Abstract
Intestinal endocrine cells release gut hormones, including glucagon-like peptides (GLPs), in response to luminal nutrients. Luminal l-glutamate (l-Glu) and 5′-inosine monophosphate (IMP) synergistically increases duodenal HCO3− secretion via GLP-2 release. Since L cells express the bile acid receptor TGR5 and dipeptidyl peptidase (DPP) IV rapidly degrades GLPs, we hypothesized that luminal amino acids or bile acids stimulate duodenal HCO3− secretion via GLP-2 release, which is enhanced by DPPIV inhibition. We measured HCO3− secretion with pH and CO2 electrodes using a perfused rat duodenal loop under isoflurane anesthesia. l-Glu (10 mM) and IMP (0.1 mM) were luminally coperfused with or without luminal perfusion (0.1 mM) or intravenous (iv) injection (3 μmol/kg) of the DPPIV inhibitor NVP728. The loop was also perfused with a selective TGR5 agonist betulinic acid (BTA, 10 μM) or the non-bile acid type TGR5 agonist 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethylisoxazole-4-carboxamide (CCDC; 10 μM). DPPIV activity visualized by use of the fluorogenic substrate was present on the duodenal brush border and submucosal layer, both abolished by the incubation with NVP728 (0.1 mM). An iv injection of NVP728 enhanced l-Glu/IMP-induced HCO3− secretion, whereas luminal perfusion of NVP728 had no effect. BTA or CCDC had little effect on HCO3− secretion, whereas NVP728 iv markedly enhanced BTA- or CCDC-induced HCO3− secretion, the effects inhibited by a GLP-2 receptor antagonist. Coperfusion of the TGR5 agonist enhanced l-Glu/IMP-induced HCO3− secretion with the enhanced GLP-2 release, suggesting that TGR5 activation amplifies nutrient sensing signals. DPPIV inhibition potentiated luminal l-Glu/IMP-induced and TGR5 agonist-induced HCO3− secretion via a GLP-2 pathway, suggesting that the modulation of the local concentration of the endogenous secretagogue GLP-2 by luminal compounds and DPPIV inhibition helps regulate protective duodenal HCO3− secretion.
Keywords: glucagon-like peptide-2, L cells, TGR5, monosodium glutamate, taste receptor
postprandial nutrient sensing in the upper gastrointestinal mucosa regulates physiological mucosal responses in addition to controlling motility and secretion during digestion via the release of gut hormones and activation of neuronal pathways (1, 7). Luminal nutrients stimulate cognate intestinal epithelial nutrient receptors in order to exert rapid physiological responses. As luminal concentrated acid and high Pco2 enhance mucosal defense mechanisms in the duodenal mucosa (2), luminal nutrients may also affect mucosal responses to counter mucosal injury during digestion. Detection of luminal chemicals by mucosal surface sensors is attributed to nutrient sensing G protein-coupled receptors (GPCRs) expressed in the apical membranes of hormone-releasing enteroendocrine cells (11). Enteroendocrine L cells produce glucagon-like peptide-1 (GLP-1) and GLP-2 simultaneously from a prohormone, proglucagon. Stimulation of nutrient sensing receptors on L cells is emerging as a treatment for diabetes, since L cells release an incretin GLP-1 (9). L cells also release GLP-2, which has an intestinotropic effect (8).
Duodenal HCO3− secretion is the most studied defense mechanism against gastric acid. We have demonstrated that intravenous (iv) infusion of GLP-2, not GLP-1, stimulates duodenal HCO3− secretion in rats (39). GLP-2 also increases mesenteric blood flow (14), another foregut mucosal defense factor. Furthermore, the activation of an amino acid receptor, taste receptor 1 family heterodimer T1R1/T1R3, known as the umami receptor, by luminal perfusion of l-glutamate (l-Glu) with 5′-inosine monophosphate (IMP) increases duodenal HCO3− secretion via GLP-2 release and GLP-2 receptor activation, followed by nitric oxide (NO) and vasoactive intestinal peptide (VIP) release (4, 39). These studies suggest that the stimulation of nutrient-sensing GPCRs on L cells by luminal chemicals not only modulates glucose metabolism but also protects the duodenal mucosa from injury via the GLP-2 pathway.
Dipeptidyl peptidase IV (DPPIV), a serine protease, rapidly deactivates hormones and cytokines by cleaving their NH2-terminal dipeptide (27). For instance, the half-life of plasma GLP-1 is ∼2 min owing to DPPIV activity. DPPIV inhibitors therefore are used as antidiabetic therapy because of their enhancement of the insulinotropic effect of GLP-1 (9). Since GLP-2 is also a substrate for DPPIV (27), we hypothesized that DPPIV inhibition potentiates the stimulated HCO3− secretion by luminal nutrients.
Bile acids along with gastric acid are major components of the postprandial duodenal lumen. Luminal perfusion of taurocholic acid increases GLP-1 release in isolated vascularly perfused rat ileum (10). L cells express the bile acid receptor TGR5 (GPBAR, GPR131) (34), and TGR5 is involved in glucose-induced GLP-1 release (23, 36). In an L cell model GLUTag cells, bile acids increase the release of GLP-1 as well as GLP-2 (33). Therefore, we further hypothesized that luminal bile acids may modulate duodenal HCO3− secretion via TGR5 activation and GLP-2 release.
