Abstract
The relation between the progression of chronic obstructive pulmonary disease (COPD) and exacerbations is unclear. Currently, no animal model of acute exacerbation of COPD (AECOPD) exists. The objectives of this study were to evaluate the effects of mechanical forces induced by deep inspirations (DIs) on short-term deterioration of lung structure and function to mimic AECOPD. At 2, 7, or 21 days after treatment with elastase, mice were ventilated with or without DIs (35 cmH2O airway pressure for 3 s, 2 times/min) for 1 h. Functional residual capacity (FRC) was measured with body plethysmography, and respiratory compliance, resistance, and hysteresivity were obtained via forced oscillations. From hematoxylin and eosin-stained sections, equivalent airspace diameters (D), alveolar wall thickness (Wt), number of septal ruptures (Nsr), and attachment density (Ad) around airways were determined. FRC, compliance, and hysteresivity statistically significantly increased with time, and both increased due to DIs. Interestingly, DIs also had an effect on FRC, compliance, resistance, and hysteresivity in control mice. The development of emphysema statistically significantly increased D and Wt in time, and the DIs caused subtle differences in D. At 21 days, the application of DIs changed the distribution of D, increased Wt and Nsr, and decreased Ad. These results suggest that once a critical remodeling of the parenchyma has been reached, acute mechanical forces lead to irreversible changes in structure and function, mimicking COPD exacerbations. Thus, the acute application of DIs in mice with emphysema may serve as a useful model of AECOPD.
Keywords: AECOPD, mechanical stress, exacerbation, irreversibility
chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema, is a slowly developing and progressive disease with episodes of acute exacerbation (AECOPD). According to the latest Global Initiative for Chronic Obstructive Lung Disease (GOLD) Summary (8a), AECOPD is an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication. Most AECOPD is triggered by bacterial (36) or viral (35) infections, by pollutants (33), or by cold temperature (4). Moreover, severe exacerbation-related hospitalization is associated with an increased risk of mortality, even after discharge (8), with a heavy health care burden worldwide.
While the frequency of AECOPD was shown to correlate with lung function decline (5, 34), irreversible structural changes behind the functional decline following AECOPD have been identified only recently. Tanabe et al. (38) reported that changes in the distribution of low-attenuation areas on computerized tomography images of COPD patients were consistent with the coalescence of neighboring low-attenuation areas due to alveolar wall destruction. Since tissue destruction is the primary mechanism that drives airspace enlargement, AECOPD should also contribute to the progression of COPD. However, a detailed understanding of how exacerbation results in irreversible functional changes is lacking. Consequently, there is an urgent need for novel animal models of AECOPD.
To our knowledge, the only study that proposed an animal model of AECOPD was by Wang et al. (40). To mimic AECOPD, the authors combined elastase-induced emphysema with viral infection. Unfortunately, they did not compare the structural or functional changes in their model with those due only to elastase. Hence, it remains unclear to what extent the viral infection exaggerated tissue destruction. Nevertheless, they did report that the animals had respiratory distress with coughing similar to human AECOPD. One should note that the main symptoms of AECOPD are always accompanied by increased mechanical stresses in the lung. Indeed, dyspnoe is characterized by hyperinflation (29), cough is preceded by a deep inspiration and followed by forced expiratory efforts with compressive and expulsive phases (22), and sputum production leads to cough. Since mechanical forces have been shown to be the governing mechanism of tissue destruction in emphysema (19, 26, 37), we hypothesized that while viral or bacterial infections are the triggers, mechanical forces during the exacerbation phase play a key role in tissue rupture leading to irreversible changes in lung structure and function. To avoid the confounding effects of viral and bacterial infections, we tested this hypothesis by superimposing deep inspirations (DIs) during mechanical ventilation on the remodeled parenchyma at different stages of emphysema. If this hypothesis is supported by data, then our experiments will also provide a novel model of AECOPD.
MATERIALS AND METHODS
Animal treatment and experimental groups.
The protocol was approved by the Animal Care and Use Committee of Boston University. Fifty-nine male C57BL/6 mice were slightly anesthetized with isofluorane vapor (3%) in oxygen (1.5 l/min). The animals were then suspended by the upper incisors using a rubber band on a 60-degree inclined platform and treated with porcine pancreatic elastase (PPE; Elastin Products, Owensville, MO) (6 IU) via aspiration (20). After treatment, the animals were allowed to recover and maintained on a 12:12-h light-dark cycle. There were no deaths associated with the PPE treatment. Additionally, 15 animals were intact controls.
