Abstract
Background
Patient navigation (PN) is an emerging strategy to overcome barriers to cancer care. We evaluated the efficacy of PN in improving time of key events in cancer care, including positive screening tests, definitive diagnosis, initiation of therapy, and completion of initial therapy.
Methods
We evaluated PN in a prospective observational study of predominantly poor Hispanic women with an abnormal breast cancer screening or untreated biopsy proven breast cancer (control = 200, intervention = 260). Controls were contemporary records-based patients with positive screening. Analyses were conducted for the entire cohort and separately by ethnic strata. We used Chi-Square tests to compare differences in proportions and Kaplan-Meier followed by Cox Regression to compare time-to-event curves of the intervention and control groups.
Results
The average days from definitive diagnosis to initiation of therapy was significantly reduced overall with PN (PN vs control, 57d vs 74d, p=.04). This effect was more pronounced in the Hispanic strata (56 vs 81 days, p=.02). More navigated Hispanic women were diagnosed within 60 days of abnormal screening (62.6% vs 47.5%, p< .01) and more began treatment within 60 days of diagnosis (80% vs 56.3%, p<.01). Navigated Hispanic and other ethnic minority women had a shorter time from positive screening test to definitive diagnosis (16 and 32 days, respectively).
Conclusions
Minority women may have benefitted from navigation with shorter times from definitive diagnosis to initiation of therapy..
Impact
PN intervention may show promise in decreasing some delays that contribute to health disparities among minority women with breast cancer.
Keywords: patient navigation, promotora, breast cancer, prevention
Introduction
The Agency for Health Care Research and Quality released its first annual National Health Care Disparities Report in 2003, highlighting differences in use of services, access to health care, and quality of health care in the population at large and within various pre-identified “target” populations (1). In 2002, the Institute of Medicine (IOM) presented evidence that racial and ethnic minorities tended to receive a lower quality of health care than majorities despite controlling for confounders (2). To address these disparities, the United States Congress passed the Patient Navigator Outreach and Chronic Disease Prevention Act in 2005. Patient navigators were envisioned as an effective intervention to improve health outcomes by reducing delays to quality care through the use of a personal guide through the health care system.
The patient navigation model was developed by Harold Freeman and showed potential as an effective tool in facilitating screening and breast diagnostic follow-up after abnormal clinical breast exams or abnormal screening mammograms and prompt initiation into oncology treatment if a malignancy was diagnosed (3). Encouraged by the successes of earlier navigation programs, the NCI funded the implementation of the Patient Navigation Research Program (PNRP) at 9 sites across the country to evaluate the efficacy and cost effectiveness of patient navigation (4). As a major metropolitan city, San Antonio, Texas, has significant underserved populations typically associated with cancer health disparities: large Hispanic population, high illiteracy rate, low socioeconomics, and a large number of uninsured (5). Our underlying hypothesis was that a patient navigation model, incorporating a patient navigator paired with a promotora, would shorten the time of key events in the cancer care continuum, particularly in our most underserved population of Hispanic women.
MATERIALS AND METHODS
Study Design
The overall general approach to navigation employed by the PNRP has previously been described (6). We utilized a quasi-experimental design comparing unmatched control and intervention participants to evaluate whether patient navigation is an effective intervention that improves the quality of breast cancer care. For study purposes, in addition to invasive cancer, ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS), were considered cancer.
A convenience sampling approach was used to recruit study participants from the county hospital system and specific local community health clinics. Individuals in the control group received usual treatment, while individuals in the intervention group received usual treatment plus patient navigation. Navigation was done prospectively from the time patients consented through end of tracking. End-of-tracking was defined as when enrolled navigated patients completed initial therapy or when definitive diagnosis resulted in resolution of the positive screening test. The Institutional Review Board at the University of Texas Health Science Center at San Antonio (UTHSCSA) approved this study. Consents were obtained for all participants enrolled in navigation. Data regarding control patients were abstracted from clinical charts.