Here we show that DPPIV inhibition enhanced l-Glu/IMP-induced HCO3− secretion. The selective TGR5 agonists had little effect on HCO3− secretion, whereas DPPIV inhibition potentiated TGR5 agonist-induced HCO3− secretion. Furthermore, the TGR5 agonist enhanced l-Glu/IMP-induced HCO3− secretion accompanied by increased GLP-2 release. Therefore, the combination of luminal nutrient sensor ligands and DPPIV inhibition potently enhances duodenal defense against acid injury.
METHODS
Chemicals and animals.
NVP DPP 728 dihydrochloride (NVP728; 6-[[2-[[2-(2S)-2-cyano-1-pyrrolidinyl]-2-oxoethyl]amino]ethyl]amino-3-pyridinecarbono nitrile dihydrochloride) and betulinic acid (BTA; (+)-(3β)-3-hydroxylup-20(29)-en-28-oic acid) were obtained from Tocris Bioscience (Ellisville, MO). 3-(2-Chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethylisoxazole-4-carboxamide (CCDC) was from BioVision Research Products (Mountain View, CA). Glycylproline 7-amido-4-methylcoumarin bromide (GlyPro-AMC) was from Santa Cruz Biotechnology (Santa Cruz, CA). Rat GLP-2(3-33) was custom synthesized by Bachem Americas (Torrance, CA). Monosodium l-Glu, IMP, HEPES and other chemicals were obtained from Sigma Chemical (St. Louis, MO). Krebs solution contained (in mM) 136 NaCl, 2.6 KCl, 1.8 CaCl2, and 10 HEPES at pH 7.0. Osmolarity was adjusted to isotonic by reducing NaCl concentration. pH of Krebs solution after dissolving the compound was adjusted to pH 7.0. All solutions were prewarmed at 37°C in a water bath, and temperature was maintained with a heating pad. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee. Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) were fasted overnight but had free access to water.
Localization of DPPIV activity in rat duodenum.
The spatial localization of DPPIV enzyme activity was determined by using frozen sections of rat duodenum with the fluorogenic DPP substrate GlyPro-AMC (29, 38), via a method modified from that used to detect intestinal alkaline phosphatase activity in rat duodenum (3). After incubation of frozen sections, cut at 8-μm thickness, with propidium iodide (1 μM) for nuclear staining, Krebs solution containing 1 mM GlyPro-AMC was applied on the frozen section for 1 min with or without NVP728 (0.1 mM). The sections were observed under a fluorescent microscope with an excitation 380 nm and emission 460 nm during the reaction. Images were recorded with a cooled charge-coupled device video camera (Hamamatsu Orca-EN; Hamamatsu USA, Bridgewater, NJ) and were analyzed with image analysis software (OpenLab; Improvision, Lexington, MA).
Measurement of duodenal HCO3− secretion.
Duodenal loops were prepared and perfused as previously described (3, 4, 31). In brief, under isoflurane anesthesia (2%), the proximal duodenal loop (perfused length 2 cm) was perfused with pH 7.0 normal saline by using a peristaltic pump (Fisher Scientific, Pittsburgh, PA) at 1 ml/min. The perfusate was bubbled with 100% O2, and stirred and warmed at 37°C with a heating stirrer (Barnstead International, Dubuque, IA). The pH of the perfusate was kept constant at pH 7.0 with a pH stat (models PHM290 and ABU901; Radiometer Analytical, Lyon, France). Furthermore, to eliminate buffering by added compounds, which would over- or underestimate the pH-stat titration volume, two sets of flow-through pH and CO2 electrodes (Lazar Research Laboratories, Los Angeles, CA) were connected in the perfusion loop where pH and CO2 concentration ([CO2]) were simultaneously and continuously measured. Since the input (perfusate) [CO2] is ∼0, the effluent [CO2] and pH were used to calculate the total CO2 output equivalent to the secreted HCO3− as previously described (3, 31). To prevent contamination of the perfusate from bile or pancreatic juice, the pancreaticobiliary duct was ligated just proximal to its insertion into the duodenal wall and cannulated with a PE-10 tube to drain the juice. After stabilization with continuous perfusion of pH 7.0 saline for ∼30 min, the time was set as t = 0. The duodenal loop was perfused with pH 7.0 saline from t = 0 min until t = 10 min (basal period). The perfusate was then changed to pH 7.0 Krebs buffer from t = 10 min until t = 35 min (challenge period), with or without chemicals. At t = 10 min, the system was gently flushed so as to rapidly change the composition of the perfusate. Duodenal HCO3− secretion was expressed as total CO2 output calculated from the measured pH and [CO2] in the effluent solution as previously reported (3, 4).
Experimental protocol.
We have reported that luminal perfusion of l-Glu or IMP alone had little effect on duodenal HCO3− secretion, whereas coperfusion of l-Glu and IMP synergistically increased HCO3− secretion (4, 39). To investigate the effect of DPPIV inhibition on amino acid-induced HCO3− secretion, the DPPIV inhibitor NVP728 was coperfused (0.1 mM) or bolus iv injected (1 or 3 μmol/kg) with the luminal perfusion of l-Glu (10 mM) and IMP (0.1 mM).
To examine the effect of TGR5 agonists on duodenal HCO3− secretion, the duodenal loop was perfused with the TGR5 selective agonist BTA (10 μM) (13) or CCDC (10 μM) (12) with or without bolus iv injection of NVP728 (3 μmol/kg). To further examine the role of TGR5 activation in the nutrient-induced HCO3− secretion, BTA or CCDC was coperfused with l-Glu and IMP.