At 2, 7, or 21 days after the treatment, animals were anaesthetized with intraperitoneally administered pentobarbital sodium (initial dose: 75 mg/kg, supporting dose: 10–20 mg/kg as needed) and received tracheostomy. The mice were then placed into a custom-built body plethysmograph and mechanically ventilated with FlexiVent (SCIREQ Scientific Respiratory Equipment, Montreal, Canada) (ventilation = 8 ml/kg, 240 br/min) at 3 cmH2O positive end expiratory pressure (PEEP) for 1 h with or without DIs defined as an inflation up to 35 cmH2O airway pressure for 3 s delivered two times per minute. The mice were distributed randomly into the following experimental groups: control (0 days DI) [n = 7; weight: 25.7 ± 0.9 g (SD)], control (0 days no-DI) (n = 8; 24.5 ± 1.3 g), 2 days treated DI (n = 8; 23.3 ± 1.0 g), 2 days treated no-DI (n = 8; 23.4 ± 1.2 g), 1 wk treated DI (n = 7; 25.3 ± 1.1 g), 1 wk treated no-DI (n = 8; 25.5 g ± 1.0 g), 3 wk treated DI (n = 7; 27.1 ± 1.5 g), 3 wk treated no-DI (n = 6; 27.6 ± 0.8 g).
At the end of the ventilation protocol, the pulmonary circulation was flushed through the right ventricle with PBS. After sacrificing the mice, the lungs and heart were resected en block and fixed at 20 cmH2O transpulmonary pressure with 10% buffered formalin.
Physiological measurements.
Functional residual capacity (FRC) was measured with a body plethysmograph (7) at 0 cmH2O PEEP before and after the 1-h ventilation (17). At end expiration, the FlexiVent was stopped, and the tracheal cannula was occluded. During spontaneous breathing efforts for about 6 s, box pressure and tracheal pressure were measured with a Validyne MP-45 (±2 cmH2O) pressure sensor (Validyne, Northridge, CA) and a miniature pressure transducer (model 8507C-2; Endevco, San Juan Capistrano, CA), respectively. FRC was calculated from the box pressure, and tracheal pressure values were corrected with the thermal characteristics of the plethysmograph (17).
Low-frequency respiratory input impedance (2–19 Hz) was measured at 3 cmH2O PEEP at the beginning and at the end of the 1-h ventilation with or without DI, using the optimal ventilation waveform (24). Respiratory compliance, Newtonian resistance (which is a surrogate of airway resistance), and hysteresivity were calculated by fitting the constant phase model to the impedance (12).
Histology.
Sections were stained with hematoxylin and eosin. Randomly selected regions were imaged and segmented using custom-made software. The equivalent diameter of alveolar airspaces (D) was computed as the diameter of a circle with the same area as the airspace. For each mouse, the area-weighted average diameter (D2) was calculated from the set of D values (31). Since D2 has been shown to be sensitive to heterogeneities and able to detect small changes in structure compared with D or mean linear intercept (31), we also characterized the airspace structure with this parameter. On the hematoxylin and eosin-stained sections, the alveolar septal wall thickness (Wt) was measured, and attachment density (Ad) was determined by dividing the number of septal walls attached to a small airway with the outer perimeter of the airway wall. Collagen and elastin fibers were visualized with modified Masson's trichrome and Verhoeff's methods, respectively (10). The total number of end tips as a measure of septal ruptures (Nsr) per image per tissue fraction was measured for the 21 days DI and 21 days no-DI groups on elastin stained samples.
Statistical analysis.
The effects of DIs and time were analyzed with two-way ANOVA, while the effects of PPE treatment before ventilation were analyzed with one-way ANOVA. Where normality failed, log transformation was applied. The effect of DIs on Nsr was compared with Mann-Whitney's rank sum test. A P value < 0.05 was considered statistically significant.
RESULTS
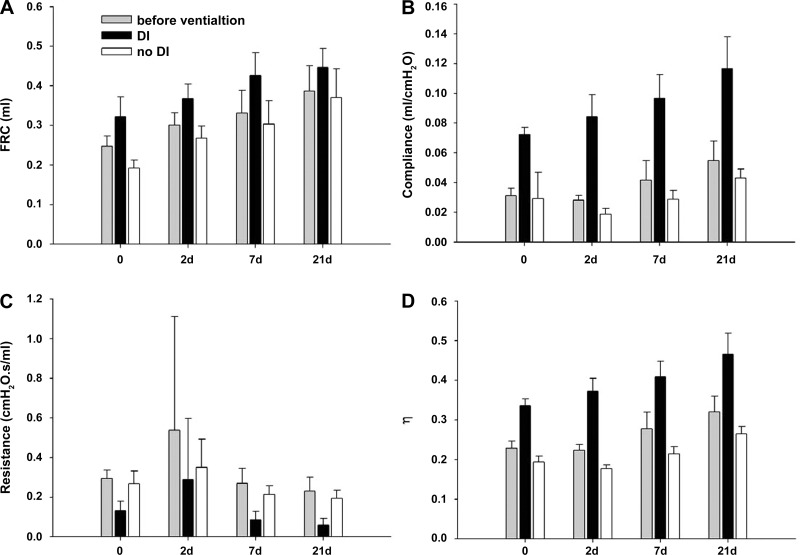
PPE significantly increased FRC before ventilation, with mean values of 0.25 ± 0.026 ml (SD) in the control mice (0 days), and 0.39 ± 0.064 ml in the PPE-treated 21 days groups (P < 0.001) (Fig. 1A). Independent of time, DIs increased FRC (P < 0.001). Following 1-h ventilation, FRC was 0.32 ± 0.05 ml and 0.19 ± 0.02 ml in the 0 days DI group and 0 days no-DI group, respectively. In the 21 days groups, FRC increased from 0.37 ± 0.07 ml in the no-DI group to 0.45 ± 0.05 in the DI group.
