Abstract
Inflammation of the distal bowel is often associated with abdominal pain and hypersensitivity, but whether and which colorectal afferents contribute to the hypersensitivity is unknown. Using a mouse model of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, we investigated colorectal hypersensitivity following intracolonic TNBS and associated changes in colorectum and afferent functions. C57BL/6 mice were treated intracolonically with TNBS or saline. Visceromotor responses to colorectal distension (15–60 mmHg) were recorded over 8 wk in TNBS- and saline-treated (control) mice. In other mice treated with TNBS or saline, colorectal inflammation was assessed by myeloperoxidase assay and immunohistological staining. In vitro single-fiber recordings were conducted on both TNBS and saline-treated mice to assess colorectal afferent function. Mice exhibited significant colorectal hypersensitivity through day 14 after TNBS treatment that resolved by day 28 with no resensitization through day 56. TNBS induced a neutrophil- and macrophage-based colorectal inflammation as well as loss of nerve fibers, all of which resolved by days 14–28. Single-fiber recordings revealed a net increase in afferent drive from stretch-sensitive colorectal afferents at day 14 post-TNBS and reduced proportions of mechanically insensitive afferents (MIAs) at days 14–28. Intracolonic TNBS-induced colorectal inflammation was associated with the development and recovery of hypersensitivity in mice, which correlated with a transient increase and recovery of sensitization of stretch-sensitive colorectal afferents and MIAs. These results indicate that the development and maintenance of colorectal hypersensitivity following inflammation are mediated by peripheral drive from stretch-sensitive colorectal afferents and a potential contribution from MIAs.
Keywords: electrophysiology, IBS, PI-IBS, single-fiber, visceral pain
irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by persistent pain and organ hypersensitivity in the absence of demonstrable gross structural or biochemical abnormalities. Although the cause(s) of IBS is unclear, postinfectious IBS (PI-IBS) seems to have a defined etiology because in those patients, an infectious gastroenteritis precedes the development of IBS symptoms (22).
Intracolonic 2,4,6-trinitrobenzene sulfonic acid (TNBS) in rodents has been widely used as a model of colitis (23), and recently TNBS has been advanced as a model of postinflammatory colorectal hypersensitivity and thus a model of PI-IBS (20). Hughes et al. (14) reported, for example, an inflammation-associated increase in colorectal afferent sensitization and a long-lasting sensitization of serosal and mesenteric class afferents after resolution of TNBS-induced colorectal inflammation. The association of these changes in afferent properties, however, with functional colorectal hypersensitivity has not been established. We therefore undertook to examine the relationship between colorectal hypersensitivity, inflammation, and afferent function after intracolonic administration of TNBS. The present study focused on the pelvic nerve innervation of the colon for two reasons: 1) behavioral assessment of colorectal hypersensitivity typically involves balloon distension of the colorectum, and responses to distension are absent after pelvic nerve transection but unaffected after lumbar splanchnectomy (15), and 2) stretch-sensitive colorectal afferents are rare in the lumbar splanchnic nerve innervation but common in the pelvic nerve innervation (4, 10). Portions of these data have been reported in abstract form (8).
MATERIALS AND METHODS
All experiments were conducted on male C57BL/6 mice (20–30 g; Taconic, Germantown, NY), and protocols were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Behavioral visceromotor response to colorectal distension.
Details have been reported previously (6). Briefly, mice were anesthetized (2% isoflurane; Hospira, Lake Forest, IL), the skin incised to expose the right abdominal musculature, and the bare ends of two segments of Teflon-coated stainless-steel wire (Cooner Wire, Chatworth, CA) inserted into the musculature and secured in place with 5-0 Vicryl sutures (Ethicon, Somerville, NJ); the other ends were tunneled subcutaneously to a small incision made at the nape of the neck and externalized for access during testing.
On the day of baseline testing (∼1 wk after surgery), mice were briefly sedated with isoflurane and a lubricated polyethylene balloon (1.5 cm long × 0.9 cm diameter) inserted transanally into the colorectum 0.5–1 cm from the anal verge and secured to the tail with tape. Mice were placed in a plastic cylinder to limit movement inside a sound-attenuating, dark chamber and allowed to recover from isoflurane (30 min) before testing. Colorectal distension (CRD) was conducted by constant pressure inflation of the balloon (15, 30, 45, or 60 mmHg; 10 s duration) and electromyographic activity was recorded for 10 s before (resting), during, and after distension. Each pressure was tested three times with a 4-min interval between distensions. Visceromotor responses to CRD were quantified as the total area of electromyographic activity during balloon inflation minus resting activity.
After establishing baseline responses to CRD (day 0), mice were anesthetized (isoflurane), and either saline or TNBS (0.2 ml @ 10 mg/ml in 50% ethanol; Sigma-Aldrich, St. Louis, MO) was administered transanally via a 22-gauge feeding needle. Colorectal distension was repeated on days 2, 7, 14, and 28; in some mice, CRD tests were extended to days 42 and 56.
Myeloperoxidase activity.
Colorectal myeloperoxidase (MPO) activity was evaluated as a biochemical indicator of inflammation. Mice were overdosed with isoflurane and the distal colorectum (∼2 cm) removed at varying times after intracolonic treatment, dissected, weighed, added to a beaker containing 1.0 ml 0.5% hexadecyl-trimethylammonium bromide (HTAB; Sigma) and finely minced using spring scissors. The contents were transferred to a 15-ml centrifuge tube with 2 ml of additional HTAB, sonicated (10 s), and then homogenized (30 s) before adding 2 ml HTAB and placing the tube on dry ice until all samples were similarly prepared. Samples then underwent three freeze-thaw cycles, were centrifuged twice, and loaded along with MPO standards (Calbiochem, San Diego, CA) on a 96-well plate. Samples and standards were reacted with O-dianisidine dihydrochloride (Sigma) and read on a plate reader at 460 nm every 20 s for 5 min. The slope for each standard was plotted and used to calculate units of MPO activity/tissue weight for each sample.
