Abstract
Asthma exacerbations can be caused by a number of factors, including the fungal allergen Alternaria, which is specifically associated with severe and near-fatal attacks. The mechanisms that trigger lung responses are unclear and might vary between allergens. A comparison between Alternaria, Aspergillus, Candida, and house dust mite, all allergens in humans, showed that only Alternaria promoted immediate innate airway eosinophilia within 12 h of inhalation in nonsensitized mice. Alternaria, but not the other allergens, induced a rapid increase in airway levels of IL-33, accompanied by IL-33 receptor (IL-33R)-positive natural helper cell (NHC) production of IL-5 and IL-13. NHCs in the lung and bone marrow constitutively expressed transcription factors [GATA-3 and E26 transformation-specific sequence-1 (ETS-1)] that could allow for rapid induction of T helper type 2 (Th2) cytokines. Lung NHC numbers and proliferation (%Ki-67), but not IL-5 or GATA-3 expression, were significantly reduced in STAT6-deficient mice 3 days after one challenge with Alternaria. Alternaria induced NHC expression of the EGF receptor ligand amphiregulin (partially dependent on STAT6), as well as EGF receptor signaling in the airway epithelium. Finally, human peripheral blood NHCs (CRTH2+CD127+ lineage-negative lymphocytes) from allergic individuals highly expressed GATA-3 and ETS-1, similar to lung NHCs in mice. In summary, Alternaria-induced lung NHC proliferation and expression of amphiregulin are regulated by STAT6. In addition, NHCs in mouse and humans are primed to express Th2 cytokines through constitutive expression of GATA-3 and ETS-1. Thus several transcription factor pathways (STAT6, GATA-3, and ETS-1) may contribute to NHC proliferation and Th2-type responses in Alternaria-induced asthma.
Keywords: innate lymphoid cells, asthma
asthma exacerbations are a major cause of morbidity for patients with severe asthma. Sensitization and exposure to the fungal allergen Alternaria alternata is a risk factor for severity of asthma symptoms, including episodes of fatal/near-fatal attacks (4, 9, 22, 28, 29, 32, 34). The spores of Alternaria are known to be a source of outdoor allergens for sensitized individuals but have also recently been detected at high levels indoors (36). Dispersion of the spores during periods of warm dry weather has been associated with epidemic, severe asthma symptoms (4, 9, 22, 28, 29, 32, 34). The unique associations with Alternaria and severe asthma exacerbations are intriguing, but the mechanisms responsible for the unique pathogenesis of Alternaria are not well understood.
Our previous studies demonstrated that Alternaria can induce an innate eosinophilia in the airway compared with other allergens that is dependent on STAT6 (7). The finding that different aeroallergens may induce unique innate responses is supported by a report that the house dust mite (HDM) Dermatophagoides pteronyssinus contains an accessory protein homolog of MD-2 that can specifically activate Toll-like receptor type 4 signaling (39). Aside from this, mechanisms whereby individual allergens can specifically activate the innate immune system are largely unknown.
Our current studies reveal that Alternaria specifically induces activation of natural helper cells (NHCs), an innate lymphoid population. Innate lymphoid cell types have recently been discovered and do not express known lineage markers (lineage-negative), including CD3, CD4, CD8, T cell receptor (TCR)-β, TCRδ, CD5, CD19, B220, NK1.1, Ter119, Gr-1, CD11c, and FcεR1 (26, 27, 33). This combination of surface markers excludes B, T, natural killer (NK), and NK T (NKT) cells, as well as mast cells, basophils, granulocytes, dendritic cells, and macrophages. Moro et al. (26) were the first to identify a novel population of small round cells with a single nucleus and scant cytoplasm they termed “natural helper cells” (NHCs) that have the innate ability to express high levels of T helper (Th) type 2 (Th2) cytokines after exposure to the proinflammatory cytokine IL-33 (26). Studies of lineage-negative lymphocytes have demonstrated their presence in mice in the gastrointestinal tract, mesenteric fat and lymph nodes, spleen, liver, bone marrow, and lung (3, 5, 24, 26, 27, 33, 44), while studies in humans have identified their presence in the gastrointestinal tract, lung, bronchoalveolar lavage (BAL), and nasal polyps (23, 24). There are a limited number of studies examining the role of lineage-negative lymphocytes in models of disease. In studies of influenza viral infection in mice associated with type 1 interferon and Th1 immune responses, a population of cells in the mouse lung that closely resemble NHCs that contribute to airway hyperreactivity, as well as tissue remodeling/repair, has been described (5, 24). Very recently, it was reported that lung NHCs contribute to papain- and Alternaria-induced airway inflammation and highlight the role of IL-33 in promoting Th2 cytokine production (2, 12, 15). These studies have largely focused on innate lymphoid cell cytokine responses or contribution to airway hyperreactivity (1, 16), but mechanisms that regulate the accumulation and proliferation of lung NHCs after allergen challenge are largely unknown.
In this study, we have examined whether Alternaria, an aeroallergen specifically associated with severe asthma, induces a unique innate immune response compared with other allergens. We were surprised to find that Alternaria alternata, but not Aspergillus fumigatus, the HDM D. pteronyssinus, or Candida albicans, induced a rapid increase in airway levels of IL-33, accompanied by IL-33 receptor (IL-33R)-positive lung NHC production of IL-5, IL-13, and the pro-remodeling/repair growth factor amphiregulin. NHCs in the lung and bone marrow of mice, as well as similar cells in allergic humans, constitutively expressed transcription factors [GATA-3 and E26 transcription-specific sequence-1 (ETS-1)] that could allow for rapid induction of Th2 cytokines. Surprisingly, NHC numbers and proliferation were reduced in lungs of STAT6-deficient (STAT6−/−) mice after a single challenge with Alternaria, despite strong GATA-3 expression. Alternaria did not induce eosinophilia in mice deficient in NHCs [IL-7 (IL-7R) receptor-deficient (IL-7R−/−) mice], and blocking the IL-33 receptor additionally inhibited the innate eosinophil response. Thus the ability of Alternaria to rapidly activate lung NHCs that express ETS-1 and GATA-3 and proliferate in a STAT6-dependent manner may contribute to Alternaria-associated severe asthma.
MATERIALS AND METHODS
Mice.
Six- to 8-wk-old male and female C57BL/6, recombination-activating gene (RAG) type 2-deficient (RAG2−/−), IL-7R−/−, and STAT6−/− mice were obtained from Jackson Laboratories and bred in-house. The mice were challenged with a single intranasal dose (25 or 100 μg) of A. alternata extract (lot nos. 136056 and 177372; LPS = 0.11–0.12 ng) or PBS and euthanized 3 h, 6 h, 12 h, or 3 days later. In some experiments, mice received a single intranasal challenge of 100 μg of D. pteronyssinus HDM extract (lot no. 165197; LPS = 0.19 ng), A. fumigatus (lot no. 118033, LPS = 0.04 ng), or C. albicans (lot no. 111797 LPS = 0.006 ng) and euthanized 12 h later. All extracts were obtained from Greer Laboratories, and LPS was measured by Limulus assay (Lonza) and reported as nanograms per 100 μg with a conversion factor of 10 endotoxin units per nanogram. In selected experiments, mice received 25 μg of Alternaria extract on days 0, 3, 6, and 9 and euthanized 24 h later. For in vivo T1/ST2 blocking studies, wild-type (WT) B6 mice received intraperitoneal injections of 0.1 mg/ml of anti-T1/ST2 (clone DJ8, rat IgG1, MD Biosciences) or rat IgG (Millipore) daily for 2 days followed by intranasal Alternaria challenge 12 h before euthanization. All studies were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Airway cellular analysis and lung, blood, and bone marrow processing.