Fig. 1.
Effects of time and deep inspirations (DIs) on physiological variables. A: functional residual capacity (FRC). Before ventilation, FRC increased with time (21 vs. 0 and 2 days: P < 0.05; 7 vs. 0 days: P < 0.05; 2 vs. 0 days: P < 0.05) and DIs had a significant effect on FRC at each time point (P < 0.001). B: respiratory compliance. Before the ventilation, compliance increased with time (21 vs. 0 and 2 days, 7 vs. 2 days: P < 0.05), and DIs had a significant effect on compliance at each time point (P < 0.001). C: respiratory resistance. Before ventilation, resistance showed a decreasing trend with time (21 vs. 0 and 2 days: P < 0.05), and DIs had a significant effect on resistance (0 days: P = 0.003, 2 days: P = 0.03, 7 and 21 days: P < 0.001). D: hysteresivity (η). Before the ventilation, hysteresivity showed an increasing trend with time (21 vs. 0 days) and DIs had a significant effect at each time point (P < 0.001). Bars denote SDs.
PPE also increased compliance with time before ventilation (P < 0.001) with values of 0.031 ± 0.005 ml/cmH2O and 0.055 ± 0.013 ml/cmH2O in the 0 days and 21 days groups, respectively (Fig. 1B). Similar to FRC, a strong effect of DIs can be seen on compliance at each time point (P < 0.001).
The parameter resistance (Fig. 1C) decreased gradually with time reaching significance at 21 days (P = 0.021). The hysteresivity (Fig. 1D) showed significant differences between the DI and no-DI groups (P < 0.001) and increased with time (P < 0.05), except from 0 days to 2 days and from 7 days to 21 days.
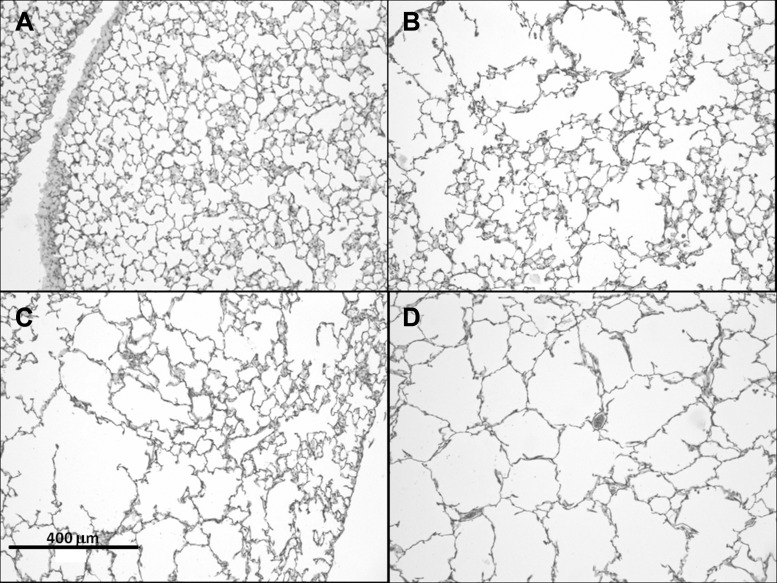
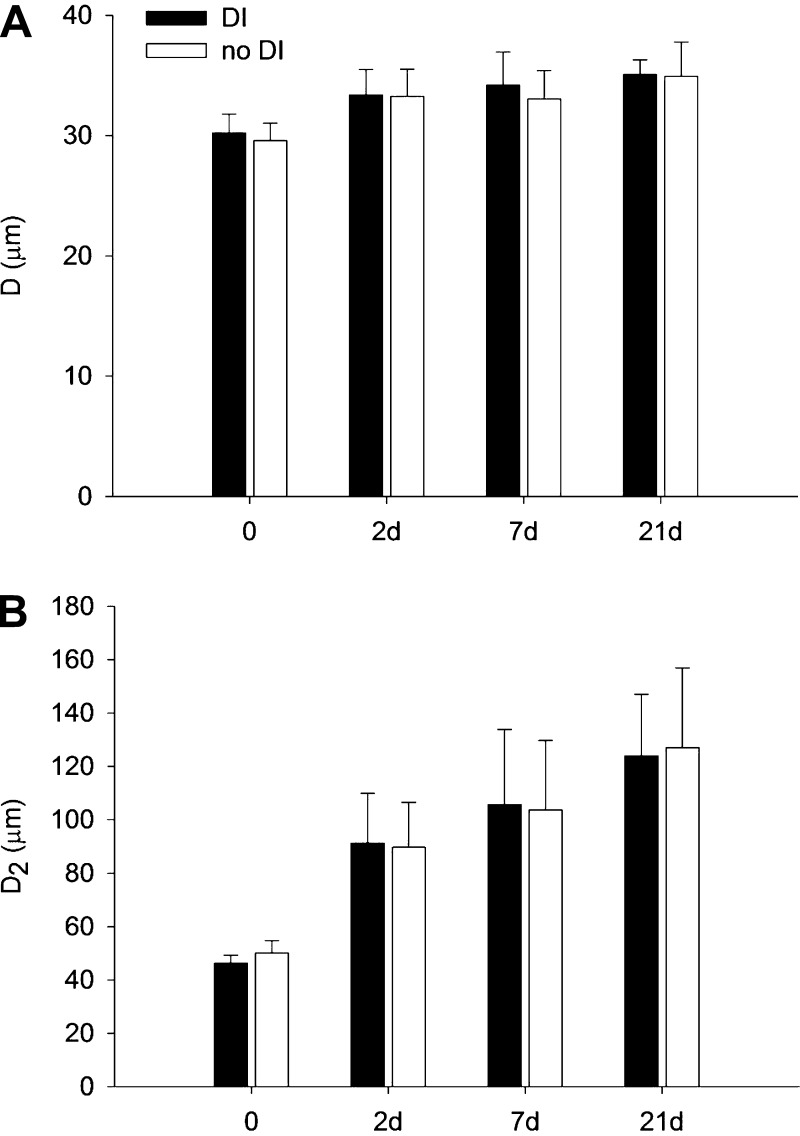
Figure 2 shows typical airspace structures in control and at each time point following treatment in the DI groups. The PPE treatment increased the mean airspace diameter (D) from 29.6 ± 1.4 μm in the control group to 34.9 ± 2.9 μm at 21 days after treatment (P < 0.001) (Fig. 3A). The D2 (Fig. 3B), which is sensitive to changes in both the mean and the heterogeneity of airspace sizes, showed much more prominent differences between the groups in time (P < 0.001). However, neither of these lumped structural indexes showed a difference between the DI and the no-DI groups at any time point.