Immunohistochemistry.
The distal colon was harvested at varying times after intracolonic treatment and fixed with 4% paraformaldehyde in 0.16 M phosphate buffer containing 14% picric acid (Sigma). After cryoprotection in 20% sucrose, fixed tissue was embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned at 20 μm. Some tissue sections were incubated with rabbit anti-PGP 9.5 antibody (1:2,000; UltraClone, Yarmouth, UK) and then with Cy3-conjugated anti rabbit IgG (1:200, Jackson Immunoresearch, West Grove, PA) for immunostaining of neuronal fibers. Monocytes and macrophages were stained in other tissue sections using rat anti-F4/80 (1:1,000, Abcam, Cambridge, MA) and Cy3-conjugated anti-rat IgG (1:200).
PGP 9.5 immunostaining was quantified by setting a brightness threshold to the 8-bit grey scale images using ImageJ and calculating the area (proportion) of PGP 9.5 immunoactivity relative to the colorectal mucosal and submucosal area as reported previously (12). Thresholds were determined from the Gaussian distribution of background grey to achieve < 0.001% probability of erroneous inclusion of background into PGP 9.5 immunopositive areas. To reduce variation between tissue slides, saline (control)- and TNBS-treated colorectums were placed on the same slides. Twenty images were randomly taken from each group and processed blind of treatment. Changes in thickness of the submucosal area following TNBS was assessed by quantifying changes in area proportions of the submucosal layer with respect to the total area (mucosal plus submucosal layers).
In vitro single-fiber recordings from colorectal afferents.
Details have been reported previously (9, 10). Briefly, mice were euthanized via CO2 inhalation and exsanguination after perforating the right atrium. The distal colorectum (∼3 cm) with pelvic nerve attached was transferred to oxygenated Krebs solution to which nifedipine (4 μM) and indomethacin (3 μM) was added. The colorectum was opened longitudinally along the antimesenteric border and pinned flat mucosal side up in a Krebs-filled tissue chamber. In the adjacent oil-filled recording chamber, the pelvic nerve was teased into fine bundles (∼10 μm thick) for single-fiber electrophysiological recordings.
Colorectal afferent fibers were excited by suprathreshold electrical current stimulation (0.5-ms duration, 0.3 Hz) using a round-tipped concentric electrode (external Φ 0.55 mm, internal Φ 0.125 mm; FHC, Bowdoin, ME) perpendicular to the mucosal surface (10).The electrode was moved systematically (∼1.5-mm steps) to examine the entire colorectal surface. Receptive endings were localized as the site requiring the least stimulus intensity to evoke an action potential (i.e., stimulus threshold), after which they were tested for mechanosensitivity and classified as serosal, muscular, mucosal, muscular/mucosal, or mechanically insensitive afferents (MIAs) as previously described (10): probing with von Frey-like nylon monofilaments (0.4, 1, and 1.4 g force), mucosal stroking with nylon filaments (10 mg force), and circumferential stretch (0–170 mN in 34 s) using a servo-controlled force actuator (Aurora Scientific, Ontario, Canada).
Data recording and analysis.
Action potentials were recorded extracellularly by a low-noise AC differential amplifier (DAM 80; World Precision Instruments, New Haven, CT). Electrical signals were filtered (0.3 to 10 kHz), amplified (×10,000), digitized at 20 kHz using a 1401 interface [Cambridge Electronic Design (CED), Cambridge, UK], monitored online by an audio monitor, and stored on a personal computer. Action potentials were clustered off line into single units using the principal component analysis in Spike 2 software (CED). To avoid erroneous discrimination, no more than two easily discriminable active units in any record were studied. The stretch threshold of the fiber was defined as the force that evoked the first action potential in the slow-ramped stretch stimulus (0 to 170 mN at 5 mN/s). The stretch-response function was quantified by binning the evoked action potentials evenly into three bins of the ramped stretch force (0–57, 57–113, and 113–170 mN).
Data are presented as means ± SE. One-way and two-way ANOVA for repeated-measures as appropriate were performed using SigmaPlot version 9.0 (Systat Software, San Jose, CA). Bonferroni-corrected post hoc comparisons were performed as appropriate when F values for main effects were significant. Differences were considered significant when P < 0.05.
RESULTS
Colorectal hypersensitivity and inflammation.