BAL fluid was obtained by intratracheal insertion of a catheter and five lavages with 0.7 ml of PBS containing 2% filtered BSA (Sigma). Cardiac puncture was performed to obtain blood. Lungs were placed in RPMI medium and digested into single-cell suspensions after incubation with 10 mg/ml collagenase D and 1 mg/ml DNase (Roche), as previously described (8). Isolation of bone marrow cells was accomplished by flushing tibias and fibulas with PBS. Live total BAL, lung, and bone marrow cells were counted using a flow cytometer (Accuri C6, BD Biosciences).
Flow cytometry.
Lung single-cell suspensions or BAL cells were first incubated with a monoclonal antibody to CD16/CD32 (24G.2) for 10 min to block Fc receptors. BAL and lung cells were stained with peridinin-chlorophyll-protein complex (PerCP)-conjugated CD45.2, phycoerythrin (PE)-conjugated Siglec-F, allophycocyanin (APC)-conjugated Gr-1, and FITC-conjugated CD11c (eBioscience). Lung cells were also stained with PerCP-conjugated CD45.2 (eBioscience) and biotinylated T1/ST2 (clone DJ8, MD Bioscience) followed by streptavidin-APC or -PE (eBioscience). Lineage staining was performed only with FITC antibodies, allowing for the exclusion of B, T, NK, and NKT cells, as well as mast cells, basophils, granulocytes, dendritic cells, and macrophages. Lineage antibodies included FITC-conjugated lineage cocktail (Biolegend), CD11c (BD Biosciences), NK1.1 (eBioscience), CD4 (BD Bioscience), CD5 (BD Biosciences), CD8 (BD Bioscience), FcεR1 (Biolegend), TCRβ (BD Bioscience), TCRγδ (Biolegend), and CD19 (Biolegend). Lung cells were also stained with various combinations of APC-conjugated Thy1.2 (Biolegend), c-Kit (eBioscience), CD44 (eBioscience), PE-conjugated Sca-1 (eBioscience), CD25 (BD Bioscience), CD127 (Biolegend), IL-4Rα (BD Pharmingen), IL-13Rα1 (eBioscience), or Thy1.2 (eBioscience). Lung NHCs were defined as lineage-negative T1/ST2-positive lymphocytes or lineage-negative Thy1.2-positive lymphocytes. PE-conjugated IL-5 and IL-13 (eBioscience) were used for intracellular staining after fixation and permeabilization (BD Biosciences). In selected experiments, lung single-cell suspensions were incubated at 37°C for 3 h with Golgi-Plug (BD Biosciences) prior to intracellular staining. ETS-1 and GATA-3 staining were performed using a FoxP3 staining kit (eBioscience) followed by addition of PE-conjugated GATA-3 (eBioscience) or ETS-1 (GeneTex) polyclonal rabbit antibody and then F(ab′)2 donkey anti-rabbit IgG (eBioscience). Ki-67 staining was performed using a FoxP3 staining kit (eBioscience) followed by APC-conjugated Ki-67 (eBioscience). Surface staining for amphiregulin was performed with polyclonal goat anti-amphiregulin antibody (R & D Systems) followed by incubation with PE-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology). Human peripheral blood mononuclear cells were incubated with Fc block and then stained with a combination of FITC-conjugated human lineage cocktail (BD Bioscience), biotin-conjugated chemoattractant receptor of Th2 cells (CRTH2; Miltenyi), FITC-conjugated CD4, TCRγδ CD235a, FcεR1, CD11b, and Alexa 647-conjugated CD127 (eBiosciences) followed by ETS-1 and GATA-3 intracellular staining. Flow cytometry was performed with an Accuri C6 cytometer (BD Biosciences), and sample data were further analyzed with Flow Jo software (Tree Star). Purified NHC were obtained using a FACS Aria cell sorter (BD Biosciences).
ELISA for BAL cytokines and serum IgE.
ELISA of BAL supernatant for IL-4, IL-5, IL-9, IL-13, IL-25, IL-33, CCL11, CCL24, IFN-γ, and amphiregulin and serum for total IgE (BD Biosciences) was performed according to the manufacturer's instructions and read with a microplate reader (model 680, Bio-Rad). All ELISA kits used for BAL studies were obtained from R & D Systems, except the kit for IL-9, which was obtained from Biolegend.
Human peripheral blood innate lymphoid cell studies.
Humans with allergic rhinitis were diagnosed on the basis of perennial or seasonal rhinitis symptoms, along with positive allergen immediate hypersensitivity skin prick testing for at least one common indoor or outdoor allergen (dust mite, cat dander, or grass pollen). The subjects did not have asthma. Peripheral blood was obtained via venipuncture in a Vacutainer tube (BD Biosciences) and centrifuged at 23°C for 30 min at 1,400 rpm. The buffy coat was removed and centrifuged again for 10 min at 1,400 rpm and then processed for flow cytometry (see Flow cytometry). All studies were approved by the University of California San Diego Human Subjects Committee.
Immunostaining.
Mouse lungs were fixed by intratracheal instillation of 4% paraformaldehyde, embedded in paraffin, and cut into 3-μm-thick sections for immunostaining. The tissue sections were deparaffinized, rehydrated in graded alcohol, and encircled with a hydrophobic film (ImmEdge PEN, Vector Laboratories). For retrieval of antigens, slides were placed in 10 mM sodium citrate buffer (pH 6.0) at a subboiling temperature for 10 min. For phosphorylated EGF receptor (p-EGFR) staining, sections were digested with proteinase K (Sigma-Aldrich) at a final concentration of 40 μg/ml in PBS for 5 min. Sections were treated in 3% hydrogen peroxide in distilled water for 10 min to quench endogenous peroxidase activity. Nonspecific protein binding was blocked with 2% goat serum in PBS for 30 min. Primary antibodies were diluted in blocking buffer and incubated overnight at 4°C at a final dilution of 1:200 for EGFR Ab and 1:25 for p-EGFR Ab, respectively. EGFR was detected using rabbit anti-human EGFR antibody (sc-03, Santa Cruz Biotechnology) directed against amino acid residues 1005–1016, which are identical to corresponding sequences in murine EGFR. p-EGFR was detected using rabbit anti-p-EGFR (Tyr845) antibody 2231 (Cell Signaling Technology) directed against phosphorylated Tyr845. After primary antibody binding, sections were washed with PBS and then incubated with biotinylated goat anti-rabbit IgG (2 μg/ml) followed by signal amplification using the Elite ABC method and 3,3′-diaminobenzidine chromogen according to the manufacturer's protocols (Vector Laboratories). Sections were counterstained with hematoxylin and mounted with glycerol gelatin (Sigma). EGFR- and p-EGFR-positive epithelial cells were counted and divided by the number of total epithelial cells to obtain the number of positive cells per airway. Six airways per mouse and ≥100 total epithelial cells per airway were analyzed.
Statistical analyses.
Statistical analysis was performed using GraphPad Prism software. Mann-Whitney test was used where indicated. P < 0.05 was considered statistically significant.
RESULTS
Alternaria specifically induces innate eosinophilia dependent on IL-33R.
