Abstract
Histone deacetylase 2 (HDAC2) is a class I histone deacetylase that regulates various cellular processes, such as cell cycle, senescence, proliferation, differentiation, development, apoptosis, and glucocorticoid function in inhibiting inflammatory response. HDAC2 has been shown to protect against DNA damage response and cellular senescence/premature aging via an epigenetic mechanism in response to oxidative stress. These phenomena are observed in patients with chronic obstructive pulmonary disease (COPD). HDAC2 is posttranslationally modified by oxidative/carbonyl stress imposed by cigarette smoke and oxidants, leading to its reduction via an ubiquitination-proteasome dependent degradation in lungs of patients with COPD. In this perspective, we have discussed the role of HDAC2 posttranslational modifications and its role in regulation of inflammation, histone/DNA epigenetic modifications, DNA damage response, and cellular senescence, particularly in inflammaging, and during the development of COPD. We have also discussed the potential directions for future translational research avenues in modulating lung inflammaging and cellular senescence based on epigenetic chromatin modifications in diseases associated with increased oxidative stress.
Keywords: cigarette smoke, oxidants, inflammation, DNA damage and repair, premature lung aging; polyphenols
the inflammaging refers to chronic low-grade inflammation with aging, which occurs in chronic inflammatory diseases, such as chronic obstructive pulmonary disease (COPD) (34, 74, 124). The inflammation and cellular senescence (a state of permanent growth arrest) are intertwined in the process of accelerated or premature lung aging. However, the mechanism and causal role of inflammation and premature aging in the development of COPD remain unknown.
Histone deacetylase 2 (HDAC2) belongs to class I histone deacetylases, which catalyze the removal of acetyl groups from ε-amino-terminal lysine tails of core histone proteins. It was first discovered as a mammalian homolog of the yeast transcriptional regulator RPD3 responsible for transcriptional repression (138). HDAC2 itself does not possess a DNA-binding domain; therefore, it requires the presence of corepressor complex proteins including mSin3, NuRD/Mi2, and NCoR to target the substrate DNA. It is tightly regulated by complex protein-protein interaction, subcellular localization, and posttranslational modification (25, 111). HDAC2 functions in regulation of various cellular processes, such as cell cycle, proliferation, differentiation, inflammation, development, and glucocorticoid function. Recently, it has been shown that HDAC2 along with HDAC1 regulates DNA damage response (DDR) and cellular senescence via an epigenetic mechanism (82, 130). The level of HDAC2 and activity is decreased in lung parenchyma, bronchial biopsies, alveolar macrophages, and peripheral blood monocytes from patients with COPD, as well as in macrophages and lungs of mice exposed to cigarette smoke (4, 20, 47, 137). Yang et al. (137) have shown that NF-κB-mediated lung inflammatory response was associated with oxidative posttranslational modifications of HDAC2 in response to cigarette smoke. These modifications led to the ubiquitination and proteosomal degradation of HDAC2 (3, 4). Other studies also showed that HDAC2 is posttranslationally modified by oxidative/carbonyl stress imposed by cigarette smoke, leading to its degradation (75, 80). Interestingly, these modifications and various cellular processes regulated by HDAC2 are shown to be involved in the pathogenesis of COPD. This implicates a pivotal role of HDAC2 in the development of COPD/emphysema. In this perspective review, we have discussed the regulation of HDAC2 in various cellular functions in translational research, particularly in the progression of COPD, and potential for targeting HDAC2 in the intervention of this disease.
HDAC2 Posttranslational Modifications by Oxidative/Carbonyl Stress
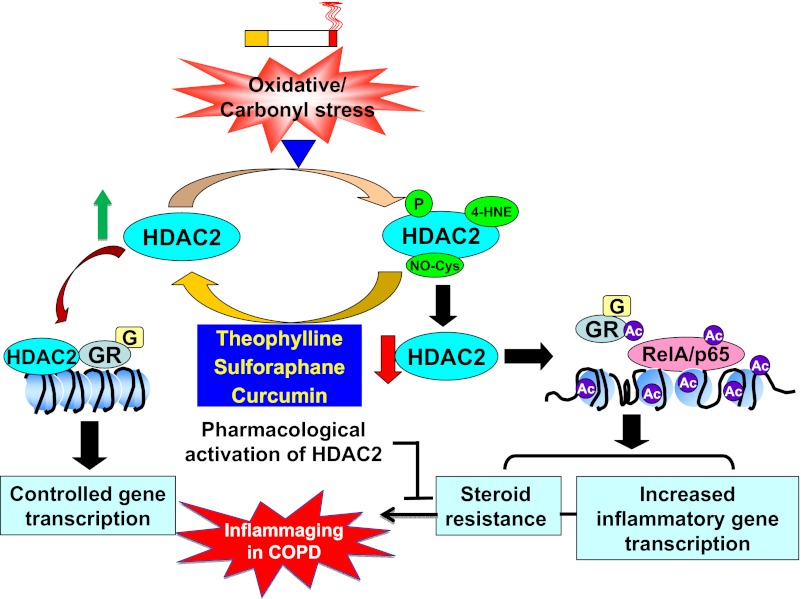
Several studies have shown that HDAC2 level and activity are closely related to its posttranslational modifications, such as phosphorylation, carbonylation, nitration, and nitrosylation (22, 88, 95, 120) (Table 1). This is corroborated by the observations that a decrease of HDAC2 level and activity in patients with COPD is associated with its posttranslational modifications, including oxidation/carbonylation, nitrosylation, acetylation, phosphorylation, and subsequent degradation in response to oxidants derived from cigarette smoke (47, 75) (Fig. 1). Tsai and colleagues (120) first identified HDAC2 as a phospho-protein with unique phosphorylation on serine, but not threonine or tyrosine site. We also found that cigarette smoke caused HDAC2 phosphorylation at Ser394, Ser411, Ser422, and Ser424 in macrophages, lung epithelial cells, and mouse lungs (3, 4). Interestingly, HDAC2 phosphorylation is required for its interaction with transcription factors (e.g., p53), cAMP-responsive element-binding protein binding protein (CBP), and corepressor complex formation as well as its acetylation on lysine residues (3). Protein kinase CK2 is responsible for HDAC2 phosphorylation induced by cigarette smoke, which leads to reduction of its deacetylase activity and level via a ubiquitination-proteasome dependent degradation (3). Furthermore, a role of phosphatidylinositol 3-kinase (PI-3K), an upstream of CK2, in regulation of HDAC2 has been shown in response to cigarette smoke (78). PI-3Kδ-null mice are more responsive to steroid treatment in inhibiting airway inflammation following cigarette smoke exposure (78), suggesting the regulation of HDAC2 modification by kinases in steroid resistance. These signaling mechanisms may be merged downstream to regulate HDAC2. Because HDAC2 has no nuclear export signal, it was initially proposed that it would be a predominantly nuclear protein. However, HDAC2 is also present in the cytoplasm, which is reduced in patients with COPD compared with nonsmokers (116, 126). Further study is required to investigate the mechanism for HDAC2 cytoplasmic localization and whether its phosphorylation is involved in lung inflammatory response incited by cigarette smoke.