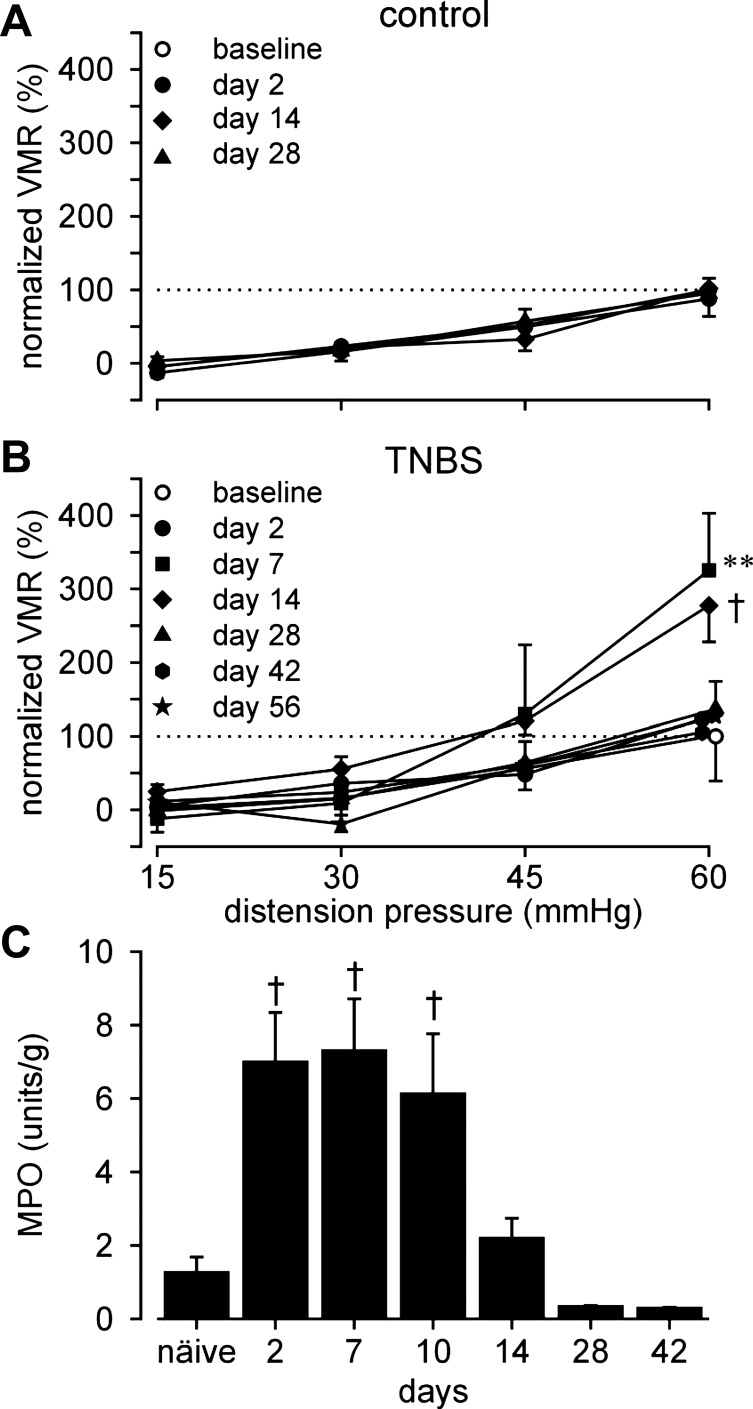
Visceromotor responses to CRD were recorded in unanesthetized mice before (baseline; day 0) and after (days 2, 7, 14, 28, 42, and 56) intracolonic administration of saline or TNBS. To eliminate between-sample variation, VMRs were normalized as percentage of the respective baseline response at 60 mmHg. Intracolonic saline treatment did not affect VMRs to distension at any time (Fig. 1A, F3,18 = 0.2, P = 0.9), whereas TNBS produced significant colorectal hypersensitivity on days 7 and 14, which resolved by day 28, after which VMRs did not differ from baseline through the end of testing at day 56 (Fig. 1B; F6,316 = 48, P < 0.001; post hoc analyses, P = 0.004 at day 7, P < 0.001 at day 14).
Fig. 1.
Visceromotor responses (VMR) to colorectal distension (CRD). VMRs were normalized to the baseline (day 0) response at 60 mmHg. A and B: responses to CRD (15–60 mmHg) recorded over time from 7 saline (control)- and 19 TNBS-treated mice, respectively. All saline-treated mice underwent CRD tests at all the 4 times in A; at least 6 TNBS-treated mice were tested at each of the 7 times in B. VMRs were significantly increased on days 7 and 14 after TNBS treatment. C: Myeloperoxidase (MPO) activity in TNBS-treated colon was significantly increased between days 2 and 10, resolving thereafter. **P < 0.01; †P < 0.001. TNBS, 2,4,6-trinitrobenzene sulfonic acid.
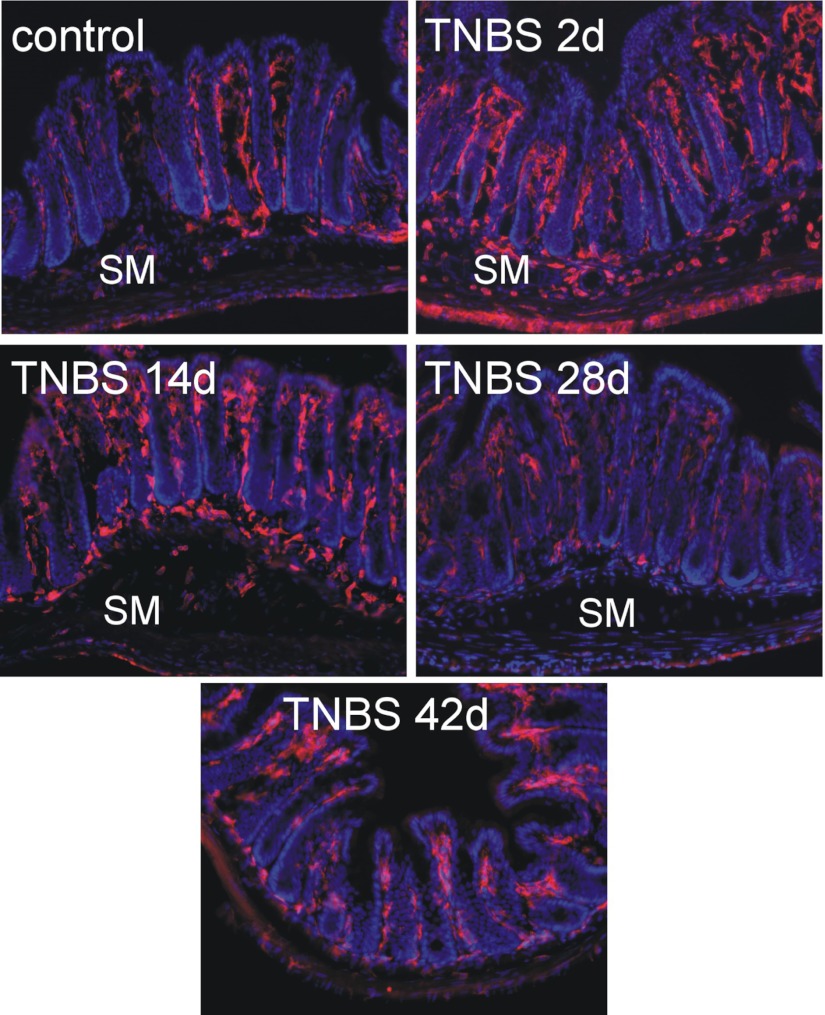
Colorectal MPO, indicative of a neutrophil-based inflammation, was significantly increased on days 2, 7, and 10 (P < 0.001; Fig. 1C), returning to resting activity (naïve) by day 14 and remaining unchanged thereafter. Macrophage-based colon inflammation was assessed by F4/80 immunohistological staining (Fig. 2). Increased numbers of F4/80-positive macrophages in mucosal villi and F4/80-positive monocytes at the bottom of crypts and in the submucosa were apparent on days 2 through 14 post-TNBS, resolving thereafter.