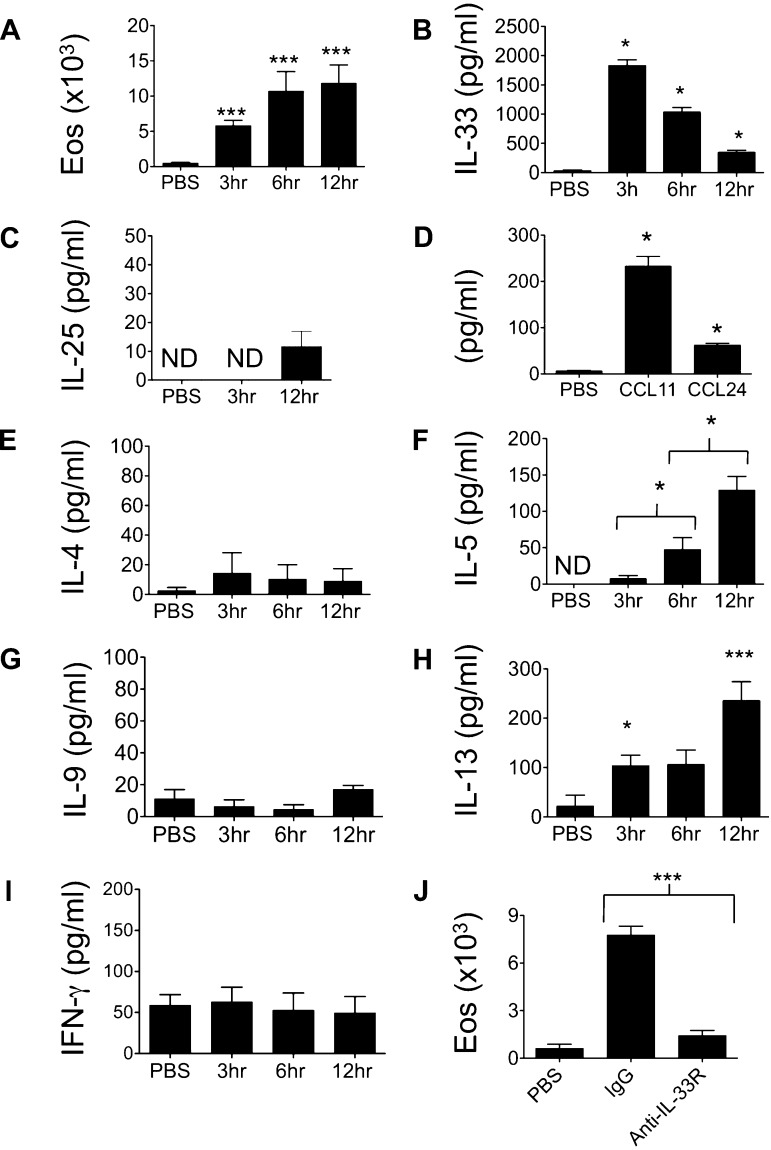
To determine the innate immune response to the fungal allergen Alternaria, naive mice were treated with a single challenge with Alternaria extract, and BAL was performed 3, 6, and 12 h later. BAL eosinophils accumulated as early as 3 h and continued to increase by 12 h after challenge (Fig. 1A). IL-33 is a proinflammatory cytokine implicated in promoting Th2-type responses that lead to eosinophilic lung inflammation, even in the absence of adaptive immune cells (19). High levels of BAL IL-33 were detected after a single Alternaria challenge (Fig. 1B), peaking at 3 h and trending downward by 12 h, and were not present after PBS challenge. In contrast, BAL levels of the proinflammatory type 2 mediator IL-25 were not significantly increased 3 and 12 h after Alternaria challenge (Fig. 1C). The chemokines CCL11/eotaxin-1 and CCL24/eotaxin-2 mediate eosinophil chemotaxis into the lung, and we detected increased BAL levels of CCL11 and CCL24 at 12 h after Alternaria challenge (Fig. 1D). Interestingly, levels of the Th2 cytokine IL-4 were not significantly increased at the time points measured (Fig. 1E), although IL-5 and IL-13 were detectable in BAL as early as 3 h and increased by 12 h (Fig. 1, F and H). IL-9 and IFN-γ were not different between PBS- and Alternaria-challenged mice (Fig. 1, G and I). We then administered IL-33R (T1/ST2) blocking antibody to mice prior to Alternaria challenge and found near-complete absence of BAL eosinophilia 12 h after challenge (Fig. 1J). Thus a single airway challenge with Alternaria induces an IL-33R-dependent innate eosinophilic response, with dramatic increases in IL-33 and the Th2 cytokines IL-5 and IL-13.
Fig. 1.
Alternaria induces innate airway eosinophilia dependent on IL-33 receptor (IL-33R). A–C: C57BL/6 wild-type (WT) mice were given a single challenge of 100 μg of Alternaria extract or PBS intranasally, and bronchoalveolar lavage (BAL) eosinophils (Eos) and the cytokines IL-33 and IL-25 were measured (n = 4–14 mice per group). *P < 0.05, ***P < 0.005 vs. PBS, by Mann-Whitney test. D: BAL chemokines CCL11 and CCL24 12 h after Alternaria or PBS challenge (n = 4–14 mice per group). *P < 0.05 vs. PBS, by Mann-Whitney test. E–I: BAL IL-4, IL-5, IL-9, IL-13, and IFN-γ levels. ND, not detectable (n = 4–14 mice per group). *P < 0.05, ***P < 0.005 vs. PBS, except IL-5 (comparison between time points) by Mann-Whitney test. J: BAL eosinophils in mice treated with anti-IL-33R (anti-T1/ST2) antibody or control IgG for 2 days followed by a single challenge with Alternaria and analyzed 12 h later (n = 6 mice per group). ***P < 0.005, by Mann-Whitney test.
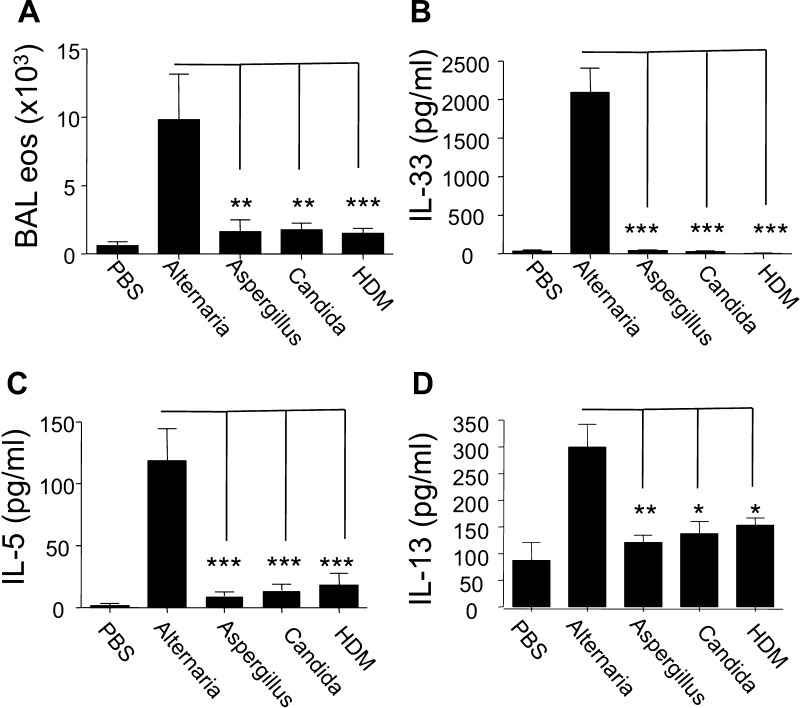
We next compared the innate airway response in naive mice receiving Alternaria with the response after challenge with the same dose of Aspergillus, D. pteronyssinus HDM, or Candida allergen. Surprisingly, an increase in BAL eosinophils, as well as in IL-33, IL-5, and IL-13, was observed only in Alternaria-challenged mice (Fig. 2). BAL neutrophils were increased in mice receiving Aspergillus, D. pteronyssinus HDM and Candida allergen challenges compared with PBS-challenged mice, suggesting that these allergens had reached the lung and were able to induce an inflammatory response (not shown). Thus Alternaria uniquely induces rapid onset of high levels of IL-33, as well as innate eosinophilia.