Table 1.
HDAC2 modifications, causal factors, and consequence
| Modification | Residue | Causal Factor | Consequence | Reference |
|---|---|---|---|---|
| Phosphorylation | Ser394, Ser411, Ser422, Ser424, and threonine | CK2 | Ubiquitination and degradation | (3, 4, 120) |
| Carbonylation | Cysteine, histidine, lysine | Aldehyde and 4-HNE | Decreased level and activity | (79, 80, 137) |
| Nitration | Tyrosine 253 | Nitric oxide | Decreased level and activity | (79, 95, 137) |
| Nitrosylation | Cys262 and Cys274 | Nitric oxide | Decreased level and activity | (22, 75, 88) |
| Acetylation | Lysine | CBP | Increased transrepression activity | (3) |
HDAC2, histone deacetylase 2; CBP, cAMP-responsive element-binding protein binding protein.
Fig. 1.
Role of histone deacetylase 2 (HDAC2) in oxidative/carbonyl stress-induced inflammation and steroid resistance. Oxidative/carbonyl stress imposed by cigarette smoke causes HDAC2 phosphorylation, carbonylation, and nitrosylation, thereby leading to the decrease of its level and activity. HDAC2 reduction increases the acetylation of histone, NF-κB, and glucocorticoid (G) receptor (GR), which results in the augmented transcription of proinflammatory genes and steroid resistance. A variety of pharmacological and dietary compounds, such as theophylline, sulforaphane, curcumin, baicalin, and quercetin are under investigation to restore steroid efficacy via regulation HDAC2 posttranslational modifications and activity. P, phosphorylation; 4-HNE, carbonylation; NO-Cys, nitrosylation; Ac, acetylation.
Apart from phosphorylation, HDAC2 can be modified by carbonyl stress, such as 4-HNE and reactive aldehydes, which are increased in lungs of patients with COPD (79, 84, 101, 137). Nitration of HDAC2 at tyrosine 253, present in the catalytic domain, has been shown to inactivate its deacetylase activity (95). Endogenous nitric oxide (NO) generation from mice expressing constitutively active endothelial NO synthase (eNOS) leads to S-nitrosylation of HDAC2 at cysteine, and subsequent loss of HDAC2 deacetylase activity (22). Nott et al. (88) observed that HDAC2 S-nitrosylation at cysteine residues 262 and 274 occurs in rat cortical neurons, which prevents its association with gene promoters (i.e., Fos and Egr1) but has no effect on its deacetylase activity (88). Thus the nitration and nitrosylation of HDAC2 have a different effect on its deacetylase and transrepression activity, although tyrosine residue 253 as well as cysteine residues 262 and 274 are at the COOH-terminal end of HDAC2 catalytic domain (57). Indeed, cigarette smoke caused upregulation of inducible NOS and eNOS in mouse lung, despite that the latter effect is transient (110). This may increase NO generation and subsequent HDAC2 nitration and nitrosylation in COPD (75). Because HDAC2 itself does not possess a DNA-binding domain but requires the presence of corepressor complex proteins to target it to substrate DNA (25), it is possible that S-nitrosylation at Cys 262 and Cys 274, as well as nitration on tyrosine 253, affects the ability of HDAC2 to interact with required DNA-binding proteins present in the corepressor complex. HDAC2 nitrosylation occurs at Cys 262 and Cys 274, which is increased in alveolar macrophages from patients with COPD. This leads to inhibition of glucocorticoid receptor (GR)-transrepression activity (75). Interestingly, treatment with Nrf2 activator sulforaphane significantly reduced HDAC2 nitrosylation and degradation, which is in agreement with the findings that reduced HDAC2 abundance is observed in lungs of Nrf2-deficient mice in response to cigarette smoke exposure (2, 75). Thus Nrf2 is an important antioxidant transcription factor that targets HDAC2 via phase II gene induction, thereby reversing corticosteroid resistance in COPD and other corticosteroid-resistant inflammatory diseases. HDAC2 is also regulated by cellular redox GSH status. Intracellular levels of GSH retain HDAC2 in a reducing environment because an increase in intracellular GSH levels can inhibit the oxidatively posttranslational modifications of HDAC2 (4, 75, 137). HDAC2 forms a complex with HDAC1 and HDAC3. Treatment of cigarette smoke extract decreases the protein levels of HDAC1, HDAC2, and HDAC3 in human monocyte/macrophage in cell culture experiments (68, 134, 137), although there is no report regarding the reduction of HDAC1 or HDAC3 in lungs of patients with COPD. Further study is required to determine whether HDAC2 reduction disrupts its complex with HDAC1 and HDAC3, leading to the change of their abundance and activity. Furthermore, it remains unknown whether other antioxidants, such as N-acetyl-l-cysteine, superoxide dismutase, and peroxynitrite scavengers, also can protect against HDAC2 oxidative modifications and nitrosylation.