To confirm that the GLP-2 pathway is involved in the stimulated HCO3− secretion, a GLP-2 receptor antagonist GLP-2(3-33) (3 nmol/kg) was bolus iv injected before the challenge period (t = 10 min) as previously described (39).
Measurement of GLP-2 in portal venous blood.
Plasma concentration of GLP-2 was measured in the portal venous blood samples. The samples were collected after 25-min challenge period using a syringe containing 1 μl each of EDTA (0.5 mM) and NVP728 (10 μM). The samples were immediately centrifuged at 3,000 g for 5 min and their plasma were stored at −80°C until measurements. Plasma was diluted with Tris·HCl buffer (50 mM, pH 7.4) containing a protease inhibitor cocktail (1 mg/ml, Sigma) and NVP728 (10 μM). Plasma concentration of total GLP-2 was measured by using a GLP-2 (rat) enzyme immunoassay kit (ALPCO Diagnostics, Salem, NH) according to the manufacturer's protocol.
Statistics.
All data are expressed as means ± SE. Data were derived from six rats in each group. Comparisons between groups were made by one-way ANOVA followed by Fischer's least significant difference test. P values of < 0.05 were taken as significant.
RESULTS
Localization of DPPIV activity in rat duodenum.
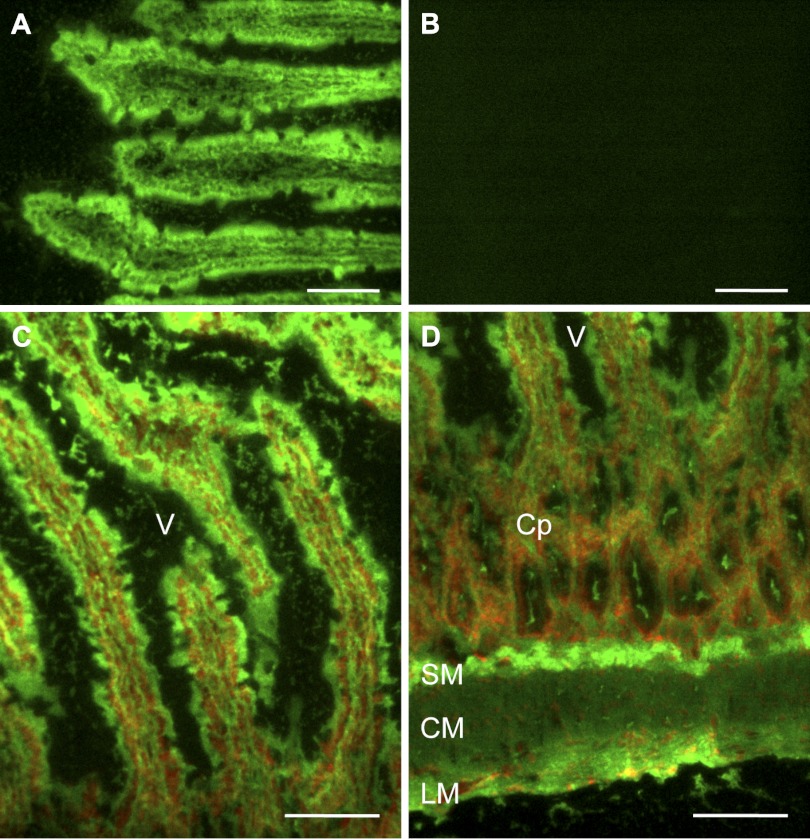
Incubation of a fluorogenic DPP substrate GlyPro-AMC on the duodenal frozen sections produced strong green fluorescent staining in the vicinity of the villous cells brush border membranes (BBM) (Fig. 1, A and C) and submucosal interstitial cells (Fig. 1D). The apical membrane of crypt cell was also weakly stained (Fig. 1D). Coincubation with a selective DPPIV inhibitor NVP728 abolished the staining (Fig. 1B), demonstrating that GlyPro-AMC fluorescence is DPPIV specific in rat duodenal mucosa. This result confirmed that DPPIV activity is mainly localized to the villous BBM as previously described (5) and in the submucosa such as in the capillaries-related structures (15).
Fig. 1.
Localization of dipeptidyl peptidase (DPP) IV (DPPIV) activity using frozen section of rat duodenum. Frozen cryostat sections were incubated with the fluorogenic DPP substrate glycylproline 7-amido-4-methylcoumarin bromide (GlyPro-AMC; 1 mM) for 1 min without (A) or with the DPPIV inhibitor NVP728 (NVP; 0.1 mM) (B). DPP activity (green) was strongly present in the villous cells (C) and in the submucosal layer (D). The section was counterstained with propidium iodide (red). V, villus; Cp, crypt; SM, submucosa; CM, circular muscle layer; LM, longitudinal muscle layer. Internal bars = 100 μm.
Effect of DPPIV inhibition on l-Glu/IMP-induced duodenal HCO3− secretion.