Fig. 2.
Alternaria, but not Aspergillus, Candida, or Dermatophagoides pteronyssinus house dust mite (HDM), induces an innate T helper type 2 (Th2)-like response. A: WT mice were given a single intranasal challenge with 100 μg of Alternaria, Aspergillus, Candida, HDM extract, or PBS, and levels of BAL eosinophilia were measured 12 h later (n = 4–8 mice per group). B–D: BAL IL-33 levels were measured 3 h and IL-5 and IL-13 12 h after Alternaria challenge (n = 4–8 mice per group). *P < 0.05, **P < 0.01, ***P < 0.005, by Mann-Whitney test. Alternaria-challenged mice were independently compared with Aspergillus, Candida, and HDM groups.
Alternaria induces Th2 cytokine production in IL-33R-expressing lung NHCs.
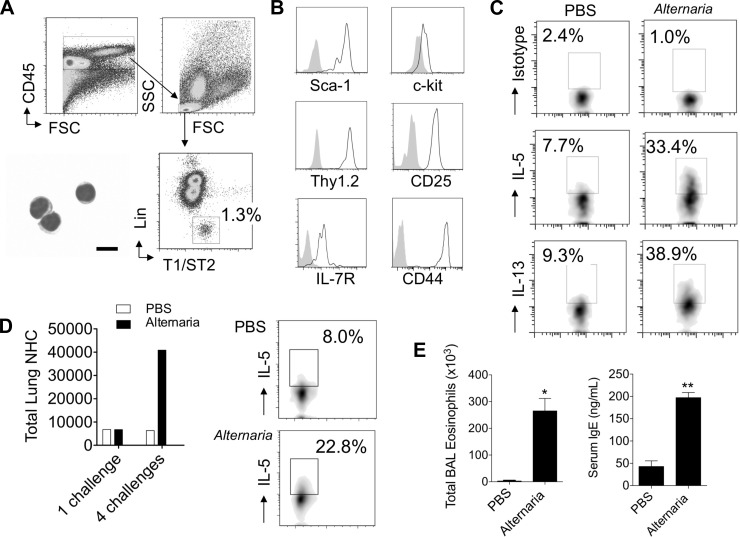
As the recently described NHCs, a lineage-negative lymphocyte population, express the IL-33R (T1/ST2) and respond to IL-33 by production of Th2 cytokines (26), we next investigated if a similar population was activated in the lungs following Alternaria challenge. Lung cells from mice that received a single challenge of Alternaria or PBS were stained with a lineage cocktail that allows for exclusion of B, T, NK, and NKT cells, as well as mast cells, basophils, granulocytes, dendritic cells, and macrophages. CD45-positive lymphocytes were gated, and lineage-negative IL-33R (T1/ST2)-positive cells were analyzed (Fig. 3A). Cytospin preparations of sorted lineage-negative IL-33R-positive cells revealed lymphocyte morphology with scant cytoplasm. Nearly all the lineage-negative cells were IL-33R-positive and expressed Sca-1, c-Kit, IL-7R, Thy1.2, CD25, and CD44 (Fig. 3B). Expression of these markers is most consistent with previously described NHCs (26).
Fig. 3.
Alternaria stimulates lung natural helper cells (NHCs) in vivo to produce Th2 cytokines. C57BL/6 WT mice were given 1 (A–C) or 4 (D and E) intranasal challenges with Alternaria or PBS, and lungs were processed for fluorescein-activated cell sorting analysis. Single-cell suspensions from lung were stained for CD45, T1/ST2, and lineage markers CD3, Gr-1, CD11b, B220, Ter-119, CD11c, NK1.1, CD4, CD5, CD8, FcεR1, TCRβ, TCRγδ, and CD19. A: cells were gated on CD45-positive cells, lymphocytes, and lineage-negative T1/ST2-positive cells; cytospin of sorted lineage-negative T1/ST2-positive cells is shown at bottom left (scale bar, 10 μm). FSC and SSC, forward and side scatter. B: surface expression of markers Sca-1, c-Kit, IL-7 receptor (IL-7R), Thy1.2, CD25, and CD44. Solid gray peak represents isotype control staining. C: lineage-negative CD45-positive T1/ST2-positive lymphocytes from lungs of mice exposed to PBS or Alternaria were gated and analyzed for IL-5 and IL-13 production or isotype staining. D: total lung NHCs after 1 and 4 challenges with PBS or Alternaria (left) and NHC intracellular IL-5 after 4 challenges with PBS or Alternaria (right). Results in A–D are representative of 2–3 independent experiments and pooled lungs from 2–4 mice per group. E: BAL eosinophils and serum IgE after 4 challenges with Alternaria or PBS (n = 4 mice per group). *P < 0.05, **P < 0.01, by Mann-Whitney test.
To determine whether lung NHCs produce the Th2 cytokines IL-5 and IL-13, we performed intracellular staining (Fig. 3C). WT mice were given one intranasal challenge with Alternaria or PBS, and lungs were processed for FACS analysis 3 h later. In PBS-challenged (control) mice, the percentage of IL-5- and IL-13-producing lung NHCs was increased compared with isotype control staining, suggesting that low levels of IL-5 and IL-13 production may be constitutive. Over one-third of the lung NHCs from Alternaria-challenged mice were IL-5- and/or IL-13-positive when measured ex vivo. Thus IL-5 and IL-13 production in lung NHCs is rapid and robust within a few hours of a single Alternaria exposure.
We further investigated whether NHC numbers and cytokine production were affected by multiple challenges with Alternaria. After four challenges over 9 days with Alternaria or PBS, NHC numbers increased nearly sevenfold in the lung in Alternaria-challenged mice (Fig. 3D). Intracellular IL-5 staining revealed that significantly more IL-5 was produced in NHCs from Alternaria- than PBS-challenged mice (Fig. 3D). BAL eosinophils and serum total IgE were also markedly elevated in mice challenged four times with Alternaria (Fig. 3E). Thus lung NHCs expand and continue to produce Th2 cytokines after multiple challenges with Alternaria.
Lung NHCs express GATA-3 and IL-5 independent of STAT6.
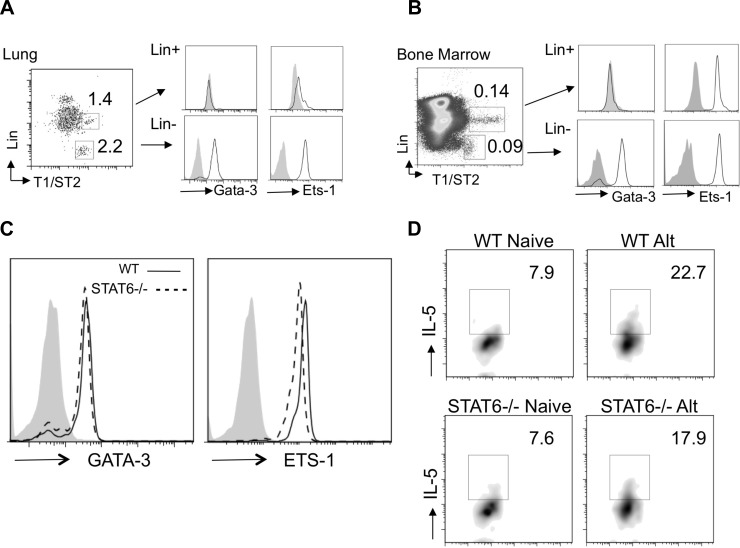
The transcription factors ETS-1 and GATA-3 bind to the IL-5 and IL-13 promoters and can synergize for Th2 cytokine production (35, 37). We performed intracellular staining for NHC GATA-3 and ETS-1 expression in lungs and bone marrow of naive mice (Fig. 4A). In the lung, GATA-3 and ETS-1 were constitutively expressed in NHCs compared with the lineage-positive T1/ST2 population. The lineage-positive T1/ST2-positive cells in the lung express c-Kit highly (not shown) and are consistent with a mast cell population, as previously reported to also express T1/ST2 (25). Similarly, in the bone marrow of naive mice, GATA-3 and ETS-1 were also constitutively expressed in NHCs (Fig. 4B), suggesting that NHCs are primed from development in unchallenged mice for rapid Th2 cytokine production.
