were submitted to RDP Classifier for taxonomic identification using a bootstrap cutoff of 50%. Operational Taxonomic Units (OTUs) were defined at an identity cutoff of 97% using mothur.
Data Analysis
For PCoA analyses, sequences were dereplicated and ClustalW was used to align the dereplicated sequences [38]. The aligned sequences were used to generate a phylogenetic tree using Phylip (University of Washington) with a weighted UniFrac distance algorithm [39]. Metastats [40] was used to detect differentially abundant taxa using taxonomic data from RDP Classifier controlling the false discovery rate at 10% for each level of the taxonomy. The Kruskal Wallace test was used to test for differences between the 3 patient groups and post hoc comparisons were conducted using the Wilcoxon test with a Bonferroni adjustment for the 3 tests. Multiple linear regression was used to test for differences between patient groups while controlling for the effect of age. All statistical tests were conducted using R version 2.15.
Supporting Information
Control and COPD Sample Rarefaction Curves. Control (top) and COPD patient (bottom) rarefaction curves show that with few exceptions, additional sequencing would not result in discovery of a significant number of additional operational taxonomic units. In the COPD patient rarefaction curve, samples from patients with Severe COPD are indicated with thicker curves.
(TIF)
Venn Diagram Analysis Demonstrates Significant Overlap Between Moderate and Severe COPD Sample Operational Taxonomic Units (OTU). A Venn diagram was created using mothur at an OTU similarity cutoff of 97%. All 10 control samples, 14 Moderate COPD samples, and 8 Severe COPD samples were merged into 3 groups–Control (pink), Moderate COPD (green) and Severe COPD (purple). The Control, Moderate COPD, and Severe COPD groups contained 202, 245, and 99 unique OTUs, respectively. Forty-six OTUs were found in all three groups. The largest number (96) of OTUs shared between two groups were shared by the Moderate and Severe COPD groups. In contrast, the Control group shared only 25 and 12 OTUs with the Moderate COPD and Severe COPD groups, respectively.
(TIF)
Principal Coordinate Analysis Demonstrates No Clustering Based on Percent Emphysema, Age, Gender, or Theophylline Use. Principal Coordinate Analysis was performed using mothur and Fast UniFrac, and the results for principal coordinates 1 and 2 are shown. A. Percent Emphysema. Percent of lung involved by emphysema was calculated from FORTE study entry chest CT scans. Patients were divided into Low (<25%, yellow), Medium (25–40%, green) and High (>40%, blue) percent emphysema tertiles. Samples do not cluster based on percent emphysema. B. Age. COPD samples were divided by median age. Samples from younger COPD patients (<66 years, yellow) and older COPD patients (≥66 years, blue) did not cluster separately. Control patient samples were not included as our two groups were not age-matched. C. Gender. Control and COPD patients were labeled as male (blue) or female (red). No clustering by gender was observed. D. Theophylline Users and Non-Users. Samples were labeled as theophylline users (blue, 4 of 22 COPD patients) and non-theophylline users (red, 18 of 22 COPD patients and 10 controls). No clustering based on theophylline use was observed.
(TIF)
Acknowledgments
We thank Klaudyna Borewicz for assistance with principal coordinate analyses. We thank the University of Minnesota BioMedical Genomics Center and the W. M. Keck Center at the University of Illinois Urbana-Champaign for assistance with sequencing and the Minnesota Supercomputing Institute for their technical support. We thank John Connett, PhD and Robert Wise, MD, along with the FORTE study for supplying the samples and clinical data.