Intervention
A care management model was used to guide the navigation process (7). The steps of our care management model included: intake, assessment, analysis of needs, development of a care plan, implementing the plan, tracking, and evaluation. We used an innovative two-member team approach to patient navigation involving a patient navigator (PN) and a promotora. We utilized 4 PNs during our study. Their professional backgrounds included one public health registered nurse, one dental hygienist, one social worker, and one masters in business administration. All PNs were trained in navigation during the study at centralized training sessions led by PNRP investigators. The PN conducted an initial assessment to identify barriers and developed a care plan to overcome them. Interventions targeted both individual and health system-related barriers and were evaluated on an ongoing basis. Patients were tracked until they achieved non-malignant diagnostic resolution, completed primary cancer treatment, withdrew from the study, died during treatment, or were considered loss-to-follow-up. We tracked patients who did not return for follow-up care a minimum of six months.
Due to the continued increase in the Hispanic population and their overrepresentation in poor health indicators, the inclusion of promotoras, or lay community health workers has been recognized as an effective strategy in reducing health disparities across the country (8). All promotoras had a background in community health education. A PN/promotora duo assigned to each patient enabled the PN to manage a larger caseload effectively compared to the single person navigation strategy. The PN primarily focused on interventions aimed at alleviating medical related barriers. For example, if a patient missed a procedure and the PN determined it was because the patient had unanswered questions or misinformation regarding risks, the PN facilitated dialog between the medical provider and the patient. The promotora's role was to assist with cultural/socioeconomic barriers. For example, if the PN determined that the patient had difficulties with childcare arrangement, then the promotora would work with the patient and her family and friends to determine an acceptable plan.
Measures
The study focused on evaluating whether the patient navigation intervention increased timeliness of diagnostic resolution and we designated T0 – T1 to represent the time from abnormal or positive screening to diagnostic resolution, or definitive diagnosis. The timeliness goals we set for diagnostic resolution and initiation of treatment were consistent with the CDC's National Breast and Cervical Cancer Early Detection Program (NBCCEDP) benchmark (9). We established 60 days as the timeframe acceptable for diagnostic resolution prompted by abnormal screenings and 60 days for the timeliness standard from diagnosis to initiation of treatment. The goal was for the time from screening to start of initial treatment to be less than 120 days. The time from diagnostic resolution to the initiation of primary therapy was designated T1–T2, and the time from initiation of primary therapy to completion of primary therapy was designated as T2–T3.
Intervention Sites
This study was conducted at University Health Systems (UHS), the county safety net hospital system in San Antonio and UTHSCSA. UHS was selected as the main clinical system because they serve a predominately low-income, uninsured, minority population. UHS provided a great opportunity to evaluate the breast cancer-related health burden of the residents of 10 zip codes in San Antonio identified by the San Antonio Metropolitan Health District as having the most need for public health services (5). Women with abnormal screening mammograms were referred to the Cancer Therapy and Research Center (CTRC) of the UTHSCSA for diagnostic mammography and then definitive therapy as appropriate for diagnosis. An active surveillance program was established to alert PNs of women with abnormal screening findings and newly diagnosed breast cancer patients at clinical sites.
Study Participants
Mentally competent patients 18 years of age and older, residing in south Texas who had abnormal breast screening testing were eligible to participate in the study and were recruited from November 2006 to May 2010. A clinical finding suspicious for cancer such as a breast mass that resulted in a referral to a specialist was an eligibility criterion. Additionally, women diagnosed with breast cancer who had not initiated treatment at the time of consent were eligible. All cancer diagnoses were confirmed by pathology. Exclusion criteria included a history of cancer treated within the last five years. Patients who have experienced navigation in the past due to an abnormal screening were ineligible because of the potential for confounding. Pregnant patients were excluded because their pregnancy status might delay diagnostic and treatment interventions until post delivery.
PNs identified potentially eligible participants from referrals provided by clinic staff, monitoring daily schedules, and routinely visiting women's health, diagnostic, and oncology clinics. Overall, the majority of navigated patients entered our study at T1, as our referral sites included over 15 outlying clinics. T0 occurred in these clinic settings where we were unable to provide navigation in an efficient manner. PNs met with patients and executed the consent, performed an initial needs assessment for care planning purposes, and began actively navigating the patient.