Fig. 4.
NHCs express GATA-3 and ETS-1 constitutively independent of STAT6. A and B: single-cell suspensions from lung and bone marrow of naive mice were stained for surface lineage, CD45, IL-33R (T1/ST2), and intracellular ETS-1 or GATA-3. Lineage-positive and -negative T1/ST2-positive lymphocytes (left) were gated, and ETS-1 and GATA-3 expression was determined (middle and right). C: intracellular staining of single-cell suspensions from naive WT and STAT6−/− mouse lungs for GATA-3 and ETS-1 after gating on lineage-negative T1/ST2-positive cells. Solid gray peaks represent isotype staining. D: WT and STAT6−/− NHC intracellular IL-5 production from naive mice and 12 h after Alternaria challenge (Alt). Results are representative of 2–3 independent experiments; lungs and bone marrow were pooled from 2–4 mice per group.
GATA-3 can be induced in STAT6-dependent and -independent manners (14, 31). We thus investigated whether GATA-3 expression in lung and bone marrow NHCs requires STAT6. Lung NHCs from WT and STAT6−/− naive mice were stained for GATA-3 and ETS-1 expression (Fig. 4C). We were surprised to find no significant difference in intracellular GATA-3 and ETS-1 expression in NHCs from STAT6−/− compared with WT mice. We also measured intracellular NHC IL-5 production in naive and Alternaria-challenged WT and STAT6−/− mice (Fig. 4D). Consistent with GATA-3 expression, there was no significant difference in NHC IL-5 before or after Alternaria challenge. This suggests that, unlike conventional Th2 cells, STAT6 does not regulate NHC GATA-3 expression or IL-5 production in vivo.
STAT6 regulates lung NHC proliferation and innate eosinophilia.
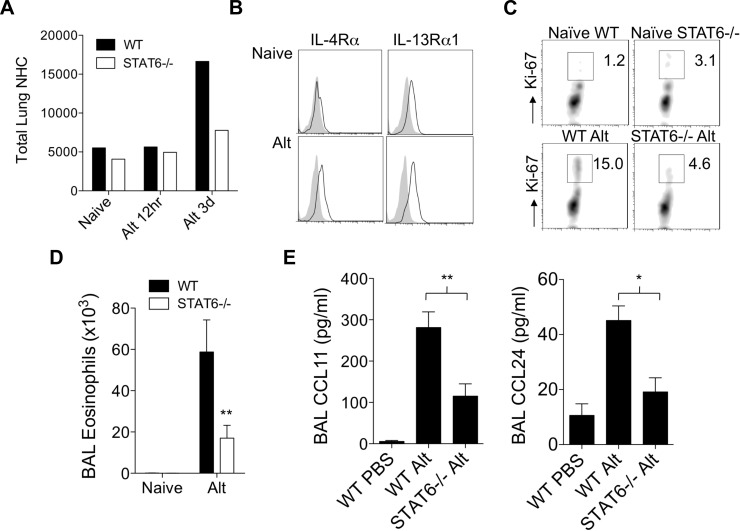
As STAT6 has been reported to regulate proliferation of many cell types, we investigated whether STAT6 regulated numbers or proliferation of lung NHCs after Alternaria challenge. In WT mice, the number of lung NHCs increased significantly 3 days after one challenge with Alternaria compared with the number of NHCs in naive mice and 12 h after challenge (Fig. 5A). In contrast, the total number of lung NHCs from STAT6−/− mice did not significantly increase 3 days after challenge (Fig. 5A). The receptors upstream of STAT6 signaling are IL-4R and IL-13R. To determine whether lung NHCs express IL-4R and IL-13R, which could induce NHC STAT6-mediated responses, we stained lung NHC for IL-4Rα and IL-13Rα1 from naive and Alternaria-challenged WT mice (Fig. 5B). We found that IL-4Rα and IL-13Rα1 were significantly upregulated by 3 days after one Alternaria challenge. This suggests that one challenge with Alternaria primes lung NHCs to respond to IL-4 and IL-13 for induction of STAT6-mediated signals and that accumulation of lung NHCs partially depends on STAT6.
Fig. 5.
STAT6 regulates lung NHC proliferation and innate eosinophilia after Alternaria challenge. A: total lung NHCs in single-cell suspensions from WT and STAT6−/− mice before challenge (naive), as well as 12 h and 3 days after Alternaria challenge. B: lung NHCs from naive mice and 3 days after Alternaria challenge (Alt) were stained for surface IL-4Rα and IL-13Rα1. Results from 1 of 2 representative independent experiments are shown for each time point (A and B). C: lung NHCs from WT and STAT6−/− naive mice and 3 days after Alternaria challenge were stained for Ki-67. Results are from 3 independent experiments; lungs were pooled from 2 WT and STAT6−/− mice. D: total BAL eosinophils from naive WT and STAT6−/− mice as well as 3 days after Alternaria challenge (n = 7–8 mice per group). **P < 0.01, by Mann-Whitney test. E: BAL CCL11 and CCL24 at 12 h (n = 7–11 mice per Alternaria group and 3 mice per PBS group). *P < 0.05, **P < 0.01, by Mann-Whitney test.
To determine whether proliferation of STAT6−/− NHCs was impaired in vivo and possibly accounts for the decreased numbers in STAT6−/− mice after Alternaria challenge, we performed NHC Ki-67 nuclear staining (Fig. 5C). Lung NHCs from naive WT and STAT6−/− mice displayed a similar and low level of proliferation (Ki-67+ NHCs). At 3 days after a single challenge with Alternaria, the percentage of proliferating lung NHCs from WT mice increased over fivefold (Fig. 5C). In contrast, only a modest increase in proliferation was observed in STAT6−/− lung NHCs from challenged mice compared with NHCs from unchallenged mice. Concomitant with a reduction in number and proliferation of lung NHCs in STAT6−/− mice, BAL eosinophils were significantly reduced compared with WT mice (Fig. 5D). BAL CCL11 and CCL24 levels were also significantly decreased in Alternaria-challenged STAT6−/− compared with WT mice (Fig. 5E). Thus STAT6 regulates NHC number and proliferation and innate eosinophilia induced by a single Alternaria challenge in nonsensitized mice. Furthermore, the receptors (IL-4R and IL-13R) upstream of STAT6 are significantly upregulated on lung NHCs after one challenge, suggesting that lung NHCs become primed to respond to IL-4/IL-13, leading to activation of STAT6 signaling.
Alternaria-induced innate eosinophilia is absent in mice lacking lung NHCs.