Funding Statement
This work was supported by National Institutes of Health grant T32-AI55433 from the National Institutes of Allergy and Infectious Diseases and the FORTE study from the National Heart, Lung and Blood Institute, contract NO10HR-96140. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gold PM (2009) The 2007 GOLD Guidelines: a comprehensive care framework. Respir Care 54: 1040–1049. [PubMed] [Google Scholar]
- 2. Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, et al. (2012) Significance of the microbiome in obstructive lung disease. Thorax 67: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirschmann JV (2000) Do bacteria cause exacerbations of COPD? Chest 118: 193–203. [DOI] [PubMed] [Google Scholar]
- 4. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaser MJ (2010) Harnessing the power of the human microbiome. Proc Natl Acad Sci U S A 107: 6125–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Relman DA (2011) Microbial genomics and infectious diseases. N Engl J Med 365: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, et al. (2011) Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 184: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, et al. (2007) Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 127: 372–381.e1–3. [DOI] [PubMed] [Google Scholar]
- 9. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, et al. (2010) Disordered Microbial Communities in Asthmatic Airways. PLoS ONE 5: 372–381.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couzin-Frankel J (2010) Bacteria and asthma: untangling the links. Science 330: 1168–1169. [DOI] [PubMed] [Google Scholar]
- 11. Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, et al. (2011) Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 184: 198–205. [DOI] [PubMed] [Google Scholar]
- 12. Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, et al. (2011) Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108: 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang YJ, Lynch SV (2011) The emerging relationship between the airway microbiota and chronic respiratory disease: clinical implications. Expert Rev Respir Med 5: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, et al. (2010) A Persistent and Diverse Airway Microbiota Present during Chronic Obstructive Pulmonary Disease Exacerbations. Omics-a Journal of Integrative Biology 14: 9–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, et al. (2011) Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6: e16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, et al.. (2012) The Lung Tissue Microbiome in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, et al. (2010) The human oral microbiome. J Bacteriol 192: 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behl D, Manchanda R, Thomas C (2008) Campylobacter empyema due to food aspiration. J Hosp Med 3: 81–83. [DOI] [PubMed] [Google Scholar]
- 19. Sapkota AR, Berger S, Vogel TM (2010) Human pathogens abundant in the bacterial metagenome of cigarettes. Environ Health Perspect 118: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jumas-Bilak E, Carlier JP, Jean-Pierre H, Citron D, Bernard K, et al. (2007) Jonquetella anthropi gen. nov., sp. nov., the first member of the candidate phylum ‘Synergistetes’ isolated from man. Int J Syst Evol Microbiol 57: 2743–2748. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao WWL, Li KL, Liu Z, Jones C (2012) Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson D, Smith AJ (2009) The microbiology of the acute dental abscess. J Med Microbiol 58: 155–162. [DOI] [PubMed] [Google Scholar]
- 23. Yang QB, Fan LN, Shi Q (2010) Polymerase chain reaction-denaturing gradient gel electrophoresis, cloning, and sequence analysis of bacteria associated with acute periapical abscesses in children. J Endod 36: 218–223. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg JP, Burd EM (2010) Other Gram-Negative and Gram-Variable Bacilli. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th Edition. Philadelphia: Elsevier, Inc. 3015–3033. [Google Scholar]
- 25. Teles FR, Teles RP, Siegelin Y, Paster B, Haffajee AD, et al. (2011) RNA-oligonucleotide quantification technique (ROQT) for the enumeration of uncultivated bacterial species in subgingival biofilms. Mol Oral Microbiol 26: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lemieszek M, Chilosi M, Golec M, Skorska C, Huaux F, et al. (2011) Mouse model of hypersensitivity pneumonitis after inhalation exposure to different microbial antigens associated with organic dusts. Ann Agric Environ Med 18: 159–168. [PubMed] [Google Scholar]