HDAC2 in Inflammation and Steroid Resistance
Corticosteroids suppress inflammation by recruiting HDAC2 to NF-κB-driven proinflammatory gene promoters, thereby inhibiting the transcription of these genes. Furthermore, HDAC2 is required for GR deacetylation, which enables GR binding to the NF-κB complex, leading to the inhibition of NF-κB-dependent proinflammatory gene transcription (38, 48). NF-κB (RelA/p65) itself can also be acetylated and activated by cigarette smoke, which is attenuated by HDAC2 (21, 137, 141). However, HDAC2 level and activity are decreased in lungs of patients with COPD (47). This renders corticosteroids unable to inhibit proinflammatory gene transcription, leading to steroid resistance in COPD (Fig. 1). This is corroborated by the findings that hypoxia-inducible factor-1α activation by hypoxia, which occurs in lung microenvironment of patients with COPD, decreases HDAC2 level, resulting in augmented inflammation and steroid resistance (18). Furthermore, HDAC2-deficient mice are not responsive to budesonide in inhibiting LPS-induced lung inflammation (LPS itself has no effect on HDAC2 level or activity) (2). Hence, the attenuation of HDAC2 posttranslational modification and reduction would restore the effectiveness of steroid in inhibiting inflammation. A variety of pharmacological and dietary compounds can influence HDAC2 activity and level (Fig. 1 and Table 2). For example, treatment of monocytes/macrophages with curcumin (active ingredient in dietary spice turmeric or Curcuma longa), a polyphenolic compound, attenuated cigarette smoke extract-induced carbonylation, phosphorylation, and proteosomal degradation of HDAC2, which was associated with the restoration of steroid effectiveness in vitro (80). Nrf2 activator sulforaphane (present in broccoli and cruciferous vegetables) restored dexamethasone sensitivity in alveolar macrophages obtained from patients with COPD and treated with cigarette smoke extract ex vivo (75). Nortriptyline, a tricyclic antidepressant, has been shown to restore corticosteroid sensitivity induced by cigarette smoke-mediated oxidative stress, which is associated with increased HDAC activity (81). Low concentration of theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammation and increases HDAC2 activity in alveolar macrophages from patients with COPD (23, 114). A recent clinical trial has demonstrated that a combination therapy with an inhaled corticosteroid and low-dose of theophylline attenuated airway inflammation in patients with COPD (32). Theophylline also suppressed peroxynitrite-induced tissue remodeling via pathways involving NF-κB/TGF-β1 and/or HDAC in the human fibroblast HFL-1 cell line (113). Erythromycin, a macrolide, restored the effect of cigarette smoke-induced decline in HDAC1, HDAC2, and HDAC3 levels associated with inhibition of NF-κB activation (68). Baicalin, a flavonoid compound isolated from the root of Scutellaria baicalenis Georgi, can influence cigarette smoke-induced inflammation and overcome steroid resistance via inhibiting HDAC2 phosphorylation in mouse lungs (67). Similarly, long-acting β2-agonists and corticosteroids restore the reduction of HDAC activity and inhibit H2O2-induced proinflammatory mediator release from human alveolar macrophages (99). All these findings provide a new avenue for the development of specific HDAC2 modulators in attenuating its posttranslational modifications and degradation so as to enhance steroid efficacy in management of COPD. However, HDAC2 is covalently modified in carbonyl/oxidative and inflammatory conditions. A simple activation of HDAC2 may not be effective in reversing steroid resistance unless its covalent posttranslational modifications are reversed.
Table 2.
Regulation of small molecules in HDAC activity and steroid resistance
| Molecule | HDAC Activity | Steroid Effectiveness and Inflammation | Reference |
|---|---|---|---|
| Curcumin | Inhibition of HDAC2 phosphorylation and degradation | Restores steroid effectiveness | (80) |
| Sulforaphane | Inhibition of HDAC2 nitrosylation and degradation | Restores dexamethasone sensitivity | (75) |
| Nortriptyline | Increases HDAC activity | Restores corticosteroid sensitivity | (81) |
| Theophylline | Increases HDAC2 activity | Increases corticosteroid effectiveness in COPD | (23, 32) |
| Erythromycin | Inhibits the reduction of HDAC1, HDAC2 and HDAC3 | Inhibits NF-κB activation | (68) |
| Baicalin | Inhibits HDAC2 phosphorylation | Overcomes steroid resistance | (67) |
| Salmeterol or formoterol | Restores HDAC activity | Inhibits inflammatory response | (99) |
COPD, chronic obstructive pulmonary disease.
HDAC2 Regulation in Histone Acetylation and Methylation as Well as in DNA Damage Response
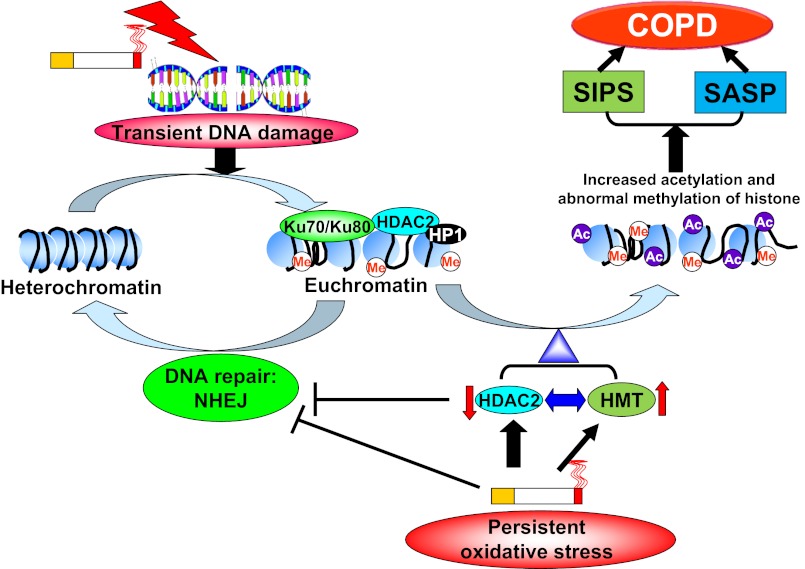
Cigarette smoke has been shown to cause DNA damage and to impair double-strand break (DSB) repair, which is aggravated in patients with COPD/emphysema (8, 15, 16, 27, 28, 97). A recent study has demonstrated that HDAC2 functions in the DNA damage response (DDR) along with HDAC1 to promote DNA nonhomologous end joining (NHEJ) repair (82). Furthermore, HDAC2 regulates chromatin plasticity and enhances DNA vulnerability, suggesting that HDAC2 inhibition can lead to DNA damage (76). This may be due to HDAC2-mediated transcriptional repression, which prevents transcription from interfering with the repair process. Another possibility is that HDAC2 recruitment influences the ability of DNA repair factors (e.g., Ku70/Ku80) to bind the damaged DNA or to function effectively (82) (Fig. 2).
Fig. 2.
Epigenetic regulation of HDAC2 in DNA damage response as well as in stress-induced premature senescence (SIPS) and senescence-associated secretory phenotype (SASP) after DNA damage. In response to transient DNA damage, chromatin structure changes, which then allows DNA repair proteins, such as Ku70 and Ku80, and cofactors including heterochromatin protein 1 (HP1) to access the damage sites. Furthermore, HDAC2 is also recruited to damaged sites to promote repair via repressing transcription or to affect the ability of DNA repair factors to bind damaged DNA. However, persistent and sustained oxidative stress induced by cigarette smoke activates the signaling pathways of DNA damage response, thereby causing SIPS. Furthermore, HDAC2 reduction and histone methyltransferase (HMT) activation induce augmented histone acetylation (e.g., H3K9) and abnormal histone methylation (e.g., H3K4) on the promoters of SASP and prosenescent genes, leading to increased transcription of these genes. Me, methylation; NHEJ, nonhomologous end joining; COPD, chronic obstructive pulmonary disease.