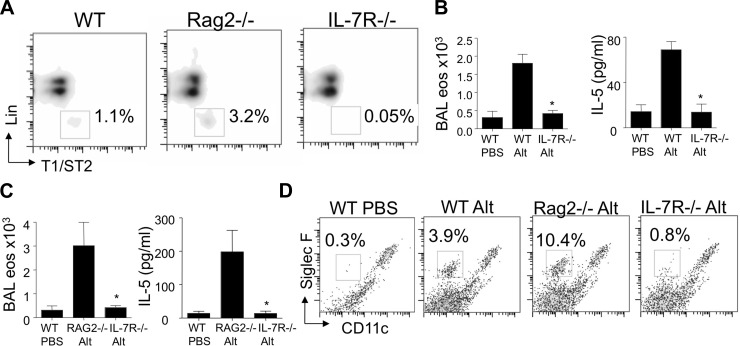
To determine whether the presence of lung NHCs was required for the innate Th2-type response to Alternaria, we challenged mice that lack lung NHCs (IL-7R−/−). IL-7R (CD127) is expressed by lung NHCs from WT mice (Fig. 3B). Signaling through IL-7R occurs through the common gamma chain (γc), which is required for the presence of NHCs in the abdominal fat-associated lymphoid clusters, as they are absent in γc-deficient, as well as IL-7R−/−, mice (26, 42). Lung NHCs were indeed absent in IL-7R−/− compared with WT and RAG2−/− mice (Fig. 6A). Next, we administered a single intranasal challenge with Alternaria or PBS to WT, RAG2−/−, and IL-7R−/− mice and determined levels of eosinophils and IL-5 at 12 h. Compared with WT mice, Alternaria-challenged IL-7R−/− mice did not mount an innate eosinophilia, and BAL IL-5 levels were similar in Alternaria-challenged IL-7R−/− mice and PBS-challenged WT mice (Fig. 6B). As IL-7R−/− mice have severely reduced T and B cell compartments, in addition to impaired NHC development, we also examined NHC responses in RAG2−/− mice that lack T and B compartments but have normal NHC responses. RAG2−/− mice had significantly increased BAL eosinophilia and IL-5 levels compared with IL-7R−/− mice at 12 h (Fig. 6C). The percentage of eosinophils in single-cell suspensions from lungs was increased in WT and RAG2−/− mice as early as 3 h after challenge, whereas eosinophils were nearly absent in PBS and IL-7R−/− mice (Fig. 6D). The percent NHCs, BAL eosinophils, and IL-5 were detected at higher levels in RAG2−/− than WT mice. BAL neutrophils were not significantly different between WT, RAG2−/−, and IL-7R−/− mice challenged with Alternaria (not shown), suggesting that NHC and IL-7R contribute specifically to the innate eosinophilic response. Taken together, IL-7R is required for the presence of lung NHCs as well as innate lung eosinophilia and IL-5 production after Alternaria challenge.
Fig. 6.
IL-7R is required for lung NHCs and Alternaria-induced innate eosinophilia. WT, RAG2−/−, and IL-7R−/− mice were challenged with 100 μg of Alternaria or PBS and analyzed 3 or 12 h later. A: presence of lung NHCs in unchallenged mice. B: BAL eosinophils and IL-5 levels 12 h after Alternaria or PBS challenge in WT or IL-7R−/− mice. C: BAL eosinophils and IL-5 levels 12 h after Alternaria or PBS challenge in RAG2−/− and IL-7R−/− mice (n = 4 mice per group). *P < 0.05, by Mann-Whitney test. D: percentage of lung eosinophils at 3 h after challenge, gated on CD45+ cells. Results are from 3 lungs pooled in Alt groups and 1 lung in PBS group.
Alternaria induces NHC amphiregulin expression and activation of EGFR signaling.
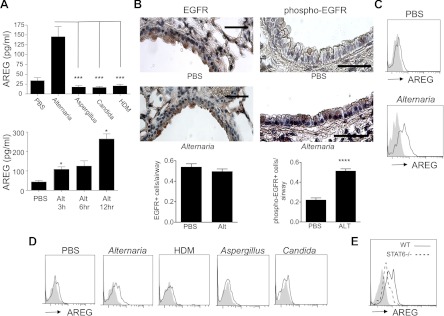
In addition to production of Th2 cytokines, innate lymphoid cells in the lung have recently been reported to produce the growth factor amphiregulin during influenza infection in mice (24). Amphiregulin has been implicated in asthma pathogenesis mainly as a proremodeling/repair mediator that was elevated during acute exacerbations (10, 17, 30, 41, 45), but whether allergens can induce amphiregulin in vivo in the lung has not been reported. We measured amphiregulin levels in the airway 12 h after a single allergen challenge with Alternaria, Aspergillus, Candida, or D. pteronyssinus HDM and found significantly increased amphiregulin levels only in mice challenged with Alternaria (Fig. 7A). BAL levels of soluble amphiregulin were detectable as early as 3 h after challenge with Alternaria and increased further at 12 h. The receptor for amphiregulin is EGFR, which is expressed primarily in the airway epithelium and is phosphorylated upon activation by EGFR ligand binding (40). Immunostaining of lung sections of Alternaria- and PBS-challenged mice revealed similar expression of EGFR in both groups and localization to the airway epithelium (Fig. 7B). In contrast, p-EGFR staining was significantly increased in the airway epithelium from Alternaria- compared with PBS-challenged mice. These data suggest that amphiregulin and activation of EGFR, the receptor for amphiregulin, are induced within 12 h after Alternaria challenge.
Fig. 7.
NHC amphiregulin expression and EGF receptor (EGFR) signaling are induced after Alternaria challenge. A: BAL amphiregulin (AREG) in WT mice challenged with PBS, Alternaria, Aspergillus, Candida, or HDM (top) and ELISA of BAL amphiregulin levels after Alternaria challenge (bottom) (n = 4–8 mice per group). *P < 0.05 vs. PBS, ***P < 0.0005, by Mann-Whitney test. B: immunostaining for EGFR and phosphorylated EGFR (phospho-EGFR) 12 h after PBS or Alternaria challenge (scale bars, 50 μm) and number of positive cells per airway (n = 13–19 airways per group). ****P < 0.0001 vs. PBS, by Mann-Whitney test. C and D: NHC surface amphiregulin expression 6 h after challenge with Alternaria or PBS and 12 h after challenge with PBS, Alternaria, Aspergillus, Candida, or HDM. E: NHC surface amphiregulin in WT and STAT6−/− mice 12 h after Alternaria challenge. Surface staining from pooled lungs is representative of 2 independent experiments. Solid gray peaks represent isotype staining.
Amphiregulin is initially upregulated on the cell surface prior to cleavage into the soluble form. Lung NHC surface amphiregulin from PBS-challenged mice revealed minimal expression, but expression was significantly increased 6 h after Alternaria challenge (Fig. 7C). In contrast to Alternaria-challenged mice, we found no difference in lung NHC surface expression or BAL levels of amphiregulin after challenges with other extracts compared with PBS-challenged mice (Fig. 7D). We then measured NHC amphiregulin expression in STAT6−/− mice after Alternaria challenge and found a significant reduction in surface amphiregulin expression compared with Alternaria-challenged WT mice (Fig. 7E). This suggests that Alternaria promotes NHC Th2 cytokine production as well as expression of the pro-remodeling/repair growth factor amphiregulin, which is partially dependent on STAT6.
Human lineage-negative CRTH2+CD127+ lymphocytes express GATA-3 and ETS-1.