- 27. Romeo L, Dalle Molle K, Zanoni G, Peretti A, Marangi G, et al. (2009) Respiratory health effects and immunological response to Thermoactinomyces among sugar cane workers in Nicaragua. Int J Occup Environ Health 15: 249–254. [DOI] [PubMed] [Google Scholar]
- 28. Hofstad T, Olsen I, Eribe ER, Falsen E, Collins MD, et al. (2000) Dysgonomonas gen. nov. to accommodate Dysgonomonas gadei sp. nov., an organism isolated from a human gall bladder, and Dysgonomonas capnocytophagoides (formerly CDC group DF-3). Int J Syst Evol Microbiol 50: 2189–2195. [DOI] [PubMed] [Google Scholar]
- 29. Al Masalma M, Raoult D, Roux V (2009) Phocaeicola abscessus gen. nov., sp. nov., an anaerobic bacterium isolated from a human brain abscess sample. Int J Syst Evol Microbiol 59: 2232–2237. [DOI] [PubMed] [Google Scholar]
- 30. Azarpazhooh A, Leake JL (2006) Systematic review of the association between respiratory diseases and oral health. J Periodontol 77: 1465–1482. [DOI] [PubMed] [Google Scholar]
- 31. Godara N, Godara R, Khullar M (2011) Impact of inhalation therapy on oral health. Lung India 28: 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth MD, Connett JE, D’Armiento JM, Foronjy RF, Friedman PJ, et al. (2006) Feasibility of retinoids for the treatment of emphysema study. Chest 130: 1334–1345. [DOI] [PubMed] [Google Scholar]
- 33. Yu Z, Morrison M (2004) Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36: 808–812. [DOI] [PubMed] [Google Scholar]
- 34. Hosono S, Faruqi AF, Dean FB, Du Y, Sun Z, et al. (2003) Unbiased whole-genome amplification directly from clinical samples. Genome Res 13: 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, et al. (2011) Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol 153: 124–133. [DOI] [PubMed] [Google Scholar]
- 37. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, et al. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamady M, Lozupone C, Knight R (2010) Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White JR, Nagarajan N, Pop M (2009) Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 5: e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control and COPD Sample Rarefaction Curves. Control (top) and COPD patient (bottom) rarefaction curves show that with few exceptions, additional sequencing would not result in discovery of a significant number of additional operational taxonomic units. In the COPD patient rarefaction curve, samples from patients with Severe COPD are indicated with thicker curves.
(TIF)
Venn Diagram Analysis Demonstrates Significant Overlap Between Moderate and Severe COPD Sample Operational Taxonomic Units (OTU). A Venn diagram was created using mothur at an OTU similarity cutoff of 97%. All 10 control samples, 14 Moderate COPD samples, and 8 Severe COPD samples were merged into 3 groups–Control (pink), Moderate COPD (green) and Severe COPD (purple). The Control, Moderate COPD, and Severe COPD groups contained 202, 245, and 99 unique OTUs, respectively. Forty-six OTUs were found in all three groups. The largest number (96) of OTUs shared between two groups were shared by the Moderate and Severe COPD groups. In contrast, the Control group shared only 25 and 12 OTUs with the Moderate COPD and Severe COPD groups, respectively.
(TIF)
Principal Coordinate Analysis Demonstrates No Clustering Based on Percent Emphysema, Age, Gender, or Theophylline Use. Principal Coordinate Analysis was performed using mothur and Fast UniFrac, and the results for principal coordinates 1 and 2 are shown. A. Percent Emphysema. Percent of lung involved by emphysema was calculated from FORTE study entry chest CT scans. Patients were divided into Low (<25%, yellow), Medium (25–40%, green) and High (>40%, blue) percent emphysema tertiles. Samples do not cluster based on percent emphysema. B. Age. COPD samples were divided by median age. Samples from younger COPD patients (<66 years, yellow) and older COPD patients (≥66 years, blue) did not cluster separately. Control patient samples were not included as our two groups were not age-matched. C. Gender. Control and COPD patients were labeled as male (blue) or female (red). No clustering by gender was observed. D. Theophylline Users and Non-Users. Samples were labeled as theophylline users (blue, 4 of 22 COPD patients) and non-theophylline users (red, 18 of 22 COPD patients and 10 controls). No clustering based on theophylline use was observed.
(TIF)