Fig. 2.
Examples of hematoxylin and eosin-stained histological sections. A: control following 1-h ventilation with DIs; B–D: 2, 7, and 21 days, respectively, after treatment following 1-h ventilation with DIs.
Fig. 3.
Morphometric analysis of airspace sizes. A: mean and SD of equivalent airspace diameters (D), in each group. Porcine pancreatic elastase (PPE) had a significant effect on D (0 vs. 2, 7, and 21 days: P < 0.001). B: mean and SD of the area-weighted mean alveolar diameter (D2) in each group. PPE had a significant effect on D2 (0 vs. 2, 7, and 21 days: P < 0.001, 2 vs. 21 days: P = 0.01).
To explore the possibility that the DIs caused a more subtle structural change in some preferential airspace size, the alveoli were pooled from all animals and grouped into small, medium, and large airspaces according to their D values, and statistics were done within these size categories (Table 1). For the smallest airspaces, significant differences were found between the DI and no-DI groups at 0 days and 21 days (P < 0.001) as well as at 7 days (P = 0.003). For the medium-sized airspaces, there were differences at 21 days (P = 0.007) and 0 days (P = 0.015), whereas for the largest airspaces, D was different only at 0 days (P < 0.001). Even though the percent differences are small, it is interesting to note that, in the control animals, the no-DI group had a larger D, but in the treated animals, the DI groups always had a larger D.
Table 1.
Mean values of alveolar diameters binned into groups of small, medium, and large
| Small |
Medium |
Large |
||||
|---|---|---|---|---|---|---|
| DI | no DI | DI | no DI | DI | no DI | |
| 0 days | 17.9 | 18.2* | 41.7 | 41.7# | 92.3 | 98.0* |
| 2 days | 27.4 | 27.4 | 142.3 | 141.6 | 329.1 | 335.1 |
| 7 days | 28.1** | 27.6 | 147.0 | 147.0 | 339.9 | 353.0 |
| 21 days | 27.6* | 27.0 | 154.5*** | 149.3 | 342.0 | 360.2 |
Values of deep inspirations (DI) are in micrometers (μm). The corresponding bins in the untreated control animals (0 days) are: 5–30 μm, 30–80 μm, and 80 μm; and in the treated animals, they are: 5–100 μm, 100–300 μm, and 300 μm,
P < 0.001,
P = 0.003,
P = 0.007,
P = 0.015.