Histone modifications play an important role in regulating DNA damage/repair, proinflammatory gene transcription, genomic instability, and premature aging (59, 93, 107, 128, 144). Apart from direct histone deacetylation (e.g., H3K56, H4K5, H4K12 and H4K16), HDAC2 can also interact with protein methyltransferases/demethylases to form a large and multiple-protein complex(es), thereby regulating histone methylation (e.g., H3K4 and H3K9 methylation) (66, 72, 127, 136) (Table 3). Histone methylation at H3K79 and H4K20 has been shown to affect DDR after DSB induction by recruiting 53BP1 and Crb2 to DNA damage foci (12, 46, 106, 131). Furthermore, H3K36 dimethylation increases the rate of the association of DNA repair proteins Ku70 and NBS1 near DSB (31, 129). In addition, H3K79 dimethylation is required for ionizing radiation-induced 53BP1 foci formation when H4K20 dimethylation is low (63, 131), suggesting a cross-talk of histone modifications to coregulate DDR. Opening, repairing, and closing of chromatin occur in response to DDR to repair the damaged DNA (96). Hence, this histone acetylation and methylation after DNA damage affect chromatin status between euchromatin and heterochromatin so as to recruit DNA repair factors and cofactors (e.g., HP1) to damaged sites. We and others have shown that cigarette smoke alters the profile of histone acetylation and methylation in lung epithelial cells, which is associated with HDAC2 reduction (3, 4, 70, 115, 140). However, it remains to be known whether cigarette smoke-induced HDAC2 reduction alters histone acetylation and methylation as well as their cross talk, thereby regulating DNA damage and repair during cellular senescence and premature aging of the lung in COPD.
Table 3.
HDAC2 regulation in histone acetylation and methylation in DDR and cellular senescence
| Histone Modification | Specific Residue | Effect of HDAC2 | Function | Reference |
|---|---|---|---|---|
| Acetylation | H3K56 | Reduce its acetylation | Involved in DDR and DNA repair | (82) |
| H4K5 | Reduce its acetylation | Involved in DNA repair | (41, 105) | |
| H4K12 | Reduce its acetylation | Regulate telomere function and premature aging | (41, 50, 105, 145) | |
| H4K16 | Reduce its acetylation | Involved in DNA repair and cellular senescence | (59, 82) | |
| Methylation | H3K4 | HDACs inhibit H3K4 methylation via LSD1 | Involved in gene activation and DDR | (30, 45, 77, 132) |
| H3K9 | HDACs increase its methylation via SUV39H1 | Involved in replication checkpoint and cellular senescence | (13, 87, 102, 127) | |
| H4K20 | Trichostatin A reduces its methylation | Influence DDR after DSB | (12, 36, 106) |
DDR, DNA damage response; DSB, double-strand break.
Histone acetyltransferase CBP/p300 has shown to acetylate histone H3 and H4 at the sites of DSB, thereby regulating DNA damage and repair (93, 128). CBP/300 is also recruited to the promoter of proinflammatory gene IL-6, leading to its transcription via acetylating histone H3K9 in mouse lung exposed to cigarette smoke (142). Therefore, CBP/p300 may be recruited on the sites of DSB in response to cigarette smoke exposure in regulating DNA damage. We have recently found that CBP interacted with HDAC2, leading to HDAC2 acetylation in response to cigarette smoke (3). Thus HDAC2 may mediate the regulation of CBP in DNA damage and repair as well as proinflammatory gene transcription. Histone acetyltransferase (CBP/p300) inhibitors may attenuate HDAC2 acetylation in lung cells and hence overcome steroid resistance in patients with COPD.
Recent studies have shown that HDAC inhibitors, not only impair NHEJ and homologous recombination, but also induce acetylation of the key components, such as Ku70, during DNA repair (5, 19, 56). The increased acetylation of Ku70 reduces its DNA-binding affinity but does not affect Ku70/Ku80 heterodimer formation. Further study is required to determine whether Ku70 and other DNA repair proteins undergo acetylation/deacetylation under oxidative stress imposed by cigarette smoke and whether HDAC2 has any effect on their posttranslational modification status for DNA repair.
HDAC2 Regulation in Cellular Senescence
Recent studies have shown that persistent DNA damage causes stress-induced premature senescence (SIPS) and senescence-associated secretory phenotype (SASP) (103, 104). SASP has been introduced to describe the senescent cells that secrete proinflammatory mediators, such as IL-6, IL-8, and matrix metalloproteinases. This is partially due to NF-κB activation by DDR. Interestingly, NF-κB inhibition in turn delays the DNA damage-induced senescence and aging in mice (118), suggesting the reinforcement of cellular senescence by SASP as well as positive feedback between SIPS and SASP. Replicative senescence occurs as a result of exhaustion of proliferative ability of the cells in concomitance with shortening of telomeres and alteration in telomerase due to organisms/cellular aging independent of environmental stress. Thus cigarette smoke-mediated DNA damage may be an important contributing factor in initiating and maintaining SIPS and SASP. Indeed, a link between cellular senescence and premature lung aging has been proposed in the pathogenesis of COPD (7, 11, 37, 52, 65, 73, 124). Cigarette smoke induces senescence in lung epithelial cells and fibroblasts in vitro (86, 91, 92, 121–123). Senescence of fibroblasts and myofibroblasts may result in fibrosis in smokers and patients with COPD. It is also likely that carbonyls/oxidants generated or present in cigarette smoke cause immunosenescence of T- and B-cells, leading to abnormal recognition of self-antigens, which is important for the development of COPD (14, 54, 62, 90, 109). The involvement of cellular senescence in the pathogenesis of emphysema is also shown in various studies using genetically altered mouse strains (55, 64, 108, 112, 139). However, the molecular mechanisms that underlie cigarette smoke-induced cellular senescence in vitro in lung cells and in vivo in mouse lung and the precise role of SIPS in the development of COPD/emphysema are unclear. HDAC2 has been shown to protect against cellular senescence via regulating prosenescent genes (i.e., p21 and p16) (69, 130, 133, 146, 147). In addition, deletion of prosenescent gene p21 protects against cigarette smoke-induced cellular senescence in lung cells and subsequent emphysema (139). Hence, reduction of HDAC2 may lead to cellular senescence and pulmonary emphysema (83). Hyperoxia occurs during oxygen therapy for patients with COPD with severe resting hypoxemia. However, hyperoxia causes DNA damage and cellular senescence as well as impairs lung development in neonatal rodents via decreasing HDAC2 level and activity (71, 148). This provides a possibility that HDAC2 is an essential factor in regulating premature cellular senescence and the susceptibility to develop COPD. Further study using HDAC2 global and cell-specific knockout mice as well as transgenic animals would identify the role of HDAC2 in cellular senescence during cigarette smoke-induced airspace enlargement/lung destruction (emphysema).