Control patients were identified from 3 potential sources: PNs, clinic staff, and system queries. The majority of control patients were identified through medical record searches using ICD-9 and CPT codes for abnormal breast screen results. For quality control purposes, the clinical chart was reviewed to confirm that the potential control participant was not receiving navigation services and this was verified with the PNs and study staff. Control patients were identified from the same local community health clinics from which navigated patients were identified. There were several reasons why patients were not offered navigation, none of which involved patient preference. The volume of patients referred for diagnostic mammography far exceeded the capacity of our navigators so that only a proportion of women referred for services were enrolled in navigation. Commonly, control women were undergoing testing while navigators were working with other participants. Additionally, study staff was often not present in the clinics for navigation training, in-services, and staff meetings. Patients who refused navigation services were also identified as control subjects, although these instances were rare. We did not collect reasons for refusal to participate. Control data was abstracted contemporaneously (during the time that women were navigated as part of this study) from both electronic medical records and paper charts. Thus, our final control sample numbered 200, and was derived from the same pool of patients as were the navigated patients.
Data Collection
Data abstracted included: dates of breast imaging, dates of biopsy, biopsy results, and if cancer diagnosis, date of referral to specialist, date of initiation of cancer treatment, treatment recommendation, and whether patient followed treatment recommendation. Data was abstracted from electronic medical records, physical charts, registration, and appointment databases. The clinical record was abstracted to obtain laboratory or imaging results, specialist's treatment recommendations, and to monitor treatment plans.
Sample Size and Power Calculation
We performed sample size and power calculations to determine optimal sample size given expected effect sizes. Estimates were based on 1) overall sample size variation between numbers of patient candidates for each disease; 2) the influence of this variation on our proposed randomization scheme. Sample size calculations were performed using Sample Power 2.0 (SPSS, Chicago, ILL). We anticipated a reduction in time from abnormal screen to diagnostic resolution of approximately 10%. Under this assumption, we targeted a power of 80% at a = .05 and determined that we would need a sample size of 115 in each group, taking into account an attrition rate of 10% or less during the course of the study.
Analytic Procedures
Analyses were conducted using SPSS v. 19.0 (SPSS, Chicago, ILL). We initially calculated descriptive statistics of group characteristics using Chi-Square, T-Tests, or Kruskal-Wallis Log-Rank Chi-Square tests as appropriate. Second, we compared overall time-to-diagnosis and time-to-treatment between navigated and control patients using a Kaplan-Meier survival model and determined mediators associated with time via multivariate Cox Regression. Based on these results, we stratified our time measures for navigated and control patients by ethnicity, which was the only independent measure associated with the dependent variable other than navigation. All other potential covariates and their interactions were also tested and were unassociated with the dependent variable; these results are not shown. We calculated P values using Kaplan-Meier survival models of median times, where larger hazards ratios (HR) indicate faster time-to-diagnosis. Third, univariate and multivariate odds ratios (OR) were generated to test the association of each characteristic with timely diagnosis within 60 days. In this case, larger OR indicates greater likelihood of timely diagnosis. A 2-sided P <.05 indicated statistical significance.
RESULTS
We navigated 261 women total with breast abnormalities and identified another 200 total control women during a contemporary time frame with breast abnormalities but were not navigated. Of navigated women, 127 (49%) initiated navigation with a cancer diagnosis, while 39 (20%) of control women initiated navigation with a cancer diagnosis. Those women who were not diagnosed with cancer ceased navigation with definitive diagnosis at T1. Of these 261 navigated women, 107 (41%) resided in the 10 zip code areas of San Antonio identified as having the greatest health disparities, while 76 of 200 control women (38%) resided in these areas. Notably, only 5% of control patients and 4.6% of navigated patients were lost to follow-up.
Table 1 shows the demographics of the navigated and control participants. Both groups demonstrated characteristics of a disadvantaged population, being largely without a college education, unemployed, and with unsettled housing status. Among the navigated participants, 99 (37.9%) were renting housing accommodations, 111 (42.5%) owned their own homes, and 44 (16.6%) were staying with family or friends. The vast majority of the navigated patients earned less than $20,000 per year (178, or 68.1%). Housing status and income was unavailable for the control patients. Regarding educational status, 71 (27.2%) had less than an 8th grade education, 37 (14.2%) had some high school education, and 66 (25.3%) received a high school diploma or equivalent. Only 21.5% (n=56) had some college and 10% (n=26) received a college or professional degree.
Table 1.