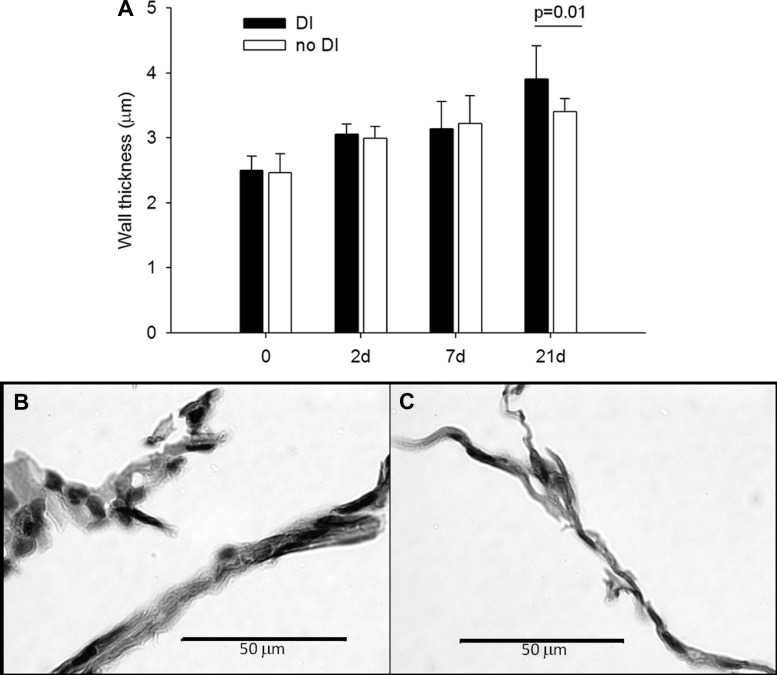
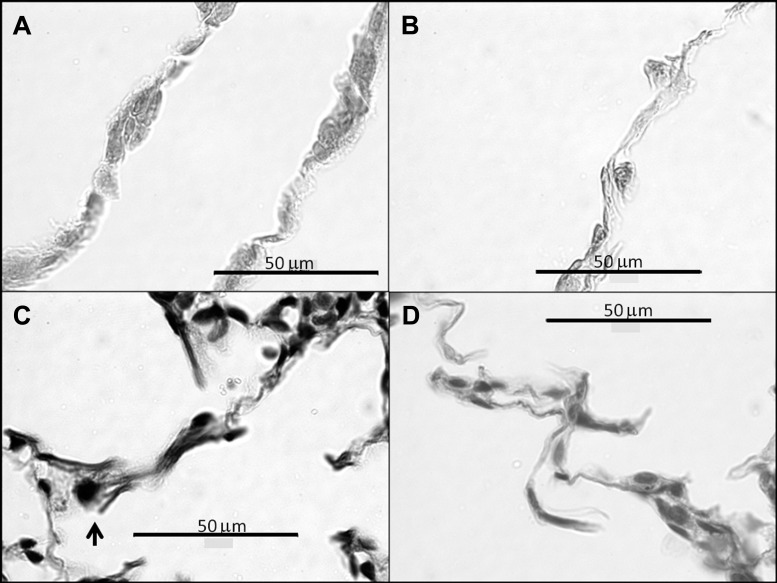
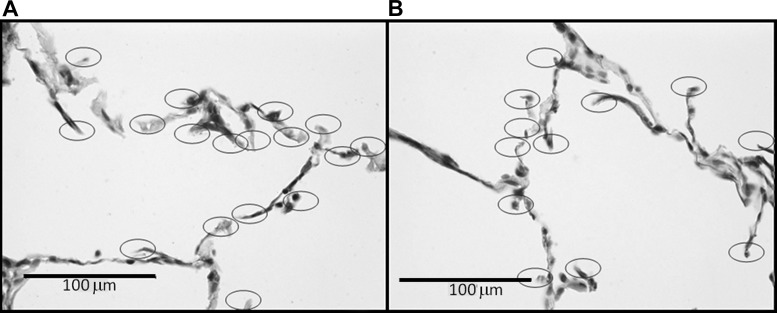
The septal Wt increased by 47% (Fig. 4) from 2.5 ± 0.3 μm at 0 days to 3.4 ± 0.2 μm at 21 days (P < 0.001). Moreover, at 21 days, the DIs induced a 13% elevation in Wt compared with the no-DI group (P = 0.01). Interestingly, there appears to be more cell nuclei in the thicker septal walls following the application of DIs (Fig. 4B). Figure 5 shows microstructural images of the septal walls with and without DIs. The increased Wt with DIs is apparent on both stainings. Furthermore, the elastin-stained images also suggest a slightly more disorganized microstructure within the septal walls. Figure 6 demonstrates the evaluation of septal wall ruptures at 21 days. Statistical analysis revealed that Nsr was significantly larger in the DI group compared with the no-DI group with values of 165 ± 63 and 134 ± 68 (P = 0.006), respectively. The perimeters of the airways in the DI and no-DI groups at 21 days for which Ad was evaluated were not different. However, compared with the no-DI group, Ad was 11% lower in the DI group (P = 0.026).
Fig. 4.
Morphometric analysis of wall thickness (Wt) of the airspaces. A: mean and SD of Wt as a function time and DI. PPE had a significant effect on Wt (0 vs. 2, 7, and 21 days; 21 vs. 2 and 7 days: P < 0.001). The DIs had a significant effect on Wt at 21 days (P < 0.01). B and C: microstructural evidences of increased Wt due to DIs 21 days after treatment. B: hematoxylin and eosin staining with DIs, C: hematoxylin and eosin staining with no-DI.
Fig. 5.
Representative images of stained histological sections. A: Masson's trichrome staining for collagen, 21 days DI, B: Masson's trichrome staining for collagen, 21 days no-DI, C: modified Verhoeff's staining showing increased Wt with stray elastin (arrow) that is a potential site of rupture, 21 days DI, D: modified Verhoeff's staining, 21 days no-DI.
Fig. 6.