HDAC2 interacts with poly(ADP-ribose) polymerase-1 (PARP-1) and reduces its acetylation that is required for full NF-κB-dependent transcriptional activity (44). PARP-1 is an important molecule that activates DNA repair programs, and PARP-1/NF-κB signaling cascade is activated during cellular senescence (94, 135). Therefore, it is possible that HDAC2 regulates SIPS and SASP via PARP-1-dependent mechanisms on DDR and telomere function in response to cigarette smoking. This contention requires further studies to translate the findings of HDAC2 regulation in replicative senescence in pathogenesis of COPD.
HDAC2 Regulation in SASP
The senescent cells are prone to secrete proinflammatory cytokines, which may in turn reinforce cellular senescence, as the deficiency of IL-6, IL-6R, or CXCR2 prevents SIPS (1, 35, 60, 103). Indeed, the percentage of proinflammatory senescent type II cells expressing both p16 and phosphorylated NF-κB (i.e., SASP) was increased in lungs of patients with COPD compared with smokers and nonsmokers (121). Increased proinflammatory gene transcription in senesced cells may be due to abnormal histone acetylation and methylation on these gene promoters. In general, histone acetylation is associated with gene activation. Unlike histone acetylation, histone H3K4 and H3K36 trimethylation activates chromatin (euchromatin), whereas histone H3K9 and H3K27 trimethylation silences gene transcription (heterochromatin) (26, 29, 33, 89). This has been shown to occur by cigarette smoke extract treatment, which increases histone H3K4 and H3K36 methylation but reduces H3K9 and H3K27 methylation in lung epithelial cells (140). It is proposed that histone deacetylation of H3K9 by HDAC2 is a prerequisite for its methylation (87, 102). Moreover, histone H3K4 methylation limits the accumulation of histone H3K4 acetylation at the gene promoter (42). These findings suggest the cross-regulation of histone methylation and acetylation, even at the same residue. Hence, the study on the pattern and cross-talk of histone acetylation and methylation at these residues on the promoters of SASP genes will unravel an epigenetic chromatin mechanism underlying abnormal inflammatory response observed in COPD. It also remains to be studied whether HDAC2 regulates histone methylation on the promoters of SASP genes in a residue-specific manner (Fig. 2). Glucocorticoids are shown to suppress selected components of the SASP (61). However, HDAC2-dependent steroid resistance occurs in patients with COPD. Hence, it is possible that steroids may not be able to suppress SASP and thereby SIPS in patients with COPD.
Administration of CXCR2 inhibitor reduces lung mucus production and goblet cell hyperplasia in animal model of pulmonary inflammation (17, 117). Genetic ablation of HDAC2 renders these mice more susceptible to cigarette smoke-induced lung inflammation (2). However, it is unknown whether CXCR2 antagonist can prevent cigarette smoke-induced lung cellular senescence and subsequently the development of emphysema, particularly in HDAC2 knockout-susceptible mice, although NF-κB inhibitor has no effect on premature senescence and airspace enlargement in mice (139).
HDAC Complex in DDR and SIPS of Lung Stem/Progenitor Cells
The function of HDAC2 relies on the formation of its corepressor complexes along with HDAC1. Deletion of NuRD complexes including HDAC1 is associated with premature aging and DDR, although ablation of mSin3A or mSin3B does not result in cellular senescence (24, 40, 82, 98). In light of the regulation of cigarette smoke on the interaction of HDAC2 with mSin3, NuRD/Mi2, and HDAC1 (3), it is possible that HDAC complexes are also involved in cigarette smoke-mediated SASP gene transcription, DDR, and SIPS.
Recent studies have demonstrated that HDAC/mSin3A complex regulates the developmental genes, such as Sox-2, Wnt/β-catenin, and bone morphogenetic protein, which are important for branching morphogenesis and epithelial cell differentiation in lungs (10, 39, 43, 53, 85). Apart from lung development, Sox-2 also regulates the maintenance and differentiation of lung progenitor cells, such as Basal and Clara cells, which are senesced in lung of cigarette smoke-exposed mice and patients with COPD (7, 100, 119, 139). Indeed, human lungs contain identifiable stem cells, which are self-renewing, clonogenic, and multipotent (51). Therefore, the study on the regulation of HDAC2 and its complexes in lung development and lung stem cells as well as progenitor cells will unravel the molecular mechanisms and susceptible factors involved in the pathogenesis of COPD. Furthermore, this will provide the potential to repair and regenerate lung tissue in this disease.
Translational Impact
HDAC2 is reduced in lungs of patients with COPD. HDAC2 reduction is associated with inflammation, steroid resistance, and DNA damage as well as lung cellular senescence. A variety of pharmacological and dietary compounds/molecules, such as theophylline, sulforaphane nortriptyline, baicalin, quercetin, erythromycin, and curcumin, are shown to target HDAC2 to reverse steroid resistance in COPD. However, all of the above agents have off-target effects. Hence, the development of a specific activator to activate HDAC2 or specific modulator to reduce HDAC2 posttranslational modification and degradation is urgently required to restore steroid efficacy in inhibiting chronic inflammation and premature cellular senescence in patients with COPD.
Conclusions and Future Directions
HDAC2 is an important deacetylase in regulating steroid function to inhibit inflammatory response in lungs. Recent studies have demonstrated a novel role of HDAC2 in regulating DNA damage/repair, premature senescence, and SASP. We and others have shown that cigarette smoke reduces the level and activity of HDAC2 in cells in vitro and in mouse lung in vivo, which may contribute to the sustained inflammation, DNA damage, and subsequently cellular senescence in lungs of patients with COPD. However, the roles of DNA damage and cellular senescence as well as their regulation by HDAC2 in the pathogenesis of chronic pulmonary diseases with lung inflammaging are unclear. Specifically, there are obviously multiple questions that remain unanswered. For example, it is unclear whether HDAC2 has a cell-specific effect in terms of steroid resistance (in macrophages, epithelial cells, and fibroblasts) as well as cigarette smoke/oxidants-induced DNA damage and cellular senescence (in lung epithelial cells and fibroblasts). Does HDAC2 protect telomere shortening, which is an important contributing factor in inducing replicative senescence that is observed in COPD (6)? Moreover, it remains to be known whether the clearance of senesced cells (e.g., p16-positive cells) in HDAC2-deficient lungs using recently developed p16-INK strategy delays or halts the progression of emphysema (9, 58, 147). Furthermore, it is not known which cells in the lung are the primary target for reduced HDAC2-mediated lung cellular senescence. Understanding the above aspects would unravel the role of DDR and premature senescence as well as HDAC2 regulation in the pathogenesis of COPD and provide a novel avenue to intervene this disease. COPD has been shown to increase the risk for developing lung cancer (143). Overexpression of HDAC2 confers oncogenic potential to human lung cancer cells (49). Thus further study is required to resolve this controversy by differentiating HDAC2 regulation and its dependent epigenetic changes in senescence during the development of COPD and lung cancer. This will provide translational HDAC2-based therapeutic approaches in lung inflammaging and premature senescence.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Y. and I.R. prepared figures; H.Y. and I.R. drafted manuscript; H.Y. and I.R. approved final version of manuscript; I.R. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH 1R01HL085613, 1R01HL097751, 1R01HL092842, and NIEHS Environmental Health Science Center grant P30-ES01247.