Fig. 2.
Resident F4/80-immunoreactive macrophages (amorphous cells, red) were present principally in lamina propria of the mucosa in control colorectums. TNBS induced an infiltration of monocytes (round, red cells) in the submucosa (SM) and an increase in macrophages in the lamina propria on day 2, which was diminished on day 14 and resolved by days 28–42. Tissue sections were counterstained with DAPI (nuclei in blue).
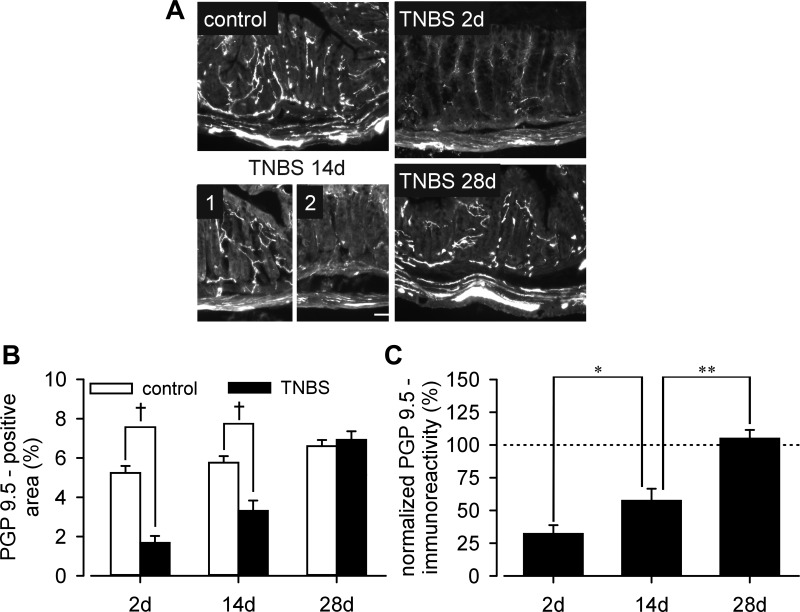
To assess the density of innervation after TNBS treatment, colorectal tissue was immunostained with anti-PGP 9.5, a pan-neuronal marker as shown in Fig. 3A. Significant (P < 0.001 vs. control) loss of PGP 9.5-immunoreacitve nerve fibers was evident on days 2 and 14 (Fig. 3B), with partial patchy recovery on day 14 (P < 0.05 vs. day 2; P < 0.01 vs. control) (Fig. 3C). Note that PGP 9.5 immunoreactivity on day 14 was comparable to control in places (Fig. 3A, TNBS 14d, 1), but relatively scarce in other areas of the colon (Fig. 3A, TNBS 14d, 2). No difference in nerve fiber density was detected 28 days after intracolonic TNBS treatment. Submucosal thickness increased following TNBS treatment; the proportion of the total area (mucosal and submucosal) contributed to by the submucosal layer was significantly increased at both day 2 (control 9.2 ± 1.2% vs. TNBS 13.7 ± 1.8%, P < 0.05) and day 14 (control 7.8 ± 1.9% vs. TNBS 12.8 ± 1.3%, P < 0.05), returning to control at day 28 (control 9.4 ± 1.3% vs. TNBS 7.5 ± 0.9%, P = 0.25).
Fig. 3.
A: PGP 9.5-immunostaining of the distal colorectal wall. PGP 9.5-immunoreactive nerve fibers were quantified as area (proportion) with respect to colorectal mucosal and submucosal areas (B), which were normalized by control mean PGP 9.5 proportions and displayed in C. Intracolonic TNBS significantly reduced the density of PGP 9.5-immunoreactive nerve fibers on day 2, which was partially restored by day 14 (compare parts 1 and 2 on day 14) and returned to normal on day 28. *P < 0.05; **P < 0.01; †P < 0.001.
Excitability of colorectal afferent fibers.
Pelvic nerve afferent endings identified by electrical stimulation were classified into four mechanosensitive subtypes and one mechanically insensitive subtype (see Fig. 4 in Ref. 12). Briefly, all mechanosensitive endings respond to blunt probing of the mucosal surface. In addition, muscular endings respond to ramped, circumferential stretch; mucosal endings respond to stroking of the mucosa; and muscular/mucosal endings respond to both stretch and stroking. Serosal endings respond only to probing and not to either stroking or stretch; MIAs do not respond to any mechanical stimulus.