Visualization of all alveolar septal end tips including possible alveolar ruptures due to DI on modified Verhoeff's stained sections. DI (A) and no-DI (B). Ellipses mark free septal ends.
DISCUSSION
Exacerbations constitute a significant component of the clinical manifestation of COPD (8a), becoming increasingly more frequent as the disease progresses (2). For example, in a large clinical study, patients with severe COPD characterized by the GOLD category IV, had a higher annual exacerbation rate (2.00) than those with more moderate COPD, having GOLD categories of III and II (1.34 and 0.85, respectively) (14). Other studies showed that patients with a forced expiratory volume in 1 s (FEV1) > 60% predicted had an exacerbation rate of 1.6 per year as opposed to patients with FEV1 between 59% and 40% and those with FEV1 < 40% predicted having annual exacerbation rates of 1.9 and 2.3, respectively (5, 30). Furthermore, patients who suffer from frequent exacerbations will likely continue to do so (9). Despite considerable effort, understanding of the irreversible nature of AECOPD is not complete.
In this study, we hypothesized that the increased mechanical forces that accompany an AECOPD event result in irreversible tissue deterioration, which in turn impairs function. Acute mechanical forces were delivered using DIs, defined as rapid inflation of the lung to 35 cmH2O airway pressure. However, peak esophageal pressure during cough can exceed 200 cmH2O (21); therefore, we consider our mechanical stimulation of the lung to be mild. Nevertheless, these acute increases in mechanical forces achieved by the frequent DIs did result in the following changes in structure and function: 1) large changes in the functional mechanical properties of the respiratory system such as FRC and compliance; and 2) noticeable structural changes in the parenchyma and airways occured only at 21 days after treatment. The former requires a careful analysis because the DIs also induce recruitments, whereas the latter suggests that in order for mechanical forces to be able to induce irreversible changes in lung structure, the ECM of the emphysematous lung should be sufficiently weakened.
The physiological variables (Fig. 1) including FRC, compliance, and hysteresivity increased with time due to the development of emphysema following PPE treatment as in previous studies (11, 16, 27). The resistance decreased with time as in the tight-skin mouse (15); however, this is in contrast to observed changes in airway resistance in human COPD patients (43). One possibility is that the lung volumes in the normal and treated mice were different, and the measurements were taken at the same PEEP and not at the same lung volume (11).
The DIs significantly increased FRC and compliance both in the control and treated mice. To interpret these results, we first note that anesthetized mice in the supine position easily derecruit large regions of the lung (1). A single DI recruits some airspace regions that, over time, tend to collapse again. Repeated DIs lead to a steady state with a higher FRC depending on the time constant of recollapse relative to the time between DIs. There are at least three ways that compliance could increase in our experiments. First, when fibers inside a wall rupture, the wall becomes softer. If such microruptures occur in many walls, compliance will go up. Second, if a wall ruptures, then there is one less “spring” in the lung resisting inflation, and hence, compliance goes up. Finally, large stretches may induce surfactant release by type II cells (42), which would result in a lower surface tension and compliance can go up again. When compliance increases, the chest wall pulls the lung outward, and FRC goes up too. Taken together, in control mice in which no structural alterations were observed, changes in compliance are due to recruitment and possibly surfactant secretion. In the treated lung, the increase in compliance can be due to mechanical failure as well as recruitment and surfactant secretion. This analysis then suggests that the immediate effect of DIs on FRC and compliance is complicated by recruitment and possibly other processes, and from FRC and compliance alone it is difficult to deduce whether the DIs have caused any irreversible change in the lung during the 1-h ventilation. Thus, a deeper understanding of the functional data requires a careful analysis of lung structure.
The progression of emphysema was accompanied by increases in both the mean alveolar airspace diameter (D) and its heterogeneity characterized by D2 (Fig. 3). Surprisingly, however, no effects of DIs could be detected on D2, a parameter highly sensitive to structural changes (31). The reason is that the subtle differences in structure due to the DIs could not be captured by a single number. Thus, in a more refined analysis, we combined D from all animals and grouped them into small, medium, and large size categories (Table 1). This analysis identified statistically significant differences between the no-DI and DI groups. Although the absolute differences were small, the statistical significances were very strong due to the large number (>10,000) of airspaces in the calculations.
In the control animals, DIs decreased the sizes of the airspaces in all three airspace size categories. This may be related to the findings of Namati et al. (28) who observed that alveoli became smaller at end inspiration and proposed that this was due to alveolar recruitment in the mouse lung, which means that at higher inflation pressures similar to those in our DI protocol, secondary (daughter) alveoli suddenly pop open and inflate via the primary (mother) alveoli through the pores of Kohn. In contrast, in the treated animals, DIs generated larger D in the two smallest size categories suggesting that at the same fixation pressure, the DIs definitely increased airspace diameters. These results then imply that the septal walls of some small- and medium-sized airspaces became softer or some septal walls actually ruptured during ventilation with DIs. A softer tissue in turn represents plastic deformation as a consequence of microruptures inside the walls induced by the mechanical stresses of the DIs.