REFERENCES
- 1. Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Adenuga D, Caito S, Yao H, Sundar IK, Hwang JW, Chung S, Rahman I. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem Biophys Res Commun 403: 452–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adenuga D, Rahman I. Protein kinase CK2-mediated phosphorylation of HDAC2 regulates co-repressor formation, deacetylase activity and acetylation of HDAC2 by cigarette smoke and aldehydes. Arch Biochem Biophys 498: 62–73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol 40: 464–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci USA 104: 19482–19487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, Tuder RM, Armanios M. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 184: 904–912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 596–601, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Aoshiba K, Zhou F, Tsuji T, Nagai A. Dna damage as a molecular link in the pathogensis of copd in smokers. Eur Respir J 39: 1368–1376, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baltus GA, Kowalski MP, Tutter AV, Kadam S. A positive regulatory role for the mSin3A-HDAC complex in pluripotency through Nanog and Sox2. J Biol Chem 284: 6998–7006, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke—an aging accelerator? Exp Gerontol 42: 160–165, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127: 1361–1373, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436: 660–665, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics 74: 2313–2323, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, Barnes PJ, Papi A. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax 66: 521–527, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Ceylan E, Kocyigit A, Gencer M, Aksoy N, Selek S. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir Med 100: 1270–1276, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Chapman RW, Phillips JE, Hipkin RW, Curran AK, Lundell D, Fine JS. CXCR2 antagonists for the treatment of pulmonary disease. Pharmacol Ther 121: 55–68, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Charron CE, Chou PC, Coutts DJ, Kumar V, To M, Akashi K, Pinhu L, Griffiths M, Adcock IM, Barnes PJ, Ito K. Hypoxia-inducible factor 1alpha induces corticosteroid-insensitive inflammation via reduction of histone deacetylase-2 transcription. J Biol Chem 284: 36047–36054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen CS, Wang YC, Yang HC, Huang PH, Kulp SK, Yang CC, Lu YS, Matsuyama S, Chen CY. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res 67: 5318–5327, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Huang P, Ai W, Li X, Guo W, Zhang J, Yang J. Histone deacetylase activity is decreased in peripheral blood monocytes in patients with COPD. J Inflamm 9: 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Wang H, Yoon SO, Xu X, Hottiger MO, Svaren J, Nave KA, Kim HA, Olson EN, Lu QR. HDAC-mediated deacetylation of NF-kappaB is critical for Schwann cell myelination. Nat Neurosci 14: 437–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, Ragone G, Pescatori M, Zaccagnini G, Antonini A, Minetti G, Martelli F, Piaggio G, Gallinari P, Steinkuhler C, Clementi E, Dell'Aversana C, Altucci L, Mai A, Capogrossi MC, Puri PL, Gaetano C. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc Natl Acad Sci USA 105: 19183–19187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med 200: 689–695, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev 19: 1581–1595, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130: 1083–1094, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Deslee G, Adair-Kirk TL, Betsuyaku T, Woods JC, Moore CH, Gierada DS, Conradi SH, Atkinson JJ, Toennies HM, Battaile JT, Kobayashi DK, Patterson GA, Holtzman MJ, Pierce RA. Cigarette smoke induces nucleic-acid oxidation in lung fibroblasts. Am J Respir Cell Mol Biol 43: 576–584, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deslee G, Woods JC, Moore C, Conradi SH, Gierada DS, Atkinson JJ, Battaile JT, Liu L, Patterson GA, Adair-Kirk TL, Holtzman MJ, Pierce RA. Oxidative damage to nucleic acids in severe emphysema. Chest 135: 965–974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Gazzar M, Yoza BK, Hu JY, Cousart SL, McCall CE. Epigenetic silencing of tumor necrosis factor alpha during endotoxin tolerance. J Biol Chem 282: 26857–26864, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Faucher D, Wellinger RJ. Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway. PLoS Genet 6: e1001082, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fnu S, Williamson EA, De Haro LP, Brenneman M, Wray J, Shaheen M, Radhakrishnan K, Lee SH, Nickoloff JA, Hromas R. Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proc Natl Acad Sci USA 108: 540–545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ford PA, Durham AL, Russell RE, Gordon F, Adcock IM, Barnes PJ. Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest 137: 1338–1344, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447: 972–978, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908: 244–254, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16: 238–246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS One 2: e763, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 6: 570–572, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol 284: L533–L540, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 317: 296–309, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Grandinetti KB, Jelinic P, DiMauro T, Pellegrino J, Fernandez Rodriguez R, Finnerty PM, Ruoff R, Bardeesy N, Logan SK, David G. Sin3B expression is required for cellular senescence and is up-regulated upon oncogenic stress. Cancer Res 69: 6430–6437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guillemette B, Drogaris P, Lin HH, Armstrong H, Hiragami-Hamada K, Imhof A, Bonneil E, Thibault P, Verreault A, Festenstein RJ. H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation. PLoS Genet 7: e1001354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC, Randell SH, Stripp BR. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J Cell Sci 125: 932–942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem 280: 40450–40464, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Huang PH, Chen CH, Chou CC, Sargeant AM, Kulp SK, Teng CM, Byrd JC, Chen CS. Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol 79: 197–206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huyen Y, Zgheib O, Ditullio RA, Jr, Gorgoulis VG, Zacharatos P, Petty TJ, Sheston EA, Mellert HS, Stavridi ES, Halazonetis TD. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432: 406–411, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 203: 7–13, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ, Xie HJ, Chang YG, Kim MG, Park H, Lee JY, Nam SW. HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J Cell Biochem 113: 2167–2177, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 133: 85–94, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med 364: 1795–1806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Karrasch S, Holz O, Jorres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med 102: 1215–1230, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Kim DW, Lassar AB. Smad-dependent recruitment of a histone deacetylase/Sin3A complex modulates the bone morphogenetic protein-dependent transcriptional repressor activity of Nkx3.2. Mol Cell Biol 23: 8704–8717, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kirkham PA, Caramori G, Casolari P, Papi AA, Edwards M, Shamji B, Triantaphyllopoulos K, Hussain F, Pinart M, Khan Y, Heinemann L, Stevens L, Yeadon M, Barnes PJ, Chung KF, Adcock IM. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184: 796–802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol 298: L784–L792, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Koprinarova M, Botev P, Russev G. Histone deacetylase inhibitor sodium butyrate enhances cellular radiosensitivity by inhibiting both DNA nonhomologous end joining and homologous recombination. DNA Repair (Amst) 10: 970–977, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Kramer OH. HDAC2: a critical factor in health and disease. Trends Pharmacol Sci 30: 647–655, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, Zhou Z. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc Natl Acad Sci USA 108: 12325–12330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, Desprez PY, Hughes RE, Campisi J. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 11: 569–578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lambers C, Hacker S, Posch M, Hoetzenecker K, Pollreisz A, Lichtenauer M, Klepetko W, Ankersmit HJ. T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 155: 466–475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Latham JA, Dent SY. Cross-regulation of histone modifications. Nat Struct Mol Biol 14: 1017–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 296: L57–L70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee J, Sandford A, Man P, Sin DD. Is the aging process accelerated in chronic obstructive pulmonary disease? Curr Opin Pulm Med 17: 90–97, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Lee MG, Wynder C, Bochar DA, Hakimi MA, Cooch N, Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol Cell Biol 26: 6395–6402, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li L, Wu J, Liu B, Sun J, Gong W, Lv Y, Zhang H, Luo Q, Wu X, Dong J. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int Immunopharmacol 13: 15–22, 2012 [DOI] [PubMed] [Google Scholar]
- 68. Li M, Zhong X, He Z, Wen M, Li J, Peng X, Liu G, Deng J, Zhang J, Bai J. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. Int Immunopharmacol 12: 643–650, 2012 [DOI] [PubMed] [Google Scholar]
- 69. Li XN, Shu Q, Su JM, Perlaky L, Blaney SM, Lau CC. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther 4: 1912–1922, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, Rao M, Meltzer PA, Schrump DS. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 29: 3650–3664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH, Rahman I. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res 69: 371–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma P, Pan H, Montgomery RL, Olson EN, Schultz RM. Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc Natl Acad Sci USA 109: E481–E489, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 37: 819–823, 2009 [DOI] [PubMed] [Google Scholar]
- 74. MacNee W. Aging, inflammation, emphysema. Am J Respir Crit Care Med 184: 1327–1329, 2011 [DOI] [PubMed] [Google Scholar]
- 75. Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, Barnes P, Biswal S. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest 121: 4289–4302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76. Marchion DC, Bicaku E, Turner JG, Schmitt ML, Morelli DR, Munster PN. HDAC2 regulates chromatin plasticity and enhances DNA vulnerability. Mol Cancer Ther 8: 794–801, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Marinova Z, Leng Y, Leeds P, Chuang DM. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology 60: 1109–1115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, Ito K, Adcock IM, Kirkham PA, Papi A. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med 179: 542–548, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 31: 633–642, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Meja KK, Rajendrasozhan S, Adenuga D, Biswas SK, Sundar IK, Spooner G, Marwick JA, Chakravarty P, Fletcher D, Whittaker P, Megson IL, Kirkham PA, Rahman I. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am J Respir Cell Mol Biol 39: 312–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mercado N, To Y, Ito K, Barnes PJ. Nortriptyline reverses corticosteroid insensitivity by inhibition of phosphoinositide-3-kinase-delta. J Pharmacol Exp Ther 337: 465–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol 17: 1144–1151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mizuno S, Yasuo M, Bogaard HJ, Kraskauskas D, Natarajan R, Voelkel NF. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol 300: L402–L413, 2011 [DOI] [PubMed] [Google Scholar]
- 84. Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 18: 1897–1899, 2004 [DOI] [PubMed] [Google Scholar]
- 85. Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18: 8–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, Krug N, Nakashima M, Branscheid D, Magnussen H, Jorres RA, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 7: 32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292: 110–113, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455: 411–415, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Nottke A, Colaiacovo MP, Shi Y. Developmental roles of the histone lysine demethylases. Development 136: 879–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nunez B, Sauleda J, Anto JM, Julia MR, Orozco M, Monso E, Noguera A, Gomez FP, Garcia-Aymerich J, Agusti A. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183: 1025–1031, 2011 [DOI] [PubMed] [Google Scholar]
- 91. Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 35: 681–688, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nyunoya T, Monick MM, Klingelhutz AL, Glaser H, Cagley JR, Brown CO, Matsumoto E, Aykin-Burns N, Spitz DR, Oshima J, Hunninghake GW. Cigarette smoke induces cellular senescence via Werner's syndrome protein down-regulation. Am J Respir Crit Care Med 179: 279–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ogiwara H, Ui A, Otsuka A, Satoh H, Yokomi I, Nakajima S, Yasui A, Yokota J, Kohno T. Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 30: 2135–2146, 2011 [DOI] [PubMed] [Google Scholar]
- 94. Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, Bille K, Robert C, Bressac-de Paillerets B, Hofman P, Rocchi S, Peyron JF, Lacour JP, Ballotti R, Bertolotto C. Senescent cells develop a PARP-1 and nuclear factor-kappaB-associated secretome (PNAS). Genes Dev 25: 1245–1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Osoata GO, Yamamura S, Ito M, Vuppusetty C, Adcock IM, Barnes PJ, Ito K. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun 384: 366–371, 2009 [DOI] [PubMed] [Google Scholar]
- 96. Palomera-Sanchez Z, Zurita M. Open, repair and close again: chromatin dynamics and the response to UV-induced DNA damage. DNA Repair (Amst) 10: 119–125, 2011 [DOI] [PubMed] [Google Scholar]
- 97. Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis 6: 209–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pegoraro G, Kubben N, Wickert U, Gohler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 11: 1261–1267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Perng DW, Su KC, Chou KT, Wu YC, Chen CS, Hsiao YH, Tseng CM, Chen YH, Hsueh TY, Lee YC. Long-acting beta(2) agonists and corticosteroids restore the reduction of histone deacetylase activity and inhibit H(2)O(2)-induced mediator release from alveolar macrophages. Pulm Pharmacol Ther 25: 312–318, 2012 [DOI] [PubMed] [Google Scholar]