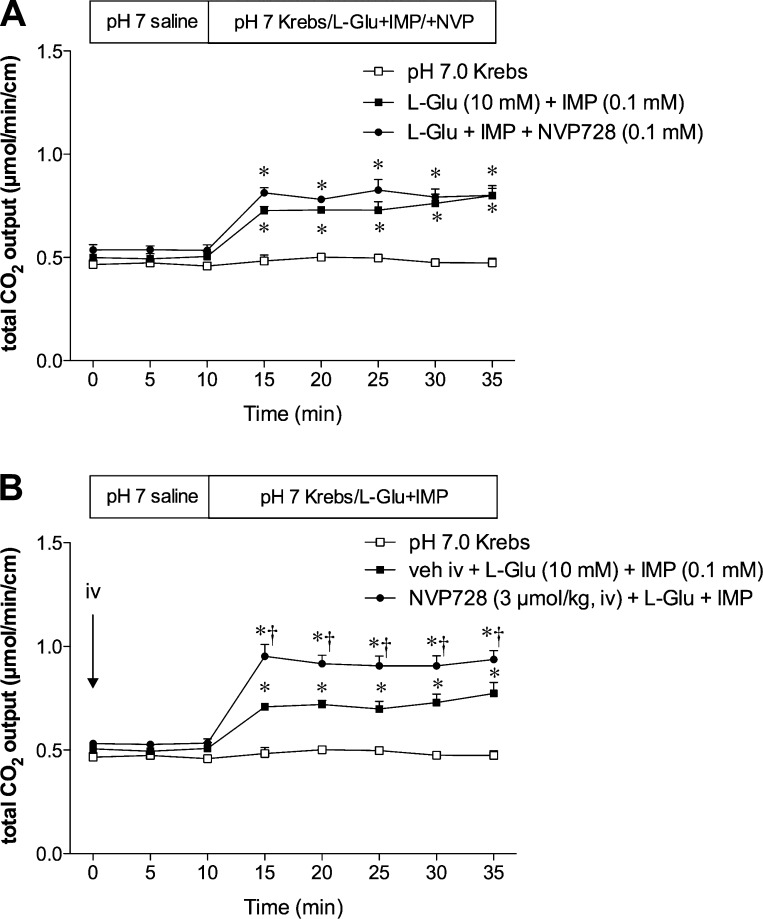
To examine the role of DPPIV on l-Glu/IMP-induced duodenal HCO3− secretion, we endeavored to inhibit BBM DPPIV with luminal perfusion of NVP728 (0.1 mM), and to inhibit submucosal DPPIV by iv injection of NVP728 (1 or 3 μmol/kg). Coperfusion of l-Glu (10 mM) with IMP (0.1 mM) increased total CO2 output (Fig. 2) measured as HCO3− secretion as previously reported (4, 39). Luminal perfusion of NVP728 had no effect on the l-Glu/IMP-induced augmented HCO3− secretion (Fig. 2A). Perfusion of NVP728 alone also had no effect on HCO3− secretion (data not shown). In contrast, iv injection of NVP728 at 3 μmol/kg enhanced the l-Glu/IMP-induced HCO3− secretion (Fig. 2B), despite having no effect on basal HCO3− secretion (t = 0–10 min). NVP728 iv at 1 μmol/kg had no effect (data not shown). Since l-Glu/IMP-induced HCO3− secretion is mediated by GLP-2 release and GLP-2 receptor activation (39), these results suggested that inhibition of the submucosal DPPIV, not BBM DPPIV, enhances the effect of released GLP-2.
Fig. 2.
Effect of DPPIV inhibition on l-glutamate (l-Glu)/5′-inosine monophosphate (IMP)-induced HCO3− secretion in rat duodenum. Duodenal HCO3− secretion was measured as total CO2 output by use of the flow-through pH and CO2 electrodes. The duodenal loop was perfused with l-Glu (10 mM) and IMP (0.1 mM). The DPPIV inhibitor NVP728 was luminally coperfused (0.1 mM) with l-Glu/IMP during the challenge period (A) or intravenously (iv) injected (3 μmol/kg) at t = 0 min (B). Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. vehicle iv + l-Glu + IMP group.
Effect of TGR5 agonists on duodenal HCO3− secretion.
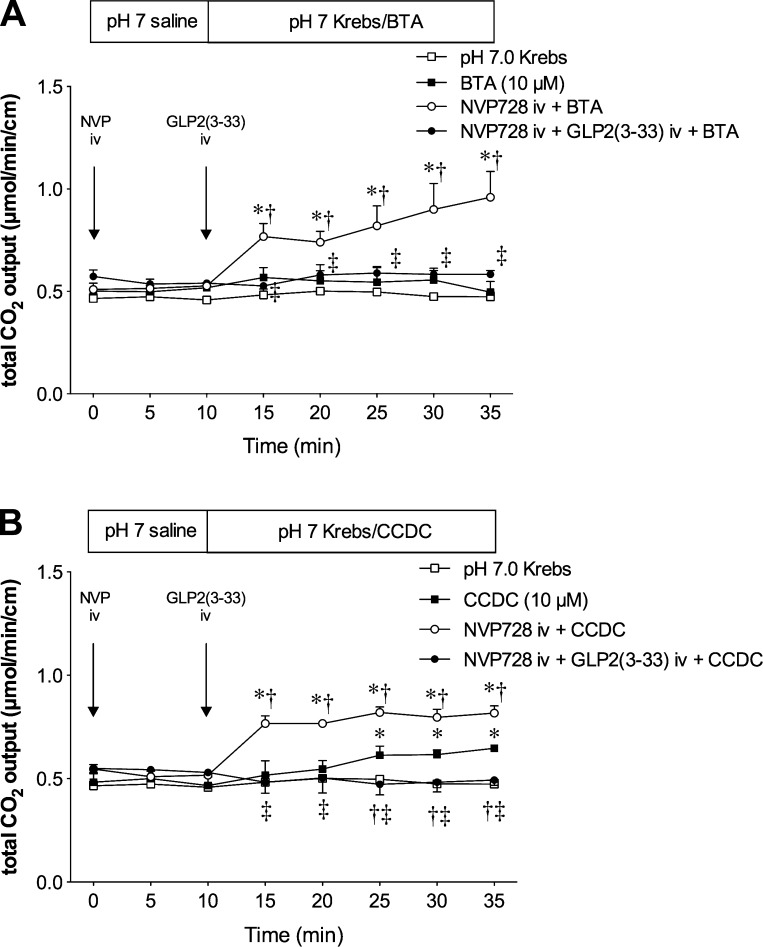
Next, we examined the effect of luminal perfusion of the selective TGR5 agonists on duodenal HCO3− secretion. Luminal perfusion of a bile acid type TGR5 agonist BTA (10 μM) had little effect on HCO3− secretion, which was enhanced by iv injection of NVP728 (Fig. 3A). Luminal perfusion of a non-bile acid type TGR5 agonist CCDC (10 μM) gradually increased HCO3− secretion, whereas iv injection of NVP728 remarkably enhanced CCDC-induced HCO3− secretion (Fig. 3B). Furthermore, the enhanced BTA- or CCDC-induced HCO3− secretion by NVP728 preinjection was abolished by injection of the GLP-2 receptor antagonist GLP-2(3-33), confirming the involvement of GLP-2 receptor activation. These results suggested that the small effect of TGR5 activation on HCO3− secretion is substantially enhanced by DPPIV inhibition and mediated by GLP-2 release.