The microstructural changes caused by the DIs were most pronounced at 21 days. Indeed, while septal Wt steadily increased with time (Fig. 4), the DIs caused a statistically significant increase only at 21 days. The increased thickness, which is a characteristic feature of severe AECOPD (1), is apparent on all three types of staining (Figs. 4 and 5). The hematoxylin and eosin staining also shows more cell nuclei (Fig. 4B), a sign of inflammation similar to the increased neutrophils in the airways of COPD patients following an exacerbation (32). Additionally, the elastin staining suggests a disorganized fiber network in the wall with possible microruptures (Fig. 5C). These findings are consistent with a sudden deterioration of lung structure that accompanies AECOPD. We also need to point out that the increase in Wt is accompanied by an increase in compliance in the DI group (Fig. 1B). Therefore, this is not an acute lung injury model in which compliance decreases. Also, the 1-h ventilation period is not enough for active cellular remodeling, hence the most likely reason for the wall thickening is capillary rupture leading to leakage and edema (41).
The fate of a septal wall in which microruptures do occur depends on several factors. Remodeling during the 1-h ventilation is not likely. However, depending on the magnitude of regional stresses, the wall can rupture. At 21 days, statistical analysis revealed that the total number of septal end-tips Nsr was significantly larger in the DI group compared with the no-DI group. Similar to Nsr, alveolar Ad around airways increased due to the DIs. In control animals, we did not see any evidence of ruptures, and hence the increase in FRC was caused by pure recruitment. On the other hand, at 21 days, the DI-induced increases in Nsr and Ad resulted in larger D (Table 1), and hence part of the increase in FRC and compliance must have resulted from ruptures. These changes in FRC and compliance represent irreversible decline in function following acute increase in mechanical forces on the parenchyma. Thus, these results provide evidence that our animal model does reproduce a number of features of AECOPD with irreversible changes in both structure and function. Furthermore, since we find evidence of Wt and fiber and septal wall rupture only at 21 days, there exists a threshold in ECM remodeling below which acute increase in mechanical forces do not seem to induce irreversible changes.
The progression of emphysema alters the architecture of the connective tissue in the septal walls as a result of collagen and elastin remodeling (23, 25, 39). As the ECM becomes progressively weaker, it is possible that DI-induced microruptures also occurred at earlier time points. For example, D increased for the smallest size category (Table 1) at 7 days. Such subtle changes in structure may not cause noticeable irreversible changes in function. However, when various ECM structures rupture, fragments of the corresponding molecules will be exposed. Both elastin (13) and proteoglycan (3) fragments are chemoattractant and hence the process of tissue breakdown by rupture can maintain and potentiate further inflammation, which is also characteristic of AECOPD (8a) and may contribute to the increased susceptibility of patients to infection-induced inflammation. If a sufficient number of structural elements of the wall mechanically fail, the entire wall also ruptures, which can influence the distribution of low attenuation areas on computerized tomography images (38) and become clinically observable. Since the parenchyma is always under tensile stress, when an alveolar wall ruptures the stress the wall carried just before failure is redistributed among the neighboring walls. Consequently, some neighbors will experience an increased stress that unfolds new binding sites for enzymes such as elastase and increases the unbinding and cleaving rate (18). These mechanisms in turn result in a higher probability for the wall to mechanically fail. Thus, a single rupture can lead to a cascade of ruptures and serve as a positive feedback to potentiate further breakdown (37). This mechanism might help explain why previous AECOPDs increase the probability of future AECOPDs leading to the emergence of the frequent-exacerbation phenotype (14).
While our model constitutes a mouse model of AECOPD in terms of irreversible changes in structure and function, we acknowledge the limitations of the study. First, despite the fact that our animal model does not involve infections, many studies have confirmed that the mechanism that triggers AECOPD in humans is the viral or bacterial infection (33). Because of the differences in the anatomy and physiology of the mouse and human lungs, the contribution of atelectasis and recruitment to human AECOPD remains uncertain. The magnitude and spatial distribution of mechanical stresses in the mouse are different from those during human AECOPD. Additionally, the PPE treatment does not reproduce the immune response due to cigarette smoke, and the characteristics of ECM remodeling is different in the human case (23, 39). Hence the threshold in ECM remodeling that is necessary for mechanical forces to induce irreversible changes in function in mice, may be different in human COPD. Nevertheless, our animal model is flexible: various knockout or transgenic mouse models can be used to mimic the background remodeling of the ECM, and future studies could also combine DIs with infection models.
In summary, we have shown that exacerbation-like irreversible changes in structure and function can be induced by acute increases in mechanical forces in the absence of bacterial or viral infections as long as the lung ECM is sufficiently weak. Thus, it is possible that in humans, the increases in mechanical stresses on the emphysematous tissue associated with cough and difficulty in breathing are also the primary reason for the irreversible changes in AECOPD. While we acknowledge that infection is the likely cause of generating mechanical stresses, it is the balance between the abnormal remodeling and the amount and strength of mechanical stresses on the ECM that determines whether an exacerbation leads to irreversible decline in function or not. Our study also suggests that clinical treatment of AECOPD should minimize mechanical stresses, for example, by attenuating coughing or carefully choosing mechanical ventilation parameters if the patient needs to be ventilated.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute grants HL-090757 and HL-098976, Hungarian Scientific Research Fund grant (OTKA) Grant 66700, and the Rosztoczy Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.V.S. and S.S. performed experiments; M.V.S., H.P., S.S., E.B.-S., and B.S. analyzed data; M.V.S., H.P., S.S., Z.H., E.B.-S., and B.S. interpreted results of experiments; M.V.S. prepared figures; M.V.S. and B.S. drafted manuscript; Z.H., E.B.-S., and B.S. conception and design of research; Z.H., E.B.-S., and B.S. edited and revised manuscript; B.S. approved final version of manuscript.