- 100. Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136: 1899–1907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 490–495, 2002 [DOI] [PubMed] [Google Scholar]
- 102. Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124: 68–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rossetto D, Truman AW, Kron SJ, Cote J. Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res 16: 4543–4552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119: 603–614, 2004 [DOI] [PubMed] [Google Scholar]
- 107. Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem 277: 39195–39201, 2002 [DOI] [PubMed] [Google Scholar]
- 108. Sato T, Seyama K, Sato Y, Mori H, Souma S, Akiyoshi T, Kodama Y, Mori T, Goto S, Takahashi K, Fukuchi Y, Maruyama N, Ishigami A. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am J Respir Crit Care Med 174: 530–537, 2006 [DOI] [PubMed] [Google Scholar]
- 109. Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, Le Corvoisier P, Rideau D, Boczkowski J, Dubois-Rande JL, Chouaid C, Adnot S. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, Nikam S, Roth M, Sydykov A, Medebach T, Klepetko W, Jaksch P, Dumitrascu R, Garn H, Voswinckel R, Kostin S, Seeger W, Schermuly RT, Grimminger F, Ghofrani HA, Weissmann N. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 147: 293–305, 2011 [DOI] [PubMed] [Google Scholar]
- 111. Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem 93: 57–67, 2004 [DOI] [PubMed] [Google Scholar]
- 112. Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro OM, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol 22: 26–33, 2000 [DOI] [PubMed] [Google Scholar]
- 113. Sugiura H, Kawabata H, Ichikawa T, Koarai A, Yanagisawa S, Kikuchi T, Minakata Y, Matsunaga K, Nakanishi M, Hirano T, Akamatsu K, Furukawa K, Ichinose M. Inhibitory effects of theophylline on the peroxynitrite-augmented release of matrix metalloproteinases by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 302: L764–L774, 2012 [DOI] [PubMed] [Google Scholar]
- 114. Sun X, Li Q, Gong Y, Ren L, Wan H, Deng W. Low-dose theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammation. Can J Physiol Pharmacol 90: 895–902, 2012 [DOI] [PubMed] [Google Scholar]
- 115. Sundar IK, Hwang JW, Yao H, Rahman I. Profiling of epigenetic chromatin modification genes and susceptibility to chronic lung disease by cigarette smoke. Am J Respir Crit Care Med 185: A3480, 2012 [Google Scholar]
- 116. Szulakowski P, Crowther AJ, Jimenez LA, Donaldson K, Mayer R, Leonard TB, MacNee W, Drost EM. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 41–50, 2006 [DOI] [PubMed] [Google Scholar]
- 117. Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 289: L322–L328, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 122: 2601–2612, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One 4: e8248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tsai SC, Seto E. Regulation of histone deacetylase 2 by protein kinase CK2. J Biol Chem 277: 31826–31833, 2002 [DOI] [PubMed] [Google Scholar]
- 121. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 80: 59–70, 2010 [DOI] [PubMed] [Google Scholar]
- 122. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174: 886–893, 2006 [DOI] [PubMed] [Google Scholar]
- 123. Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol 31: 643–649, 2004 [DOI] [PubMed] [Google Scholar]
- 124. Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc 9: 62–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vashisht Gopal YN, Arora TS, Van Dyke MW. Tumour necrosis factor-alpha depletes histone deacetylase 1 protein through IKK2. EMBO Rep 7: 291–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vaute O, Nicolas E, Vandel L, Trouche D. Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Res 30: 475–481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vempati RK, Jayani RS, Notani D, Sengupta A, Galande S, Haldar D. p300-mediated acetylation of histone H3 lysine 56 functions in DNA damage response in mammals. J Biol Chem 285: 28553–28564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol 13: 115–126, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wagner M, Brosch G, Zwerschke W, Seto E, Loidl P, Jansen-Durr P. Histone deacetylases in replicative senescence: evidence for a senescence-specific form of HDAC-2. FEBS Lett 499: 101–106, 2001 [DOI] [PubMed] [Google Scholar]
- 131. Wakeman TP, Wang Q, Feng J, Wang XF. Bat3 facilitates H3K79 dimethylation by DOT1L and promotes DNA damage-induced 53BP1 foci at G1/G2 cell-cycle phases. EMBO J 31: 2169–2181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Whyte WA, Bilodeau S, Orlando DA, Hoke HA, Frampton GM, Foster CT, Cowley SM, Young RA. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature 482: 221–225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wilting RH, Yanover E, Heideman MR, Jacobs H, Horner J, van der Torre J, DePinho RA, Dannenberg JH. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J 29: 2586–2597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Winkler AR, Nocka KN, Williams CM. Smoke exposure of human macrophages reduces HDAC3 activity, resulting in enhanced inflammatory cytokine production. Pulm Pharmacol Ther 25: 286–292, 2012 [DOI] [PubMed] [Google Scholar]
- 135. Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair (Amst) 7: 1077–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 136. Yang L, Mei Q, Zielinska-Kwiatkowska A, Matsui Y, Blackburn ML, Benedetti D, Krumm AA, Taborsky GJ, Jr, Chansky HA. An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription co-repressors mSin3A/B. Biochem J 369: 651–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006 [DOI] [PubMed] [Google Scholar]
- 138. Yang WM, Inouye C, Zeng Y, Bearss D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA 93: 12845–12850, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yao H, Chung S, Sundar IK, Rahman I. Cigarette smoke extract treatment caused a residue-specific histone methylation and its cross-talk with histone acetylation in human lung epithelial cells. Am J Respir Crit Care Med 185: A3474, 2012 [Google Scholar]
- 141. Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 142. Yao H, Hwang JW, Moscat J, Diaz-Meco MT, Leitges M, Kishore N, Li X, Rahman I. Protein kinase C zeta mediates cigarette smoke/aldehyde- and lipopolysaccharide-induced lung inflammation and histone modifications. J Biol Chem 285: 5405–5416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol 9: 375–383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yuan J, Pu M, Zhang Z, Lou Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle 8: 1747–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhou BO, Wang SS, Zhang Y, Fu XH, Dang W, Lenzmeier BA, Zhou JQ. Histone H4 lysine 12 acetylation regulates telomeric heterochromatin plasticity in Saccharomyces cerevisiae. PLoS Genet 7: e1001272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhou Q, Wang Y, Yang L, Chen P, Dong X, Xie L. Histone deacetylase inhibitors blocked activation and caused senescence of corneal stromal cells. Mol Vis 14: 2556–2565, 2008 [PMC free article] [PubMed] [Google Scholar]
- 147. Zhou R, Han L, Li G, Tong T. Senescence delay and repression of p16INK4a by Lsh via recruitment of histone deacetylases in human diploid fibroblasts. Nucleic Acids Res 37: 5183–5196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhu L, Li H, Tang J, Zhu J, Zhang Y. Hyperoxia arrests alveolar development through suppression of histone deacetylases in neonatal rats. Pediatr Pulmonol 47: 264–274, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.