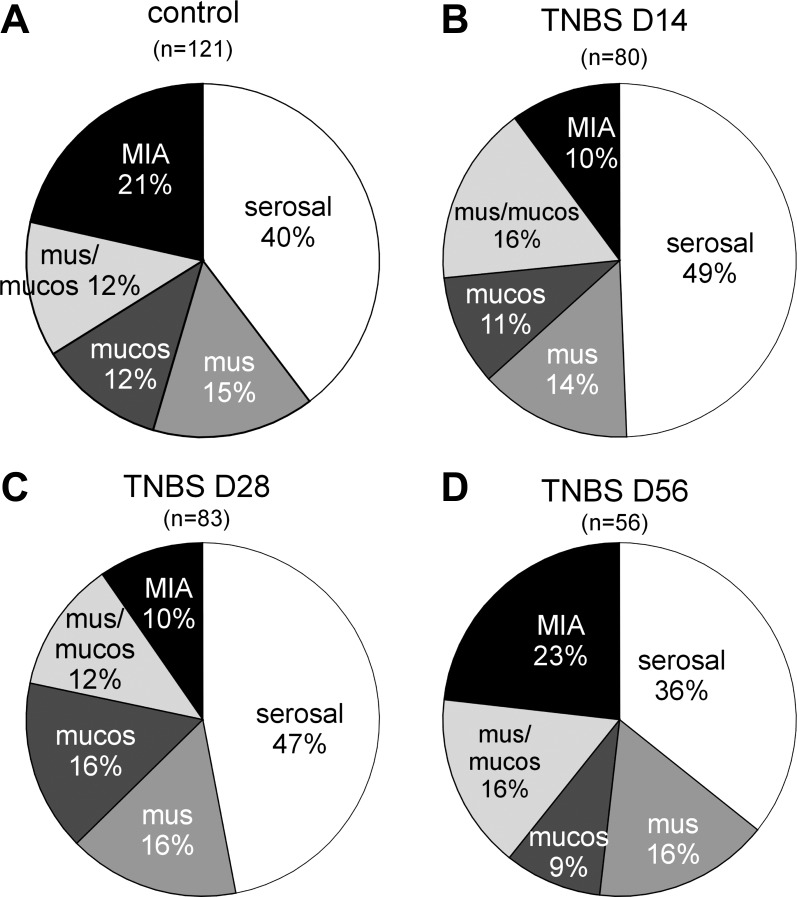
Fig. 4.
Proportions of colorectal pelvic nerve afferents after intracolonic instillation of saline (control; A) or TNBS. Afferent proportions were studied at days 14, 28, and 56 post-TNBS and reported in (B–D), respectively. mus/mucos, Muscular/mucosal.
Between days 14 and 28 post-TNBS, mice appeared to transit from a colorectal hypersensitive to normosensitive state (Fig. 1B) consistent with resolution of macrophage-based inflammation (Fig. 2) and recovery of nerve fiber loss in the colon (Fig. 3). Thus, to investigate changes in afferent properties, mice were killed at days 14 and 28 post-TNBS or post-saline treatment, the colorectum was harvested, and pelvic nerve endings were studied in vitro with investigators blind to treatment. Single-fiber recordings were also carried out at day 56 after intracolonic TNBS in another group of mice that had been assessed behaviorally on day 28 for colorectal hypersensitivity. The proportions of afferent classes in saline-treated mice at days 14 and 28 posttreatment were not different statistically [or different from previous results (12); χ2, P = 0.3] and thus pooled together in Fig. 4A. In colorectums taken from TNBS-treated mice, the proportion of MIAs was significantly decreased by ≥ 50% relative to their saline-treated counterparts at both at days 14 and 28 post-TNBS (χ2 vs. control, both P < 0.04; Figs. 4, B and C), which returned to control proportions (∼20%) at day 56 post-TNBS (P > 0.7, Fig. 4D). The reduced MIA proportions appeared to be accompanied by increased (though statistically insignificant) proportions of serosal afferents at days 14 and 28 post-TNBS. The proportions of mechanosensitive muscular, muscular/mucosal, and mucosal afferents did not differ between saline- and TNBS-treated mice at days 14, 28, or 56 posttreatment (χ2 vs. control, all P > 0.7).
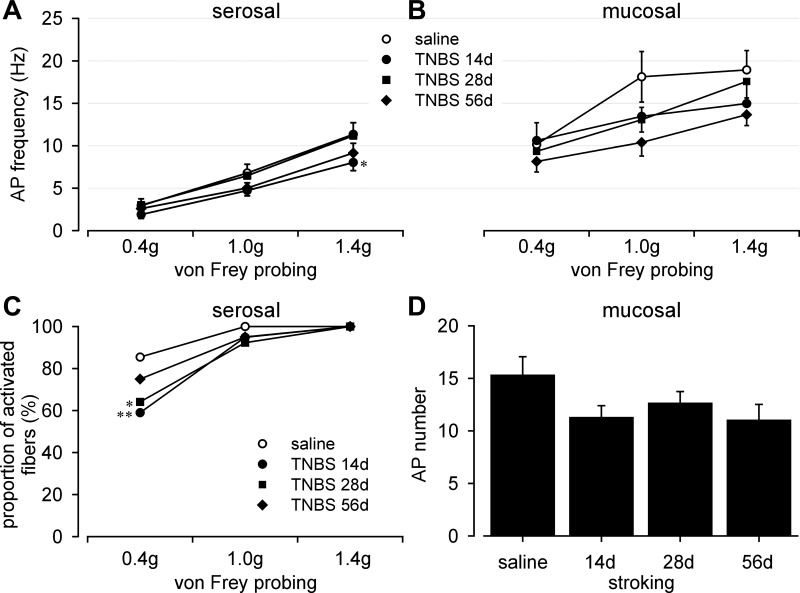
Serosal and mucosal endings were tested by punctate probing with von Frey-like monofilaments at three forces (0.4, 1, and 1.4 g; 3-s duration); probing was conducted twice at each force for calculation of average response frequency (Hz). Responses of mucosal afferents to 10-mg stroking were quantified as the number of evoked action potentials per 10 consecutive strokings at ∼1-s intervals. Compared with responses (magnitude) of serosal afferents to probing in saline-treated mice, TNBS-treated mice showed significantly reduced responses at day 14 posttreatment, but did not differ from saline-treated mice at days 28 or 56 posttreatment (Fig. 5A, F3,426 = 3.1, P < 0.05; post hoc comparison, P = 0.021 at day 14). In addition, probing with the 0.4 g force activated a significantly smaller proportion of serosal afferents in TNBS-treated mice (days 14 and 28) relative to saline-treated controls (Fig. 5C, Fisher's exact test, P < 0.01 at day 14, P < 0.05 at day 28); no significant difference was observed at day 56 (P = 0.31). There were no differences between groups in the proportions of responders to von Frey-like probing at 1 and 1.4 g forces, which activated most, if not all serosal afferents regardless of treatment. Mucosal afferents showed significantly greater baseline responses to probing than serosal afferents (Fig. 5B saline vs. Fig. 5A saline, F1,180 = 43.2, P < 0.001). Responses of mucosal afferents in TNBS-treated mice to probing (Fig. 5B, F3,108 = 2, P = 0.12) or stroking (Fig. 5D, F3,36 = 1.61, P = 0.2) did not differ from responses in their saline-treated counterparts.