REFERENCES
- 1. Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L710–L717, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 19: 113–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cantor JO, Nadkarni PP. Hyaluronan: the Jekyll and Hyde molecule. Inflamm Allergy Drug Targets 5: 257–260, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Donaldson GC, Seemungal T, Jeffries DJ, Wedzicha JA. Effect of temperature on lung function and symptoms in chronic obstructive pulmonary disease. Eur Respir J 13: 844–849, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57: 847–852, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois AB, Botelho SY, Bedell GN, Marshall R, Comroe JH., Jr A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest 35: 322–326, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 66: 585–590, 2011 [DOI] [PubMed] [Google Scholar]
- 8a. Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf
- 9. Gompertz S, Bayley DL, Hill SL, Stockley RA. Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax 56: 36–41, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamakawa H, Bartolak-Suki E, Parameswaran H, Majumdar A, Lutchen KR, Suki B. Structure-function relations in an elastase-induced mouse model of emphysema. Am J Respir Cell Mol Biol 45: 517–524, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hantos Z, Adamicza A, Janosi TZ, Szabari MV, Tolnai J, Suki B. Lung volumes and respiratory mechanics in elastase-induced emphysema in mice. J Appl Physiol 105: 1864–1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116: 753–759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Ito S, Bartolak-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol 34: 688–694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol 97: 204–212, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Janosi TZ, Adamicza A, Zosky GR, Asztalos T, Sly PD, Hantos Z. Plethysmographic estimation of thoracic gas volume in apneic mice. J Appl Physiol 101: 454–459, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Jesudason R, Sato S, Parameswaran H, Araujo AD, Majumdar A, Allen PG, Bartolak-Suki E, Suki B. Mechanical forces regulate elastase activity and binding site availability in lung elastin. Biophys J 99: 3076–3083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kononov S, Brewer K, Sakai H, Cavalcante FS, Sabayanagam CR, Ingenito EP, Suki B. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med 164: 1920–1926, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res 32: 181–199, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavietes MH, Smeltzer SC, Cook SD, Modak RM, Smaldone GC. Airway dynamics, oesophageal pressure and cough. Eur Respir J 11: 156–161, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Leith DE. Cough. Phys Ther 48: 439–447, 1968 [DOI] [PubMed] [Google Scholar]
- 23. Lucey EC, Goldstein RH, Stone PJ, Snider GL. Remodeling of alveolar walls after elastase treatment of hamsters. Results of elastin and collagen mRNA in situ hybridization. Am J Respir Crit Care Med 158: 555–564, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Lutchen KR, Yang K, Kaczka DW, Suki B. Optimal ventilation waveforms for estimating low-frequency respiratory impedance. J Appl Physiol 75: 478–488, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Mercer RR, Crapo JD. Structural changes in elastic fibers after pancreatic elastase administration in hamsters. J Appl Physiol 72: 1473–1479, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, Nishimura K, Oku Y, Chin K, Ohi M, Nakamura T, Bates JH, Alencar AM, Suki B. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 96: 8829–8834, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mouded M, Egea EE, Brown MJ, Hanlon SM, Houghton AM, Tsai LW, Ingenito EP, Shapiro SD. Epithelial cell apoptosis causes acute lung injury masquerading as emphysema. Am J Respir Cell Mol Biol 41: 407–414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Namati E, Thiesse J, de Ryk J, McLennan G. Alveolar dynamics during respiration: are the pores of Kohn a pathway to recruitment? Am J Respir Cell Mol Biol 38: 572–578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 155: 109–115, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 351: 773–780, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol 100: 186–193, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 968–975, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 61: 250–258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 1608–1613, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 16: 677–683, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stockley RA, O'Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. 2000. Chest 136: e30, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med 168: 516–521, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, Kinose D, Ogawa E, Hoshino Y, Mishima M. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183: 1653–1659, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med 160: 2086–2092, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Wang Y, Liu YN. Experimental pulmonary infection and colonization of Haemophilus influenzae in emphysematous hamsters. Pulm Pharmacol Ther 23: 292–299, 2010 [DOI] [PubMed] [Google Scholar]
- 41. West JB. Pulmonary capillary stress failure. J Appl Physiol 89: 2483–2489, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Zamel N, Hogg J, Gelb A. Mechanisms of maximal expiratory flow limitation in clinically unsuspected emphysema and obstruction of the peripheral airways. Am Rev Respir Dis 113: 337–345, 1976 [DOI] [PubMed] [Google Scholar]