Demographic Features of Navigated and Control Patients
| Navigated, n = 261 n (%) | Control, n = 200 n (%) | P-value | |
|---|---|---|---|
| Abnormal Eligibility | 258 (98.9) | 199 (99.5) | 0.456 |
| Cancer Eligibility | 3 (1.1) | 1 (.5) | |
| Median Age | 52 | 49.6 | 0.024 |
| Race/Ethnicity | 0.013 | ||
| Hispanic | 93.0 (44.1) | 117 (60.4) | |
| White | 97 (46.0) | 56 (29.0) | |
| Othera | 16 (6.1) | 21 (10.5) | |
| Primary language spoken | 0.105 | ||
| English | 162 (62.5) | 140 (70.7) | |
| Spanish | 94 (36.3) | 55 (27.8) | |
| Other | 3 (1.1) | 3 (1.5) | |
| Country of Origin | 0.489 | ||
| US | 158 (60.5) | 129 (64.5) | |
| Mexico | 85 (32.6) | 55 (27.5) | |
| Other | 18 (6.9) | 16 (8.0) | |
| Marital Status | 0.001 | ||
| Single/Never Married | 47 (18.2) | 45 (25.6) | |
| Married/Living as married | 113 (43.8) | 88 (50.0) | |
| Divorced or Separated | 78 (30.2) | 23 (13.1) | |
| Widowed | 18 (7.0) | 17 (9.7) | |
| Chose not to answer | 2 (.8) | 3 (1.7) | |
| insurance status | <0.001 | ||
| Medicare | 16 (6.2) | 21 (10.5) | |
| Private insurance | 24 (9.3) | 42(21.1) | |
| Other local governmental payment assistance plan | 105 (40.7) | 132 (66.3) | |
| Total Insured | 145 (56.2) | 195 (97.9) | |
| Employment status | <0.001 | ||
| Employed | 123 (47.5) | 109 (60.6) | |
Other race/ethnic women included African-Americans, Asians, and native Americans
In the overall population, we found no difference in the times of T0–T1. However, for Hispanic women who experienced navigation the mean time to diagnosis (36.65 ± 5.17 days) was shorter than for the total population of control women (52.96 ± 4.28 days, p < 0.05). This difference was also noted for other ethnic minorities (navigated 37.68 ± 6.53 vs. 70 ± 44.4 days, p < 0.05). Hispanic women were more likely to have a definitive diagnosis within 60 days of screening if they had experienced navigation (62.6% for navigated women vs. 47.5% for control women, p < 0.05). Similar results were seen for definitive diagnosis at less than 90 and less than 120 days. Moreover, the average days from definitive diagnosis to initiation of therapy was significantly reduced overall with PN (PN vs control, 57d vs 74d, p=.04) and, significantly more navigated Hispanic women began treatment within 60 days of diagnosis when compared to controls (80% vs 56.3%, p<.01).
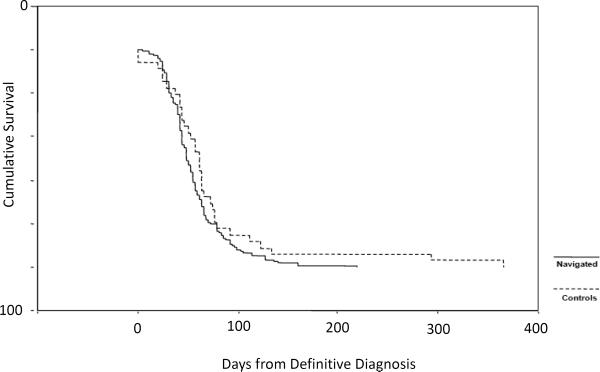
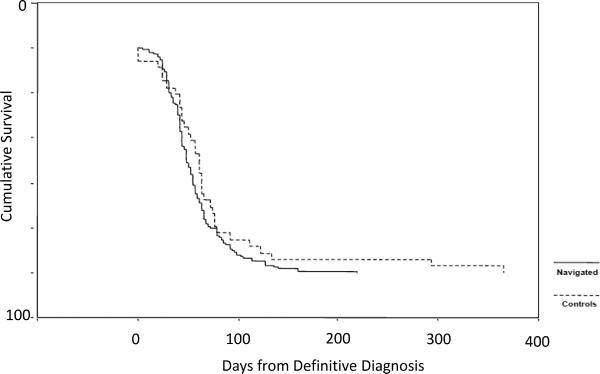
Figure 1 and 2 are Kaplan-Meier curves showing T1–T2 timing for the general population (figure 1) and Hispanic women with a cancer diagnosis (figure 2). For women who were navigated from T1–T2, all three groups were navigated for approximately 55–60 days, similar findings for T0-T1 where all women where navigated for 35 to 38 days on average. In general, race or ethnicity did not alter meeting critical milestones in the cancer continuum in women who were navigated. For our entire population, we found that navigation decreased T1–T2 by 27 days on average (populations (74.0 ± 17.3 days vs. 56.8 ± 2.9 days, p=0.042). Stratification by ethnicity revealed a more pronounced decrease in T1 - T2 among Hispanics (56 vs 81 days, HR = 1.45 [1.09–1.67], p=.02). For the time period T1–T2, control white women again had the same time in care as those being navigated (55.84 ± 9.0 days), and minority women had relatively longer periods of time from definitive diagnosis until initiating therapy (Hispanic women 81.45 ± 18.37 days, other ethnicities 73.67 ± 25.06 days).