Fig. 5.
Effect of TNBS on responses of serosal and mucosal afferents. A: TNBS transiently (at day 14 posttreatment) reduced responses of serosal afferents to graded probing (0.4, 1, and 1.4 g); responses did not differ from saline on days 28 and 56. TNBS treatment was accompanied by reduced proportions of fibers activated by 0.4 g probing at days 14 and 28 after treatment, which recovered by day 56 (C). Responses of mucosal afferents to either graded probing (B) or mucosal stroking (D) were not affected by TNBS treatment. AP, action potential; *P < 0.05; **P < 0.01.
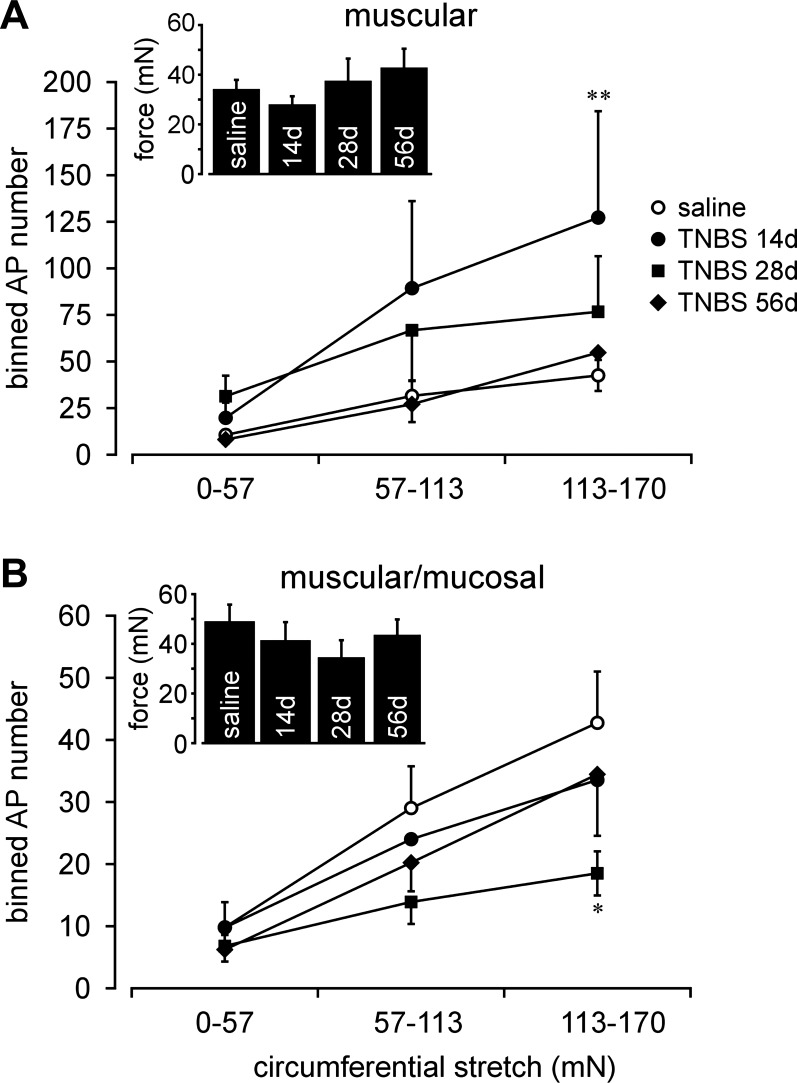
Following TNBS treatment, muscular afferents exhibited significantly greater responses to stretch (i.e., were sensitized) relative to saline-treated controls at day 14 (Fig. 6A; F3,138 = 4.8, P < 0.01; post hoc comparison, P = 0.003 at day 14) and somewhat enhanced responses at day 28, which, however, were not statistically significant (P = 0.09 at day 28); stretch sensitivity at day 56 did not differ from control (P = 1.0). Responses of muscular-mucosal afferents were not increased (sensitized) at any time of testing after intracolonic TNBS; rather, their responses to stretch tended to be less than control, significantly so on day 28 (Fig. 6B, F3,129 = 2.89, P < 0.05; post hoc comparison, P = 0.014 at day 28, P > 0.3 at days 14 and 56). The threshold for response to stretch was defined as the force (mN) at which the first action potential was produced (stretch-sensitive afferents were not spontaneously active) and did not differ between mice treated with saline or TNBS at any time of testing (Fig. 6A, inset, F3,46 = 0.69, P = 0.56; Fig. 6B, inset, F3,43 = 0.54, P = 0.66).