Fig. 3.
Effect of luminal perfusion of TGR5 agonists on duodenal HCO3− secretion. Duodenal loop was perfused with a bile acid type TGR5 agonist betulinic acid (BTA, 10 μM) (A) or a non-bile acid type TGR5 agonist 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethylisoxazole-4-carboxamide (CCDC, 10 μM) (B). BTA or CCDC alone had little effect, whereas NVP728 (3 μmol/kg iv) potentiated BTA- or CCDC-induced HCO3− secretion, the effect abolished by GLP2(3-33) (3 nmol/kg, iv). Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. BTA group, ‡P < 0.05 vs. NVP+BTA or NVP+CCDC group.
Effect of coperfusion of l-Glu/IMP with TGR5 agonists on HCO3− secretion.
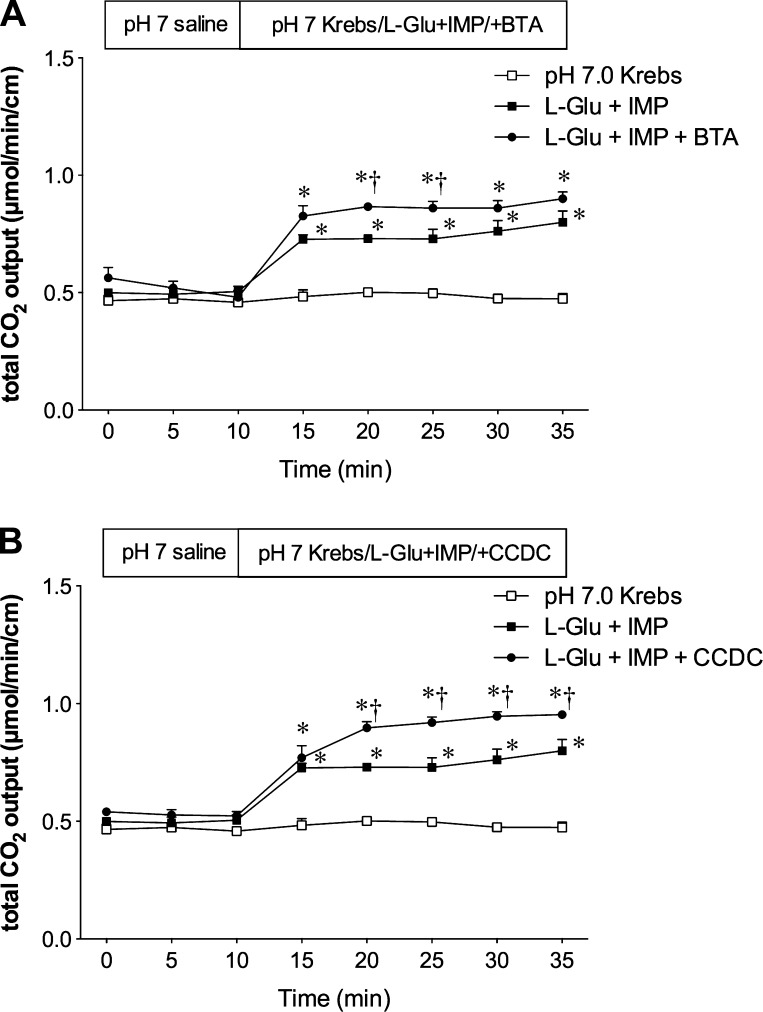
Since TGR5 activation increases intracellular cAMP (25) and enhances glucose-induced GLP-1 release, but alone had a little effect (36), we further hypothesized that TGR5 activation augments the nutrient-induced GLP2 release and consequent HCO3− secretion. BTA alone had little effect on HCO3− secretion, whereas coperfusion of BTA enhanced l-Glu/IMP-induced HCO3− secretion (Fig. 4A). Similarly, CCDC alone had a small effect on HCO3− secretion (Fig. 3B), whereas coperfusion of CCDC apparently enhanced l-Glu/IMP-induced HCO3− secretion (Fig. 4B).
Fig. 4.