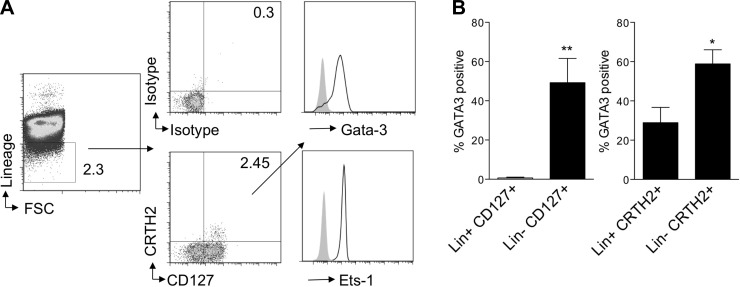
Limited reports are available that demonstrate the presence lineage-negative innate lymphoid cells in humans. A recent study demonstrated expression of the prostaglandin D2 receptor CRTH2, a marker for human Th2 cells, on innate lymphoid cells in human peripheral blood and tissues (23). We investigated whether lineage-negative lymphocytes that were positive for CRTH2 and CD127 constitutively expressed the Th2 transcription factors GATA-3 and ETS-1 similar to NHCs in mouse lung. We found that lineage-negative CRTH2+CD127+ lymphocytes in human blood from allergic individuals highly express GATA-3 and ETS-1 (Fig. 8A). As GATA-3 can be expressed in many cell types, including Th2 cells, we compared the percentage of GATA-3-expressing cells in the lineage-negative CRTH2+CD127+ lymphocyte population with lineage-positive CD127+CRTH2+ populations (Fig. 8B). Very little GATA-3 was expressed in the lineage-positive CD127+ population, whereas >50% of lineage-negative CD127+ cells expressed GATA-3. Approximately 30% of lineage-positive CRTH2+ cells expressed GATA-3 compared with 60% of the lineage-negative CRTH2+ cells. This suggests higher expression of GATA-3 in CRTH2+CD127+ lineage-negative lymphocytes in the blood of allergic individuals than in the respective lineage-positive populations. Overall, transcription factors involved in IL-5 and IL-13 production were constitutively expressed in lung and bone marrow NHCs in mice, as well as in peripheral blood CRTH2+ lineage-negative lymphocytes in humans, suggesting that NHCs are primed from development for Th2 cytokine production in mice and humans.
Fig. 8.
Human lineage-negative CRTH2+CD127+ lymphocytes from allergic individuals express GATA-3 and ETS-1. A: human peripheral blood mononuclear cells from allergic patients were stained for lineage, lineage-negative population was gated and analyzed for CRTH2 and CD127 expression or isotype, and lineage-negative CRTH2+CD127+ cells were analyzed for intracellular GATA-3 and ETS-1 expression. Results are representative of 6 patients. B: human peripheral blood mononuclear cells stained for lineage (Lin), CD127, CRTH2, and GATA-3. Percentage of GATA-3-positive lineage-positive and lineage-negative CD127+ cells and GATA-3-positive lineage-positive and lineage-negative CRTH2+ cells (n = 6 allergic individuals). *P < 0.05, **P < 0.005, by Mann-Whitney test.
DISCUSSION
Our studies demonstrate that Alternaria, an allergen specifically associated with severe asthma exacerbations (4, 9, 22, 28, 29, 32, 34), can activate NHCs to produce the Th2 cytokines IL-5 and IL-13, as well as upregulate the expression of IL-4R and IL-13R and the pro-remodeling/repair growth factor amphiregulin, which is elevated during asthma exacerbations (10, 17, 30, 41, 45). In addition, we demonstrated an important role for STAT6 in NHC proliferation, but not Th2 cytokine production, suggesting that the alternate transcription factors (GATA-3 and ETS-1) we identified to be expressed constitutively in mouse and human NHCs may play a more important role than STAT6 in rapid innate Th2 cytokine responses to Alternaria. In contrast to the very important role of STAT6 in Th2 cytokine production by lineage-positive conventional T cells, lineage-negative NHC production of Th2 cytokines occurred independent of STAT6.
Innate lymphoid cell types that produce large amounts of the Th2 cytokines IL-5 and IL-13 after IL-33 stimulation have only recently been described (26, 27, 33). Lung innate lymphoid cells are activated during influenza viral infection and contribute to airway hyperresponsiveness and tissue repair (5, 24). Our studies suggest that the fungal allergen Alternaria can specifically induce high levels of IL-33 and activate NHCs in the lung to produce Th2 cytokines. Influenza and other respiratory viruses can precipitate severe/life-threatening asthma exacerbations, similar to Alternaria exposure in some cases (4, 29, 32, 34), suggesting that NHC activation may be a link in asthma exacerbation.
We compared the innate response to Alternaria extract with the response to other allergens and found that Alternaria specifically induced high levels of IL-33 in the airway compared with Aspergillus, Candida, and D. pteronyssinus HDM. A previous report demonstrated that Alternaria induced airway IL-33 release that was dependent on the danger signal extracellular ATP (20), but comparative studies with other allergens in vivo have not been reported. The same group very recently showed that innate lymphoid cells in the lung mediate the eosinophilic response after three Alternaria challenges over 6 days (2). Our findings extend the important role of lung NHCs in the eosinophilic airway response to even earlier time points (within 12 h) and after only one challenge with Alternaria. The innate eosinophilia induced by Alternaria was abrogated by IL-33R blockade, suggesting that IL-33-induced NHC activation is the dominant mechanism after Alternaria challenge, although other direct or indirect mechanisms of NHC activation may be present. The finding that different aeroallergens may induce unique innate responses is supported by a report that the HDM D. pteronyssinus contains an accessory protein homolog of MD-2 that can activate Toll-like receptor 4 signaling (39). Our data suggest that Alternaria may specifically or more robustly direct innate responses through an IL-33/NHC pathway compared with other allergens, including HDM.
We found that NHCs in the lung and bone marrow from unchallenged mice constitutively express the transcription factors GATA-3 and ETS-1, which can synergistically induce Th2 cytokine transcription (35, 37). A recent study demonstrated that GATA-3 was required for accumulation of IL-13-producing innate Th2 cells in the lungs after helminth infection, suggesting that GATA-3 does regulate innate lymphoid cells in the lung (21). The finding that NHCs in the bone marrow of mice, and a similar population in human blood, express GATA-3 and ETS-1 suggests that the cells are primed from development for rapid and robust Th2 cytokine production. This notion is supported by a recent report showing that a similar lineage-negative T1/ST2-positive population in the bone marrow is responsive to IL-33 and produces IL-5 and IL-13 (3). The aim of our studies of NHCs in human blood was to determine whether ETS-1 and GATA-3 are constitutively expressed. Future investigation is required to determine whether there are differences in NHC numbers or responses between patients with allergic rhinitis and healthy controls or patients with asthma.
The transcription factor GATA-3 can be induced in STAT6-dependent and -independent manners (14, 31). We found that lung NHC GATA-3 expression was not reduced in STAT6−/− mice, suggesting that lung NHCs maintain GATA-3 expression in a STAT6-independent manner. In Th2 cells, IL-4 and IL-13 signaling activates STAT6 and GATA-3 to promote Th2 cytokine production and Th2 differentiation (13). Our findings of similar GATA-3 expression and IL-5 production from STAT6−/− lung NHCs suggest a differential regulation of Th2 cytokine production in NHCs compared with Th2 cells that are largely STAT6-dependent.
Interestingly, we detected a reduction in NHC numbers and proliferation in the lungs of STAT6−/− mice after one challenge with Alternaria compared with WT mice, suggesting a novel role for STAT6 in the regulation of NHCs. This finding is consistent with a recent report showing that STAT6 regulated the total numbers of innate Th2 cells in the lungs of helminth-infected mice (21). The upregulation of receptors for IL-4 and IL-13 on NHCs and reduction in proliferation in STAT6−/− NHCs after Alternaria challenge suggest that STAT6 directly regulates NHC proliferation, although we cannot exclude contributions to NHC proliferation by STAT6 signaling in other cell types such as macrophages, dendritic cells, and airway epithelium. Our findings are consistent with a known proliferative role of STAT6 in many cell types (reviewed in Ref. 11). Additionally, our previous studies demonstrated that bone marrow-derived cells expressing STAT6 are required for eosinophil accumulation after a single Alternaria challenge (7). Our current studies show that STAT6 is not required for NHC IL-5 production and suggest that other cell types may produce eosinophil chemoattractants, such as eotaxins, in a STAT6-dependent manner, which may be responsible for the reduction of eosinophils in STAT6−/− mice (18, 43, 46). Thus NHCs are likely an important source of IL-13 that may subsequently induce STAT6-dependent eotaxin-1 and eotaxin-2 production in other cell types, such as dendritic cells and macrophages, that promote eosinophilia. This is consistent with a recent report showing that depletion of macrophages and dendritic cells in the lung resulted in a significant reduction of BAL eotaxin levels after allergen challenge (6).