Figure 1. Kaplan-Meier Function for T1–T2, Total Population (Time from Definitive Diagnosis to Initiation of Treatment).
There was a significant difference in between control and navigated populations (74.0 ± 17.3 days vs. 56.8 ± 2.9 days, p=0.042).
Figure 2. Kaplan-Meier Function for T1–T2, Women Diagnosed with Cancer (Time from Definitive Diagnosis to Initiation of Treatment).
There was a significantly shorter time from T1 to T2 among women who were diagnosed with cancer (71.7 ± 12.3 days for control women vs. 56.8 ± 2.9 days for navigated women, p=0.042).
Given that our study design precluded us from accessing the majority of patients at T0, we speculated that a more relevant time interval was T0–T2. The mean number of days for different time frames from navigated women who met the diagnostic criteria for cancer was compared to control women. For T0–T2 (time from positive screen to initiation of therapy), white women were in navigation for 82.1 ±8.7 days, Hispanic women for 93.13 ± 6.35 days, and other ethnic women 99.56 ± 10.37 days. For T0–T2 intervals for control women, white women averaged 79.33 ± 7.63 days, essentially the same as for white women who were navigated. Conversely, Hispanic women (127.33 ± 18.84 days, p < 0.05) and women of other ethnicities (143.67 ± 51.91 days, p < 0.05) had significantly longer time periods from abnormal screen to initiating definitive therapy. There were no differences between navigated and control patients for T2–T3 (data not shown).
DISCUSSION
Overall, we found no salutary effects for navigation in our overall study population, and in particular in white women. In a subgroup analysis, we found that navigation significantly shortened the time from definitive diagnosis until initiation of primary therapy in minority population of predominantly Hispanic women, thus supporting our primary hypothesis that navigation could potentially benefit our most underserved population. Moreover, navigation eliminated race/ethnic differences for critical milestones in cancer care for those women who met our criteria for a cancer diagnosis. Notably, in the control women, there were marked race/ethnic differences in meeting these key time points. However, with navigation these racial and ethnic differences disappeared. Moreover, the time frame for these critical milestones was shortened for Hispanic and other ethnicities. Our results show that navigation may have eliminated the disparity in time from definitive diagnosis to initiation of the therapy for breast cancer diagnoses between white women and minority women.
One significant limitation we encountered was the relative lack of access to patients at the time of initial screening. Women were funneled from a number of outlying clinics into a single diagnostic center (CTRC). Since our navigation capacity was limited, we focused our efforts at the site where diagnostic mammography, and hence definitive diagnosis, was performed. We found that referrals from the outlying clinics did occur relatively promptly and that there was virtually no problem with patients being lost to follow-up. Late in the study we received IRB approval to contact women at the time of scheduling for diagnostic mammography. Given these limitations, we are not surprised to find little effect on the time from initial positive screening test until the time of definitive diagnosis (T0–T1).