Fig. 6.
Effect of TNBS on stretch-sensitive afferents. Responses of muscular afferents to circumferential stretch (0–170 mN @ 5 mN/s, binned into 3 bins and plotted as stretch-response functions) were significantly increased (sensitized) at day 14 after TNBS treatment (A), returning to baseline (control, saline) by days 28 and 56. Responses of muscular/mucosal afferents to stretch were unchanged at day 14, significantly reduced at day 28, and recovered by day 56 after TNBS treatment. Insets present response thresholds of muscular (A) and muscular/mucosal (B) afferents, which did not differ from control (saline) at any time after TNBS treatment. *P < 0.05; **P < 0.01.
DISCUSSION
Studies in IBS patients reveal that colorectal afferent input contributes to colorectal as well as somatic (abdominal) hypersensitivity and pain, the major complaints of IBS patients, which are promptly and reversibly relieved by intracolonic instillation of the local anesthetic lidocaine (24). Recently, we documented long-lasting sensitization of stretch-sensitive colorectal afferents in parallel with persistent behavioral colorectal hypersensitivity produced by intracolonic zymosan, a treatment not associated with changes in MPO activity, macrophages/monocytes infiltration or reduced colorectal innervation (12). In that same study, MIAs exhibited a phenotypical switch and acquired mechanosensitivity as in the present study. Here, we employed a different, PI-IBS-like model produced by intracolonic instillation of TNBS to examine the relationship between behavioral hypersensitivity and afferent sensitization.
It has been reported previously that afferent sensitization persists beyond the period of TNBS-produced acute colorectal inflammation [behavioral hypersensitivity was not assessed (14)]. We found good correspondence in the present study between TNBS-produced behavioral hypersensitivity of the colorectum (which persisted for 14 days), indices of colorectal inflammation (macrophage infiltration/monocyte accumulation and MPO activity, which similarly persisted for 10–14 days), and sensitization of stretch-sensitive colorectal muscular afferents at day 14 after TNBS (but not longer). We also noted a significant reduction in the proportion of MIAs at both days 14 and 28 after TNBS, apparently reflecting a complementary increase in the proportion of serosal afferents. It is not clear at present what role MIAs might play in colorectal hypersensitivity as we showed previously that their sensitization in vitro, while including acquisition of mechanosensitivity to probing, does not include acquisition of stretch-sensitivity (10). While it has been advanced that TNBS models PI-IBS because colorectal afferents exhibit sensitization beyond the resolution of colorectal inflammation (14), we found neither behavioral hypersensitivity nor afferent fiber sensitization beyond the time colorectal inflammation resolved by day 14 posttreatment. The duration of behavioral colorectal hypersensitivity reported here is wholly consistent with a previous study in C57BL/6 mice where resolution of TNBS colitis was associated with normalization of visceromotor responses to colorectal distension (16). In rats, however, intracolonic TNBS reportedly induces two phases of colorectal hypersensitivity with the second phase lasting for weeks post-TNBS (1, 13). It has also been reported in rats that only a portion (24%) develop long-lasting colorectal hypersensitivity following TNBS, consistent with PI-IBS (25).This apparent discrepancy could be accounted for by different concentrations of TNBS used, methods of testing and particularly by the relatively low sensitivity of C57BL/6 mice to TNBS colitis compared with other mouse strains (7).
The important finding reported here is the close correlation between the duration of afferent fiber sensitization and behavioral colorectal hypersensitivity (7–14 days) following intracolonic TNBS treatment and the ensuing resolution of afferent fiber sensitization and return to baseline visceromotor responses to CRD (28–56 days). These results are consistent with a previous study (12) in which pelvic nerve afferent fiber sensitization in mice treated intracolonically with zymosan paralleled behavioral colorectal hypersensitivity. These models differ significantly, however, in that TNBS does and zymosan does not produce significant colorectal inflammation as assessed by histological examination, MPO activity or monocytes infiltration. Furthermore, unlike zymosan-treated mice that exhibit persistent colorectal hypersensitivity to CRD after resolution of brief macrophage accumulation in the submucosa (12), TNBS-treated mice in this study were not hypersensitive to colorectal distension after recovery from colitis (day 14). The current findings suggest that a previous episode of intestinal inflammation is not sufficient for development of persistent visceral hypersensitivity to mechanical stimulation, which supports previous suggestions that colon inflammation may not necessarily correlate with colorectal hypersensitivity (17, 21). The present findings are also consistent with clinical observations that colon hypersensitivity is generally absent in patients either with Crohn's disease with inflammation limited to the ileum (2) or ulcerative colitis in quiescent or mildly active phases of the disease (5), two major subgroups of inflammatory bowel diseases.
Significant reduction in PGP 9.5 immunostaining in the colon wall, particularly in the mucosal layer, was observed at day 2 after TNBS treatment, consistent with a previous report in the rat (19). The significant reduction (67% at day 2, 43% at day 14) in PGP 9.5 immunostaining following TNBS cannot be attributed to the increase in submucosal thickness as the proportion of the total area quantified that is contributed to by the submucosal layer is small (7.5–13.7%). This may be interpreted either as loss of extrinsic and/or intrinsic nerve fibers or loss of the efficacy of PGP 9.5 antibodies in inflamed tissue. The latter is unlikely as a previous study (19) reported that CGRP staining in the mucosal layer had a similar time course of reduction after TNBS, which is in agreement with our unpublished observations in mice (La, Feng, and Gebhart). A reduction in both PGP 9.5 and CGRP immunostaining in the mucosal layer suggests loss of extrinsic colorectal afferent innervation after TNBS between days 2 and 14, further suggesting a reversible neuropathic-like insult to colorectal afferent endings. Although recovery of fiber density was not fully complete at day 14 post-TNBS, afferent proportions were not different from proportions at day 28, by which time innervation density was not different from control.