Effect of coperfusion of TGR5 agonists on l-Glu/IMP-induced duodenal HCO3− secretion. Luminal coperfusion of BTA (A) or CCDC (B) with l-Glu (10 mM)/IMP (0.1 mM) further enhanced l-Glu/IMP-induced HCO3− secretion. Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu + IMP group.
GLP-2 release and duodenal HCO3− secretion.
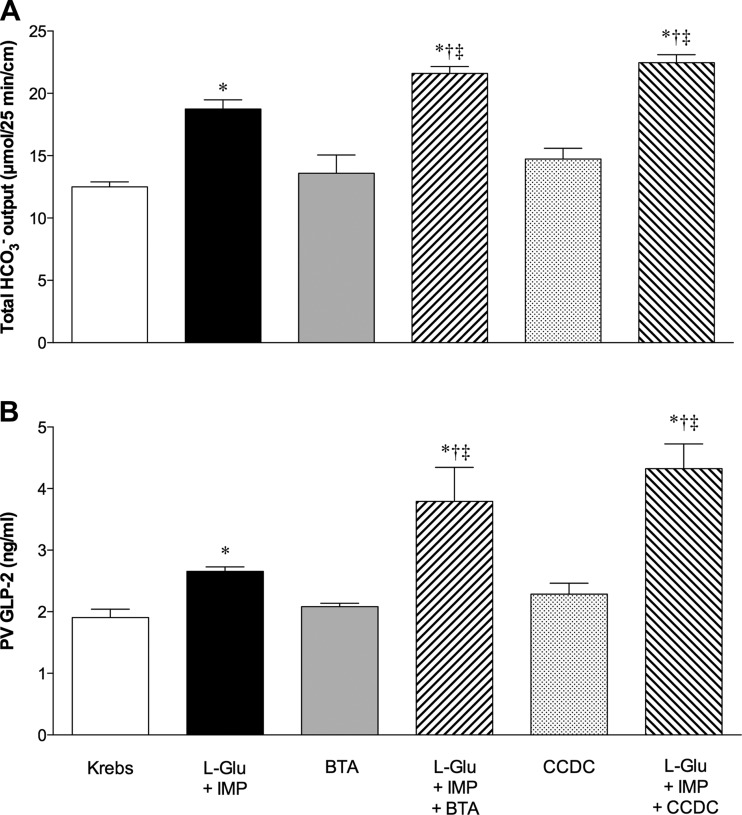
Total HCO3− secretion and plasma GLP-2 concentrations measured during or after the challenge period are summarized in Fig. 5. Increased GLP-2 release was correlated to the increased HCO3− secretion. l-Glu/IMP increased HCO3− secretion accompanied by augmented GLP-2 release as previously reported (39). BTA or CCDC alone had no effect on HCO3− secretion and GLP-2 release, whereas BTA or CCDC enhanced l-Glu/IMP-induced HCO3− secretion accompanied by enhanced GLP-2 release, suggesting that TGR5 activation amplifies the effect of l-Glu/IMP.
Fig. 5.
Effect of luminal perfusion of l-Glu/IMP or TGR5 agonists on HCO3− secretion and glucagon-like peptide-2 (GLP-2) release into portal vein (PV). A: total HCO3− output during the challenge period was calculated from Figs. 2–4. B: PV plasma concentration of GLP-2 was measured after 25-min perfusion of l-Glu (10 mM)/IMP (0.1 mM) and/or BTA (10 μM), or CCDC (10 μM). Each data point represents mean ± SE (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group, †P < 0.05 vs. l-Glu + IMP group, ‡P < 0.05 vs. the corresponding TGR5 agonist group.
DISCUSSION
We demonstrated that DPPIV inhibition potentiated l-Glu/IMP- and TGR5 agonist-induced duodenal HCO3− secretion via GLP-2 release and that TGR5 activation enhanced the effect of l-Glu/IMP on GLP-2 release followed by the stimulation of HCO3− secretion. This is the first study to demonstrate that DPPIV inhibition enhances duodenal physiological mucosal defenses and that combined activation of nutrient receptor and TGR5 enhances GLP-2 release in vivo. These results suggest that activation of chemosensors on L cells combined with a DPPIV inhibitor may protect the mucosa from injury via enhanced effects of GLP-2.
DPPIV inhibitors have been used clinically to improve insulin secretion and treat Type 2 diabetes via the enhancement of the effect of GLP-1 secreted from L cells (9). DPPIV inhibitors also prolong the half-life of GLP-2 (16). The intestinotropic effect of exogenous GLP-2 has been applied clinically to treat short bowel syndrome (21, 22), and the DPPIV inhibitor sitagliptin increases intestinal adaptation following intestinal resection in mice (32). We showed that although DPPIV activity was present on the brush borders and in the submucosa, enhancement of l-Glu/IMP-induced duodenal HCO3− secretion occurred only after iv injection of the DPPIV inhibitor. Since l-Glu/IMP-induced HCO3− secretion is mediated by a GLP-2 pathway (39), this result suggests that the DPPIV inhibitor increases the local, submucosal concentration of active GLP-2, enhancing HCO3− secretion, an important foregut defense factor.