Another significant limitation of our study was the lack of randomization of our sample. Prior to initiation of the study, we analyzed our clinical system and determined that a randomized sample would be highly subject to a Hawthorne effect. Therefore, we opted to use contemporaneous controls from the same clinics, acknowledging that this may lead to selection bias. Our navigators usually had full caseloads, so they were unable to navigate all potential patients because of patient volume. Therefore, our controls were women who accessed CTRC for diagnostic services and who were not contacted by our navigators. Thus, we doubt that there is a significant Hawthorne effect in our study and control populations, and our methodology of identifying control women seems unlikely to result in a significant selection bias.
Our study design does not allow us to conclude that navigation as performed in this population was the reason for this improvement, as there are many confounding factors that could have accounted for this improvement that could not be controlled. For example, control women may have been managed using navigation concepts by clinic staff uninvolved in the study as they noted the activities of the navigators. Also, women with more resources may not have availed themselves of navigation services and so may not have benefited from navigator activities.
Patient navigation programs are gaining momentum nationwide (10, 11). To date, there is no standardized skill set or minimum level of education required to designated as a patient navigator. To our knowledge, patient navigation based on the profession of the person providing navigation services, i.e., clinical professional, social worker, paraprofessional, or lay community workers, has not been empirically evaluated. However, we believe that a common set of skills recognized by a credentialing professional body is necessary to elevate the navigator to gain professional status (12). The rationale for pairing the promotora and PN was to capitalize on the promotora's liaison role between the clinical population and the healthcare system. Consequently, their ability to build rapport with the target population and provide health information in a culturally sensitive way has been very helpful in furthering many public health goals such as increasing various routine screening practices and improving childhood vaccination rates.
In summary, we found that combined navigator/promotora patient navigation may have decreased the time from diagnostic resolution after abnormal mammogram to time of initiating treatment after a cancer diagnosis in a predominantly Hispanic population. Navigation may be an effective method to improve time from definitive diagnosis to initiation of therapy in a population of predominantly underserved Hispanic women.
Acknowledgements
The authors acknowledge our navigators and promotoras, including Katherine Eisermann, Eva Garza, Sandra Rodriguez, Arlene (Harmony) Rodriguez, Guadalupe Cornejo, Mary Elizabeth Perez, and Angela Rodriguez.
Funding: This project was funded by the National Institutes of Health National Cancer Institute through the Center to Reduce Cancer Health Disparities (U10 CA11685-01).
REFERENCES
- 1.National Healthcare Disparities Report, Agency for Healthcare Research and Quality. US Department of Health and Human Services; 2003. [Google Scholar]
- 2.National Research Council . Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 3.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3:19–30. [PubMed] [Google Scholar]
- 4.Wells K, Battaglia TA, Dudley DJ, Garcia R, Greene A, Calhoun E, et al. Patient Navigation: state of the art, or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healthy Profiles 2002 San Antonio Metropolitan Health District. Electronic publication accessed at www.sanantonio.gov/health/profiles/index.asp.
- 6.Freund K, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, et al. National Cancer Institute Patient Navigation Research Program: Methods, Protocols, and Measures. Cancer. 2008;113:3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longest B, Young GJ. Coordination and communication. In: Shortell S, Kaluzny AD, editors. Health Care Management: Organization Design and Behavior. Thomson Delmar Learning; Albany NY: 2000. pp. 210–243. [Google Scholar]
- 8.Reinschmidt KM, Hunter JB, Fernandez ML, Lacy-Martinez CR, Guernsey de Zapien J, Meister J. Understanding the success of promotoras in increasing chronic disease screening. J Health Care Poor Underserved. 2006;17:256–264. doi: 10.1353/hpu.2006.0066. [DOI] [PubMed] [Google Scholar]
- 9.Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Bernard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health. 2010;100:1769–1776. doi: 10.2105/AJPH.2009.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson-White S, Conroy B, Slavish KH, Rosenweig M. Patient navigation in breast cancer: a systematic review. Cancer Nurs. 2010;33:127–140. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JE, Green E, Lankshear S, Hughes E, Burkoski V, Sawka C. Nurses as patient navigators in cancer diagnosis: review, consultation and model design. Eur J Cancer Care. 2011;20:228–236. doi: 10.1111/j.1365-2354.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 12.Alvillar M, Quinlan J, Rush CH, Dudley DJ. Report from the Field: Recommendations for developing and sustaining community health workers. J Health Care Poor Underserved. 2011;22:745–750. doi: 10.1353/hpu.2011.0073. [DOI] [PubMed] [Google Scholar]