An increased percentage of NHCs in RAG2−/− compared with WT mice is in part due to the absence of B and T cells in the lineage-positive lymphocyte gate in RAG2−/− samples, leading to an increase in the percentage of all non-B non-T cells, including NHCs. We did detect increased BAL IL-5 and eosinophils in RAG2−/− compared with WT mice. This is consistent with another report that identified an increased percentage of lineage-negative lymphocytes, as well as BAL IL-5 and IL-13 production, in RAG−/− compared with WT mice after multiple Alternaria challenges (2). We also detected an increase in total lung NHCs in RAG2−/− mice (not shown) compared with WT C57BL/6 mice. These findings may be due to an increase in non-B non-T lymphocyte compartments as a result of increased homeostatic proliferation, as is well documented after lymphocyte transfer to RAG−/− mice and is IL-7-dependent (38). An increase in RAG−/− NHCs may account for increased cytokines and eosinophilia, thus further highlighting the important role of non-B non-T NHCs.
We found that Alternaria induces amphiregulin in the airway and on NHCs in the lung and induces activation of EGFR, the receptor for amphiregulin in bronchial epithelium. Thus lung NHC amphiregulin expression in response to Alternaria challenge is associated with activation of its receptor on airway epithelium, suggesting a mechanism for NHC to modulate epithelial cell function in asthma. Amphiregulin has been found to be elevated in asthma patients during exacerbations and may contribute to airway remodeling features, including mucus production (10, 17, 30, 41, 45). Importantly, a recent report showed that amphiregulin was highly expressed in microarray studies of sorted innate lymphoid cells in the lung compared with lymphoid tissue inducer cells in the spleen of naive mice (24). In vivo administration of recombinant amphiregulin after influenza viral infection led to improved tissue repair in mice depleted of innate lymphoid cells, suggesting that amphiregulin has a protective role. In contrast, in vitro studies (10, 30, 41, 45) suggest that amphiregulin may contribute to pathological remodeling responses. The precise role of amphiregulin in protecting or contributing to airway remodeling in asthma requires further investigation, although a novel finding from our studies is that NHC surface amphiregulin is significantly upregulated after Alternaria challenge and is partially dependent on STAT6.
In summary, our studies demonstrate the novel finding that Alternaria, an allergen specifically associated with severe asthma exacerbations, induces an innate NHC-mediated rapid burst of Th2 cytokine (IL-5 and IL-13) production, as well as expression by NHCs of IL-4R, IL-13R, and amphiregulin, and initiates eosinophilic inflammation compared with other allergens such as Aspergillus, D. pteronyssinus HDM, or Candida. In addition, we have demonstrated an important role for STAT6 in NHC proliferation, but not Th2 cytokine production, suggesting that alternate transcription factors (GATA-3 and ETS) we identified to be expressed constitutively in mouse and human NHCs may play a more important role than STAT6 in rapid innate Th2 cytokine responses to Alternaria. In contrast to the very important role of STAT6 in Th2 cytokine production by lineage-positive conventional T cells, lineage-negative NHC production of Th2 cytokines occurred independent of STAT6. The ability of Alternaria to rapidly induce IL-33, as well as the constitutive expression by NHCs of GATA-3 and ETS-1, may contribute significantly to a rapid Th2 innate response that could trigger asthma exacerbations.
GRANTS
This study was supported by National Institute of Allergy and Infectious Diseases Grant 1K08 AI-080938 (to T. A. Doherty) and Grants AI-38425, AI-70535, and AI-72115 (to D. H. Broide).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.A.D., N.K., M.C., and D.H.B. are responsible for conception and design of the research; T.A.D., N.K., J.E.C., H.-K.K., and P.R. performed the experiments; T.A.D., N.K., J.E.C., H.-K.K., and P.R. analyzed the data; T.A.D., N.K., J.E.C., H.-K.K., P.R., and D.H.B. interpreted the results of the experiments; T.A.D., N.K., and J.E.C. prepared the figures; T.A.D. and N.K. drafted the manuscript; T.A.D., N.K., M.C., and D.H.B. edited and revised the manuscript; T.A.D. and D.H.B. approved the final version of the manuscript.
REFERENCES
- 1. Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 129: 191–198, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 188: 1503–1513, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage-Sca1+c-Kit− CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol 187: 5795–5804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol 113: 227–234, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol 12: 631–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crapster-Pregont M, Yeo J, Sanchez RL, Kuperman DA. Dendritic cells and alveolar macrophages mediate IL-13-induced airway inflammation and chemokine production. J Allergy Clin Immunol 129: 1621–1627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol 188: 2622–2629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med 17: 596–603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, Xuan W, Downie SR, Tobias A, Peat JK. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med 164: 455–459, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Enomoto Y, Orihara K, Takamasu T, Matsuda A, Gon Y, Saito H, Ra C, Okayama Y. Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol 124: 913–920, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res 50: 87–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36: 451–463, 2012 [DOI] [PubMed] [Google Scholar]
- 13. Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 111: 677–690; quiz 691, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol 9: 125–135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol 188: 703–713, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol 129: 216–227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim KW, Jee HM, Park YH, Choi BS, Sohn MH, Kim KE. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest 136: 805–810, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Knott ML, Matthaei KI, Foster PS, Dent LA. The roles of eotaxin and the STAT6 signalling pathway in eosinophil recruitment and host resistance to the nematodes Nippostrongylus brasiliensis and Heligmosomoides bakeri. Mol Immunol 46: 2714–2722, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 20: 791–800, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186: 4375–4387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 13: 58–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyons TW, Wakefield DB, Cloutier MM. Mold and Alternaria skin test reactivity and asthma in children in Connecticut. Ann Allergy Asthma Immunol 106: 301–307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12: 1055–1062, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol 161: 4866–4874, 1998 [PubMed] [Google Scholar]
- 26. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463: 540–544, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol 103: 709–711, 1999 [DOI] [PubMed] [Google Scholar]
- 29. O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med 324: 359–363, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. FcεRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol 115: 272–279, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity 12: 27–37, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Plaza V, Serrano J, Picado C, Cosano J, Ancochea J, de Diego A, Martin JJ, Sanchis J. Clinical characteristics of the fatal and near-fatal asthma in Alternaria alternata sensitized patients. Med Clin (Barc) 121: 721–724, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA 107: 11489–11494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol 120: 610–617, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Russell L, Garrett-Sinha LA. Transcription factor Ets-1 in cytokine and chemokine gene regulation. Cytokine 51: 217–226, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol 118: 892–898, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strempel JM, Grenningloh R, Ho IC, Vercelli D. Phylogenetic and functional analysis identifies Ets-1 as a novel regulator of the Th2 cytokine gene locus. J Immunol 184: 1309–1316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA 98: 8732–8737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457: 585–588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, Holtzman MJ. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116: 309–321, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang SW, Oh CK, Cho SH, Hu G, Martin R, Demissie-Sanders S, Li K, Moyle M, Yao Z. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J Allergy Clin Immunol 115: 287–294, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORα is critical for nuocyte development. Nat Immunol 13: 229–236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-α chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 25: 522–530, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: natural helper cells derive from lymphoid progenitors. J Immunol 187: 5505–5509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol 40: 610–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, Finkelman FD, Rothenberg ME. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol 165: 5839–5846, 2000 [DOI] [PubMed] [Google Scholar]