The pathophysiological roles of bile acids are still controversial. Physiologically, bile acids are essential for long-chain fatty acid absorption and also can suppress bacterial growth in the upper intestinal lumen (30, 40). Oral administration of chenodeoxycholic acid increases plasma GLP-2 level and prevents intestinal villous atrophy induced by total parenteral nutrition (20). Cholic acid feeding alters gut microbiota composition, similar to the composition induced by high-fat diets (18). Bile acid exclusion or diversion reduces nonsteroidal anti-inflammatory drug-induced enteropathy (19). These opposite effects of bile acids may be due to their “detergent” effects owing to their amphipathic structure; beneficial for anti-bacterial effect and micelle formation, but potentially harmful owing to increased paracellular permeability. Furthermore, bile acids may modify cellular responses via the nuclear receptor farnesoid X receptor FXR (28). Therefore, we attempted to perfuse the selective TGR5 agonists, a bile acid-type BTA and non-bile acid-type CCDC, but not natural bile acids. Our preliminary data showed that luminal perfusion of lithocholic acid, taurolithocholic acid, or taurodeoxycholic acid at 10 μM had no effect on HCO3− secretion, whereas taurocholic acid (10 μM) increased HCO3− secretion, possibly due to anion exchanger-mediated absorption (Inoue Y, Akiba Y, Kaunitz JD, unpublished data). Nevertheless, BTA or CCDC had little effect on HCO3− secretion, whereas DPPIV inhibition with luminal BTA or CCDC enhanced HCO3− secretion, suggesting that TGR5 activation rather than the detergent or “nuclear” effects of bile acids mediates GLP-2 release. This result is consistent with a previous study showing that the TGR5 agonist INT-777 has little effect on GLP-1 release, whereas a DPPIV inhibitor with INT-777 enhances GLP-1 release, the effect abolished in TGR5 knockout mice (36). Furthermore, the enhanced TGR5 agonist-induced HCO3− secretion by DPPIV inhibitor preinjection was abolished by a GLP-2 receptor antagonist, supporting our hypothesis that DPPIV inhibition potentiates TGR5 activation-induced GLP-2 effect, followed by GLP-2 receptor activation on myenteric neurons (14), then releasing secretagogues NO and VIP (39) (Fig. 6). Capsaicin-sensitive afferent and vagal efferent nerves may not be involved in this pathway (39). Finally, the sequence of cellular mechanisms augments HCO3− output from epithelial cells including cAMP or cGMP, Na+: HCO3− cotransporter, carbonic anhydrases, and cystic fibrosis transmembrane regulator (24, 35).
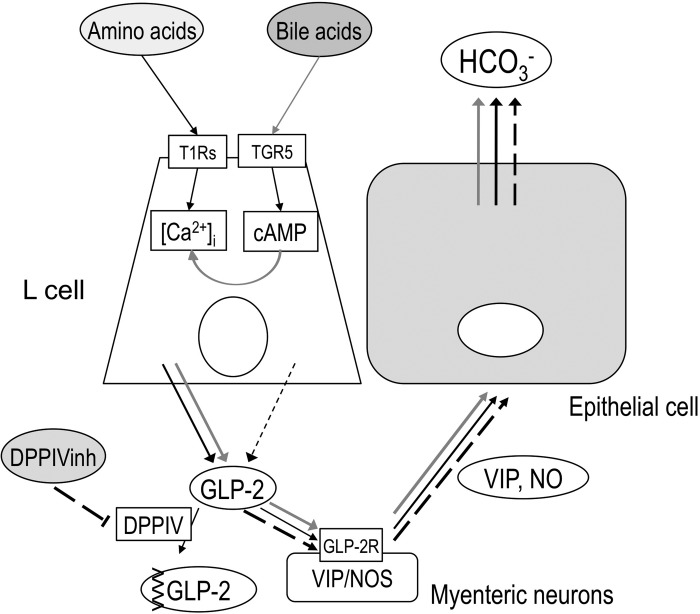
Fig. 6.
Proposed mechanisms of T1Rs- and TGR5-mediated GLP-2 release and HCO3− secretion. Luminal amino acid may activate T1Rs on L cells, releasing GLP-2 via raising intracellular Ca2+ concentration ([Ca2+]i), followed by activation of GLP-2 receptors (GLP-2R) on myenteric neurons containing VIP or NO synthase (NOS) (Ref. 14), increasing VIP and NO release (Ref. 39), and then increasing HCO3− secretion (black arrows). Luminal bile acids may activate TGR5 on L cells, increasing cAMP, but have little effect on GLP-2 release (black dashed arrow), whereas TGR5 activation may amplify the effect of amino acid-T1Rs-[Ca2+]i pathway, then enhance GLP-2 release, resulting the enhanced HCO3− secretion (gray arrows). DPPIV inhibition inhibits GLP-2 degradation, enhancing the effect of GLP-2 on HCO3− secretion (long dashed arrows).
In GLUTag cells, bile acids alone have a little effect on GLP-1 release, whereas TGR5 agonists enhance glucose-induced GLP-1 release (33), suggesting that TGR5 activation amplifies nutrient-induced GLP-1 release. We showed that BTA or CCDC enhanced l-Glu/IMP-induced HCO3− secretion with enhanced GLP-2 release into PV, consistent with the previous study. Since L cells express numerous nutrient GPCRs (34), these results suggest that combined activation of nutrient GPCR and TGR5 enhances GLP-2 release. The lack of GLP-2 release and HCO3− secretory response by TGR5 agonist alone, but the enhancement by DPPIV inhibition suggests that TGR5 activation at concentrations used in this study may not be adequate to release GLP-2 sufficient to augment the rate of HCO3− secretion in the presence of DPPIV activity.