The proportions of pelvic nerve colorectal afferents in control, saline-treated mice are comparable to those from previous studies (10, 12). Mechanosensitive muscular, mucosal, and muscular-mucosal afferent proportions were not affected by TNBS treatment, consistent with a previous report in which intracolonic zymosan was used to produce colorectal hypersensitivity (12). The proportion of MIAs, however, was significantly reduced by ≥ 50% at both days 14 and 28 post-TNBS, while the serosal proportion proportionally increased, suggesting that MIAs acquired a serosal phenotype and mechanosensitivity to punctuate probing of the receptive field. This interpretation is consistent with a previous study in which MIAs were acutely sensitized by application of an inflammatory soup to the receptive ending, acquiring mechanosensitivity to probing of the receptive ending, but not mechanosensitivity to stretch (10). We would thus interpret the present results as sensitization of MIAs, but cannot be certain because it is not possible to do the experiment in a way that permits tracking changes in the same afferent fiber over weeks. A phenotypic switch is, however, supported by the reduced proportion of serosal afferents activated by low-intensity (0.4 g) punctuate probing in TNBS-treated mice, because sensitized MIAs appear to have high thresholds to mechanical probing (>1 g) (10). In a recent study (12), mice developed long-lasting visceral hypersensitivity after intracolonic zymosan treatment and a similar pattern of reduced MIA and increased serosal afferent proportions was observed that lasted throughout the duration of the experiment (24 days posttreatment), suggesting persistent sensitization of MIAs. In the present study, TNBS-treated mice displayed colorectal hypersensitivity only for a limited time (14 days), whereas apparent MIA sensitization persisted for 28 days. This observation draws into question the role of MIAs in the maintenance of colorectal hypersensitivity.
Stretch-sensitive muscular and muscular-mucosal afferents respond to circumferential colorectal stretch and subserve the sensory encoding of colorectal distension/tension in vivo (9, 11). Here we report that responses of muscular afferents were significantly increased (i.e., sensitized) at day 14 post-TNBS, whereas responses of muscular-mucosal afferents were unchanged. Collectively, responses of stretch-sensitive afferents were thus increased at a time when colorectal hypersensitivity was documented behaviorally. Colorectal hypersensitivity was no longer present on day 28 after TNBS, a time at which responses of stretch-sensitive afferents were no longer sensitized, but rather significantly reduced relative to control. At day 56 post-TNBS, responses of muscular and muscular-mucosal afferents did not differ from control, corresponding to the absence of behavioral colorectal hypersensitivity. These findings add to a growing literature reinforcing the key role of increased peripheral activity from colorectal afferents in the development and maintenance of behavioral visceral hypersensitivity (3, 12, 18).
In summary, we found that intracolonic TNBS produced a significant neutrophil- and macrophage-based inflammation and significant structural damage that persisted for 14 days after treatment. Mice exhibited behavioral colorectal hypersensitivity to distension at days 7 and 14 post-TNBS in concert with evidence of inflammation. Indices of colorectal inflammation, reduced innervation and behavioral hypersensitivity were all resolved by day 28 post-TNBS. Significantly, stretch-sensitive pelvic nerve muscular afferents were sensitized at day 14 post-TNBS, suggesting their contribution to the behavioral colorectal hypersensitivity. Colorectal hypersensitivity normalized at day 28 post-TNBS, and responses of stretch-sensitive fibers were no greater than control at that time. Extending beyond the time of colorectal hypersensitivity, the proportion of colorectal MIAs (and proportionate increase in the serosal afferent proportion) were reduced at both 14 and 28 days post-TNBS, suggesting that sensitization of MIAs alone is not sufficient to maintain colorectal hypersensitivity.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-093525 and UL1-RR-024153 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.F. and G.F.G. conception and design of research; B.F., J.H.L., T.T., E.S.S., T.P.M., and G.F.G. performed experiments; B.F., J.H.L., T.T., E.S.S., T.P.M., and G.F.G. analyzed data; B.F., T.T., and G.F.G. interpreted results of experiments; B.F. and G.F.G. prepared figures; B.F. drafted manuscript; B.F., J.H.L., T.T., E.S.S., and G.F.G. edited and revised manuscript; B.F., J.H.L., T.T., E.S.S., T.P.M., and G.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michael Burcham for preparation of figures.
Present address of T. Tanaka: Takeda Pharmaceutical, Osaka, Japan.
REFERENCES
- 1.Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 123: 179–186, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 66: 151–161, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Beyak MJ. Visceral afferents-determinants and modulation of excitability. Auton Neurosci 153: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut 47: 497–505, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol 157: 2174–2185, 1996 [PubMed] [Google Scholar]
- 8.Feng B, La JH, Tanaka T, Gebhart GF. Silent afferents and colorectal hypersensitivity (Abstract). Program No.682.19/VV3.2010, Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2010 [Google Scholar]
- 9.Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng B, La JH, Schwartz ES, Gebhart GF. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: G1085–G1098, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gschossmann JM, Liebregts T, Adam B, Buenger L, Ruwe M, Gerken G, Holtmann G. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 49: 96–101, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58: 1333–1341, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Larsson MH, Rapp L, Lindstrom E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil 18: 144–152, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res 971: 73–82, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Miampamba M, Sharkey KA. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil 10: 315–329, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol 46: 164–174, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Sharkey KA. Visceral sensation and colitis: inflammation and hypersensitivity do not always go hand in hand. Neurogastroenterol Motil 18: 87–90, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 136: 1979–1988, 2009 [DOI] [PubMed] [Google Scholar]
- 23.te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis 12: 995–999, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 134: 9–15, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]