The mechanism underlying TGR5-induced potentiation of nutrient chemosensing is still unclear. TGR5 activation increases intracellular cAMP concentration (25). Since nutrient GPCRs expressed in L cells are mostly Gαq-coupled or phospholipase Cβ2-related receptors (37), activation of nutrient receptors such as T1Rs with l-Glu/IMP may increase intracellular Ca2+ concentration ([Ca2+]i), which triggers hormone release from L cells (34). Increased cellular cAMP by Gαs-coupled receptor activation or exogenous cAMP analogs enhance the effect of [Ca2+]i via exchange protein directly activated by cAMP (Epac) (6, 26). Epac2 is present in islet β cells (17). In GLUTag cells transfected with Epac2-based FRET probe, the TGR5 agonist activates Epac2, followed by the enhanced [Ca2+]i and GLP-1 release in response to glucose (33), suggesting that the Epac pathway in L cells may explain how TGR5 activation amplifies T1Rs-mediated GLP-2 release.
In conclusion, DPPIV inhibition and TGR5 activation enhanced amino acid-induced HCO3− secretion in rat duodenum via GLP-2 pathway (Fig. 6). The combination of luminal nutrients, TGR5 agonists, and DPPIV inhibition may be a novel approach to GLP-2 related therapeutics for such conditions as intestinal ulcers, mucosal atrophy, and short bowel syndrome.
GRANTS
This research was supported by a research grant from Ajinomoto, Japan (Y. Akiba), Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J. Kaunitz), and the animal core of NIH-NIDDK P30 DK0413 (J. E. Rozengurt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.I., J.-H.W., M.H., S.R., and Y.A. performed experiments; T.I., J.-H.W., M.H., S.R., and Y.A. analyzed data; T.I., J.D.K., and Y.A. drafted manuscript; T.I., J.-H.W., M.H., S.R., K.H., P.H.G., E.E., J.D.K., and Y.A. approved final version of manuscript; K.H., J.D.K., and Y.A. edited and revised manuscript; P.H.G., E.E., J.D.K., and Y.A. interpreted results of experiments; J.D.K. and Y.A. conception and design of research; Y.A. prepared figures.
ACKNOWLEDGMENTS
We thank Coleen Palileo for assistance with manuscript preparation.
REFERENCES
- 1. Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion 83, Suppl 1: 25–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akiba Y, Kaunitz JD. Luminal chemosensing in the duodenal mucosa. Acta Physiol (Oxf) 201: 77– 84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293: G1223– G1233, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol 297: G781– G791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darmoul D, Rouyer-Fessard C, Blais A, Voisin T, Sapin C, Baricault L, Cibert C, Geraud G, Couvineau A, Laburthe M. Dipeptidyl peptidase IV expression in rat jejunal crypt-villus axis is controlled at mRNA level. Am J Physiol Gastrointest Liver Physiol 261: G763– G769, 1991 [DOI] [PubMed] [Google Scholar]
- 6. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474– 477, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol 54, Suppl 4: 9–17, 2003 [PubMed] [Google Scholar]
- 8. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93: 7911– 7916, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696– 1705, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139: 3780– 3786, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab 8: 447– 449, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Evans KA, Budzik BW, Ross SA, Wisnoski DD, Jin J, Rivero RA, Vimal M, Szewczyk GR, Jayawickreme C, Moncol DL, Rimele TJ, Armour SL, Weaver SP, Griffin RJ, Tadepalli SM, Jeune MR, Shearer TW, Chen ZB, Chen L, Anderson DL, Becherer JD, De Los FM, Colilla FJ. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J Med Chem 52: 7962– 7965, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem 53: 178– 190, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150– 164, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140: 5356– 5363, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hansen L, Hare KJ, Hartmann B, Deacon CF, Ugleholdt RK, Plamboeck A, Holst JJ. Metabolism of glucagon-like peptide-2 in pigs: role of dipeptidyl peptidase IV. Regul Pept 138: 126– 132, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53: 5– 13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773– 1781, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Jacob M, Foster R, Sigthorsson G, Simpson R, Bjarnason I. Role of bile in pathogenesis of indomethacin-induced enteropathy. Arch Toxicol 81: 291– 298, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 302: G218– G224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60: 902– 914, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806– 815, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386– 390, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kaunitz JD, Akiba Y. Review article: duodenal bicarbonate-mucosal protection, luminal chemosensing and acid-base balance. Aliment Pharmacol Ther 24, Suppl 4: 169–176, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435– 9440, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275– 2279, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40: 209– 294, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89: 147– 191, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Leiting B, Pryor KD, Wu JK, Marsilio F, Patel RA, Craik CS, Ellman JA, Cummings RT, Thornberry NA. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J 371: 525– 532, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorenzo-Zuniga V, Bartoli R, Planas R, Hofmann AF, Vinado B, Hagey LR, Hernandez JM, Mane J, Alvarez MA, Ausina V, Gassull MA. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 37: 551– 557, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573: 827– 842, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery 150: 217– 223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid stimulated glucagon-like peptide-1 secretion. Br J Pharmacol 165: 414– 423, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532– 539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther 130: 59– 70, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167– 177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587: 27– 32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Villhauer EB, Brinkman JA, Naderi GB, Burkey BF, Dunning BE, Prasad K, Mangold BL, Russell ME, Hughes TE. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem 46: 2774– 2789, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther 339: 464– 473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson FA, Sallee VL, Dietschy JM. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science 174: 1031– 1033, 1971 [DOI] [PubMed] [Google Scholar]